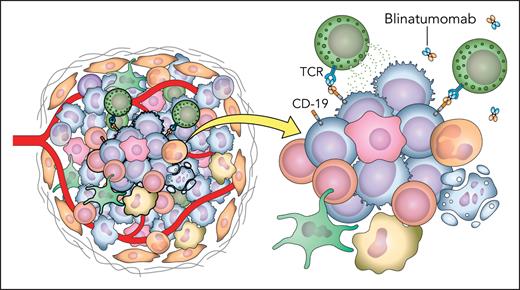
In this issue of Blood Advances, Long et al1 describe a novel mouse model with humanized CD3 that makes it ideal for studying bispecific antibodies (BsAbs). The authors provide proof-of-concept that this model recapitulates an immune architecture that closely resembles the human one. They transplant a B-cell leukemia cell line that expresses humanized CD19 and use blinatumomab in their experiments to show kinetics similar to what is seen in clinic.
The term “bispecific antibodies” refers to molecules with 2 separate binding domains that typically engage the immune system (T cell or natural killer cell) on 1 hand and a malignant cell on the other, with notable exceptions. This proximity is hypothesized to induce cell killing. Blinatumomab, the first bispecific T-cell engager (BiTE) on the market, initially received breakthrough designation and was then approved by the Food and Drug Administration in 2014 for treatment of Philadelphia chromosome–negative relapsed and refractory B-cell acute lymphoblastic leukemia (ALL).2 Preclinical studies for development were done in immunodeficient (NOD/SCID) mice using lymphoma cell lines and was efficacious.3 It was then trialed in patients with non-Hodgkin lymphoma, but responses were modest, likely because of low doses and interrupted infusions. A phase 2 study in patients with ALL with measurable residual disease showed 80% response rate.4 This launched a series of trials for relapsed/refractory disease, and upfront therapy in various combinations, including maintenance, which have all been fairly successful and well-received by the medical and regulatory community.5 This success ushered in an era of investigation into these molecules across a multitude of malignancies and targets. At the date of the preparation of this manuscript, there are 11 approved BsAbs with 7 being T-cell BsAbs (T-BsAbs) for oncological indications (see table).6 These T-BsAbs have shown remarkable efficacy in malignancies that are highly treatment-resistant.
List of BsAbs used to treat malignancies
| Name . | Approval date . | Target . | Indication . |
|---|---|---|---|
| Blinatumomab | 2014 | CD3 × CD19 | ALL |
| Amivantamab | 2021 | MET × EGFR | Non–small cell lung cancer |
| Tebentafusp | 2022 | CD3 × HLA-A∗02:01 | Uveal melanoma |
| Teclistamab | 2022 | CD3 × BCMA | Multiple myeloma |
| Mosunetuzumab | 2022 | CD3 × CD20 | Follicular lymphoma |
| Epcoritamab | 2023 | CD3 × CD20 | Diffuse large B–cell lymphoma |
| Talquetamab | 2023 | CD3 × GPRC5D | Multiple myeloma |
| Glofitamab | 2023 | CD3 × CD20 | Diffuse large B–cell lymphoma |
| Name . | Approval date . | Target . | Indication . |
|---|---|---|---|
| Blinatumomab | 2014 | CD3 × CD19 | ALL |
| Amivantamab | 2021 | MET × EGFR | Non–small cell lung cancer |
| Tebentafusp | 2022 | CD3 × HLA-A∗02:01 | Uveal melanoma |
| Teclistamab | 2022 | CD3 × BCMA | Multiple myeloma |
| Mosunetuzumab | 2022 | CD3 × CD20 | Follicular lymphoma |
| Epcoritamab | 2023 | CD3 × CD20 | Diffuse large B–cell lymphoma |
| Talquetamab | 2023 | CD3 × GPRC5D | Multiple myeloma |
| Glofitamab | 2023 | CD3 × CD20 | Diffuse large B–cell lymphoma |
BCMA, B-cell maturation antigen; EGFR, epidermal growth factor; MET, mesenchymal epithelial transition factor.
Blinatumomab in the initial phase 2 study had unusual toxicities and unique pharmacokinetics. Patients had infusion reactions, cytokine release syndrome, and neurotoxicity, including seizures attributed to polyclonal activation of T cells. The effect on T and B cells was measured in peripheral blood at various times points and shows T-cell redistribution shortly after infusion followed by an expansion of T cells, most notably effector memory T cells. This was also hypothesized to be the cause of the long remissions seen with blinatumomab.4 Relapses occurred, and there was an effort to elucidate the mechanisms of failure and resistance early in development. Some of the more commonly described mechanisms are loss of CD19 through a variety of mechanisms, such as alternate splicing of CD19 and lineage switch.7 Data for these hypotheses are derived from very few relapsed patient samples and/or cell lines. Immune escape mechanisms were also hypothesized with 1 study describing activation of regulatory T cells by blinatumomab in the peripheral blood and association with resistance to therapy.8 The timing of emergence of the CD19 clones, the activity of blinatumomab in extramedullary disease, and the defined T-cell subset that drives remissions and relapses all remain controversial.
There is no doubt that T-BsAbs have revolutionized, at the very least, the treatment of hematological malignancies. However, there are still many unanswered questions. How much do secondary immune mechanisms, such as macrophages and natural killer cells, contribute to the eradication of the tumor? What kind of reprogramming does the T cell undergo during this process? What drives this activation and homing into secondary lymphoid organs and can that be used to guide T cells to other solid tumors and extramedullary disease? Which T-cell subset(s) is (are) the most effective and can we design better T-BsAbs to specifically target that? Conversely, which are detrimental, and can we predict which tumors will be resistant and the hosts who will experience significant toxicities? Does T-cell exhaustion contribute to treatment failure, and can we overcome that by simply modifying the schedule of administration; what about synergistic combinations? These questions and many more are posed in every forum when 1 of these studies is presented. There are many working hypotheses for these questions, but they are generally an inference of associative data gathered from peripheral blood and a handful of patient samples for whom these therapies failed, and now we have a model that will help answer these.
The mouse model described herein offers an immunocompetent canvas with the whole coffer of secondary lymphoid organs and native microenvironment allowing us to look closely at the interactions between the different immune subsets. The authors carefully selected litters that have CD4 to CD8 ratios that approximate wild-type littermates. They not only humanized the leukemia cell lines but also accounted for rejection by crossing the human target antigen into the litter to have it recognized as self. They transfected humanized T cells and showed that they are independently capable of inducing cell killing. To quote the authors for the final product, “syngeneic models offer a native, fully matched microenvironment and secondary lymphoid systems for T cell interactions.” The humanized T cells redistributed to secondary lymphoid organs, and the authors were able to show that they are immunophenotypically different from T cells in peripheral blood. The animals also experienced cytokine release syndrome with a cytokine profile that closely mirrors that of patients. This model can shed light on the complex interactions between T cells and the rest of the immunome and allows high-level analysis for future research (see figure).
Depiction of the complex immune interactions and tumor microenvironment present in vivo. TCR, T-cell receptor. Professional illustration by Patrick Lane, ScEYEnce Studios.
Depiction of the complex immune interactions and tumor microenvironment present in vivo. TCR, T-cell receptor. Professional illustration by Patrick Lane, ScEYEnce Studios.
Conflict-of-interest disclosure: J.E.M.’s institution receives research funding on his behalf from Gilead, Atara Bio, CRISPR, Precision Biosciences, and Scripps Research Institute. The author declares no competing financial interests.