Knockdown of IGF2BP3 sensitizes MLL-AF4 leukemia to menin-MLL inhibition.
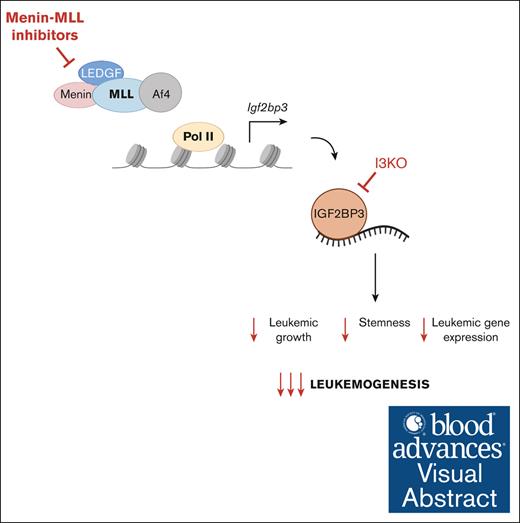
These data support the role of IGF2BP3 as an oncogenic amplifier in MLL-AF4 leukemogenesis and its therapeutic potential.
Visual Abstract
RNA-binding proteins (RBPs) are emerging as a novel class of therapeutic targets in cancer, including in leukemia, given their important role in posttranscriptional gene regulation, and have the unexplored potential to be combined with existing therapies. The RBP insulin-like growth factor 2 messenger RNA–binding protein 3 (IGF2BP3) has been found to be a critical regulator of MLL-AF4 leukemogenesis and represents a promising therapeutic target. Here, we study the combined effects of targeting IGF2BP3 and menin-MLL interaction in MLL-AF4–driven leukemia in vitro and in vivo, using genetic inhibition with CRISPR-Cas9–mediated deletion of Igf2bp3 and pharmacologic inhibition of the menin-MLL interaction with multiple commercially available inhibitors. Depletion of Igf2bp3 sensitized MLL-AF4 leukemia to the effects of menin-MLL inhibition on cell growth and leukemic initiating cells in vitro. Mechanistically, we found that both Igf2bp3 depletion and menin-MLL inhibition led to increased differentiation in vitro and in vivo, seen in functional readouts and by gene expression analyses. IGF2BP3 knockdown had a greater effect on increasing survival and attenuating disease than pharmacologic menin-MLL inhibition with small molecule MI-503 alone and showed enhanced antileukemic effects in combination. Our work shows that IGF2BP3 is an oncogenic amplifier of MLL-AF4–mediated leukemogenesis and a potent therapeutic target, providing a paradigm for targeting leukemia at both the transcriptional and posttranscriptional level.
Introduction
Leukemias driven by translocations in the mixed-lineage leukemia 1 gene (MLL1/KMT2A) represent a subgroup of particular interest because of their unique clinical and biological characteristics and poor prognosis. Chromosomal rearrangements in the MLL1 gene result in fusion proteins, with several partners that are part of the superelongation complex, leading to aberrant gene expression.1-3 Comprehensive genomic studies of patient-derived MLL-rearranged leukemia cells show that these leukemias have very few genetic alterations,4,5 suggesting that epigenetic and transcriptional dysregulation driven by the fusion proteins are largely responsible for leukemogenesis. This is further borne out by the demonstration of the sufficiency of MLL fusion protein overexpression, for example, MLL-AF9 and MLL-Af4, to drive leukemia in both murine and human hematopoietic stem and progenitor cells (HSPCs).6-8 MLL-leukemogenesis requires the interaction of the N-terminal end of the fusion protein with menin, in addition to its interactions with downstream chromatin modifiers and the superelongation complex.9-11 Menin is a molecular adapter protein that in turn interacts with epigenetic regulators and cell signaling molecules, driving massive dysregulation of gene expression.
In addition to epigenetic and transcriptional mechanisms, the importance of posttranscriptional regulation by factors such as microRNAs and RNA-binding proteins (RBPs) has been increasingly appreciated in leukemogenesis. Our group implicated the oncofetal RBP insulin-like growth factor 2 messenger RNA (mRNA)–binding protein 3 (IGF2BP3) as a critical regulator of MLL-AF4–mediated leukemogenesis.12,13 We found that MLL-AF4 transcriptionally induced Igf2bp3 and that IGF2BP3 regulates important leukemogenic transcripts, amplifying the aberrant gene expression initiated by MLL-AF4.13 Furthermore, IGF2BP3 is required for the efficient initiation of MLL-Af4–driven leukemia and the function of leukemic initiating cells (LICs), thereby establishing a critical role of IGF2BP3 in the pathogenesis of MLL–driven leukemia and its potential as a promising therapeutic target.
Existing strategies for treating MLL-rearranged leukemia include chemotherapy, small molecule inhibitors, and CD19-directed therapies. Novel therapeutic strategies for MLL-rearranged leukemia have been directed toward targeting the menin-MLL interaction as well as various epigenetic factors.14,15 Multiple potent small molecule inhibitors that have been developed to target the menin-MLL1 interaction, including MI-503, MI-463, MI-3454, and VTP-50469, have demonstrated impressive single-agent activity in preclinical studies, with derivatives entering phase 1 to 2 clinical trials.16-20 Nonetheless, combinatorial strategies remain a cornerstone of cancer-directed therapy to combat issues with dose-limiting toxicities and drug resistance from single-agent therapy. Therefore, we sought to explore a novel therapeutic strategy of combinatorial targeting of leukemia at the transcriptional and posttranscriptional level. Our approach combined the pharmacologic inhibition of the menin-MLL interaction with the genetic inhibition of IGF2BP3.
Here, we tested the combined effects of IGF2BP3 knockdown via CRISPR/Cas9–mediated deletion and menin-MLL inhibition using commercially available menin-MLL inhibitors in human B-cell acute lymphoblastic leukemia (B-ALL) cell lines and a murine model for MLL-Af4–driven leukemia.6 Depletion of IGF2BP3 sensitized MLL-AF4 leukemia to the effects of menin-MLL inhibition on cell growth and LICs. Mechanistically, we found that both knockdown of IGF2BP3 and menin-MLL inhibition led to increased differentiation of MLL-Af4 leukemia, with a more dramatic effect seen when both were combined. Concordantly, gene expression analyses demonstrated an upregulation of differentiation genes with MI-503 treatment and IGF2BP3 knockdown. Lastly, we found decreased leukemic engraftment and significantly increased survival with IGF2BP3 knockdown in comparison to MI-503 treatment and enhanced antileukemic effects with the combination. Together, our work supports the idea that IGF2BP3 is an oncogenic amplifier of MLL-AF4–mediated leukemogenesis, and IGF2BP3 represents an exciting therapeutic target. Our data also support a novel combinatorial therapeutic strategy of targeting leukemia at both the transcriptional and posttranscriptional levels.
Methods
Cell culture
RS4;11 (American Type Culture Collection (ATCC) CRL-1873), NALM6 (ATCC CRL-3273), and SEM (DMZ-ACC 546) were cultured as previously described.21 Immortalized MLL-Af4–transformed HSPCs derived from mouse bone marrow (BM; MLL-Af4 lineage-negative [Lin–] cells) were cultured in Iscove modified Dulbecco medium with 15% fetal bovine serum, supplemented with murine 100 ng/mL stem cell factor, 4 ng/mL murine interleukin-6, 50 ng/mL human Fms-like tyrosine kinase 3 ligand (FLT3L), and 50 ng/mL mouse thrombopoietin (mTPO).
Plasmids and viral transduction
The MSCV-MLL-FLAG-Af4 plasmid was generously provided by Michael Thirman (University of Chicago) through a material transfer agreement.21 Single-guide RNAs (sgRNAs) against mouse Igf2bp3 and nontargeting (NT) guides were cloned into an inhouse MSCV-hu6-sgRNA-EFS-mCherry vector.21 Single-guide RNAs against human IGF2BP3 and nontargeting guides were cloned into pLKO.sgRNA.EFS.tRFP (Addgene 57823). Sequences are available in supplemental Table 1. Generation of retroviral and lentiviral supernatants and viral transduction were performed according to standard procedures.
CRISPR/Cas9–mediated deletion of IGF2BP3 in cell lines
SEM, RS4;11, and NALM6 were depleted for IGF2BP3 using lentiviral delivery of CRISPR/Cas9 components in a 2-vector system as previously described.21 MLL-Af4 Lin– cells were initially isolated from Cas9–green fluorescent protein (Cas9-GFP) mouse BM and immortalized after transduction with MLL-Af4 retrovirus, as previously described.21 Cells then underwent a second transduction with retroviral supernatant containing sgRNA against Igf2bp3 (I3sg2 and I3sg3) or nontargeting (NT-1 and NT-2) and sorted on GFP and mCherry positivity.
Molecular biology assays
mRNA extracts and cell lysates were prepared, and western blot and reverse transcription quantitative polymerase chain reaction (qRT-PCR) were performed as previously described.12 Reverse transcription quantitative polymerase chain reaction (RT-qPCR) primer sequences are listed in supplemental Table 1. Antibodies used were IGF2BP3 anti-rabbit polyclonal (RNP009, Medical and Biological Laboratories) and β-actin anti-mouse monoclonal (A5441, Sigma-Aldrich).
In vitro assays for cell viability and apoptosis, with drug treatment
Cells were treated with drug or 0.1% dimethyl sulfoxide (DMSO) control, using concentrations and time periods specified in figure legends. The following menin-MLL inhibitors were used: MI-503 (SML2520, Sigma-Aldrich), MI-463 (HY-19809, MedChemExpress), and MI-538 (HY-19810, MedChemExpress). Cell viability assays were performed using a luminescent assay based on adenosine triphosphate quantitation (CellTiterGlo, Promega). To measure apoptosis, cells were analyzed for annexin V by flow cytometry (BV421, BD Biosciences, BDB563873) and caspase-3/7 activity by a luminescent-based assay (Caspase-Glo 3/7 Assay, Promega).
Methylcellulose-based colony-forming unit assays
The assay was performed by seeding drug-treated MLL-Af4 Cas9 Lin– cells into MethoCult colony-forming media (STEMCELL Technologies, M3434) at seeding densities of 250 to 2500. Cells were cultured in MethoCult media for 10 to 12 days and counted for total colony number and morphologic subtypes.
Flow cytometry
Isolation of single-cell suspensions and staining with fluorochrome-conjugated antibodies were performed per standard procedures.12 Antibodies are provided in supplemental Table 2. Flow cytometry was performed on a BD LSRII and analyzed using FlowJo software.
RNA sequencing
Paired-end RNA sequencing was performed on an Illumina NovaSeq S4 (UC Davis Genome Center). RNA-sequencing reads were mapped to the mouse genome assembly mm18 using STAR 2.5.3 and Bowtie 2.30,31 Differentially expressed genes were identified using DESeq2.25 Multiple testing correction was performed using the Benjamini–Hochberg method. Significant differentially expressed genes have adjusted P value ≤.05 and absolute log2FC ≥1. Enrichment analyses were completed with Metascape.29 Overlap between differentially expressed genes and publicly available gene sets for MLL-AF4 chromatin immunoprecipitation (CHIP) and IGF2BP3 cross-link immunoprecipitation (CLIP) in CD11b+ and Lin– leukemic cells was evaluated using Fisher exact test. Raw data have been deposited to the NCBI Short Read Archive (accession number PRJNA918326).
Mice and BM transplantation
C57BL/6J, B6.SJL-PtprcaPepcb/BoyJ (B6 CD45.1), and B6J.129(Cg)-Gt(ROSA)26Sortm1.1(CAG-cas9∗,-EGFP)Fezh/J (Cas9-GFP BL/6J) mice were obtained from The Jackson Laboratory. MLL-Af4 Lin– cells were transplanted into 8- to 10-week-old CD45.1 female recipients (or C57BL/6J in pilot experiments). Recipients were conditioned with busulfan 30 mg/kg intraperitoneally twice, three days and two days prior to transplantation, followed by retro-orbital injection of 2x105 Lin– cells with 105 CD45.1 carrier marrow cells (with the exception of the experiment in supplemental Figure 4, which used a dose of 3x105 Lin– cells). This study received approval from the Chancellor's Animal Research Committee at UCLA, the responsible body for animal use approvals at UCLA. No human participants were involved in the research.
Histopathology
Fixation, sectioning, and analysis were performed as previously described (DSR).32
Statistics
Data shown represent mean ± standard deviation for continuous numerical data. Two-tailed Student t tests or 1-way analysis of variance followed by Bonferroni multiple comparisons test were performed using GraphPad Prism software and conducted as described in the figure legends. Survival analyses were performed using the Kaplan-Meier method with comparisons made using log-rank tests, followed by Bonferroni correction for multiple comparisons.
Results
IGF2BP3 knockdown increases sensitivity of MLL-r leukemia cells to menin-MLL inhibition in vitro
We assessed the effects of combining menin-MLL inhibition with IGF2BP3 knockdown in human B-ALL cell lines and murine immortalized HSPCs transformed with MLL-Af4 (referred herein as MLL-Af4 Lin–) as a means of inhibiting MLL-AF4 leukemogenesis at the transcriptional and posttranscriptional level. To knock down IGF2BP3 in human B-ALL cell lines, we used a 2-vector lentiviral system as previously described to deliver Cas9 and sgRNA targeting IGF2BP3 (I3-sg2) or NT in 2 MLL-AF4–expressing human B-ALL cell lines, RS4;11 and SEM, and an additional human B-ALL cell line without MLL-AF4 translocation, NALM6. To deplete IGF2BP3 in MLL-Af4 Lin– cells, MLL-Af4 Cas9-GFP Lin– cells were transduced with a retroviral vector containing sgRNA targeting Igf2bp3 (I3-sg2 or I3-sg3) as previously described13,21 (Figure 1A; supplemental Figures 1B and 2A). MLL-Af4 Lin– cells treated with menin-MLL inhibitor, MI-503, for 4 days showed a dose-dependent decrease in IGF2BP3 expression, consistent with our prior findings that MLL-Af4 drives Igf2bp3 expression13 (Figure 1B).
IGF2BP3 knockdown increases sensitivity of MLL-r leukemia cells to menin-MLL inhibition. (A) Western blot analysis showing IGF2BP3 knockdown in RS4;11, NALM6, and SEM cell lines and MLL-Af4 Lin– cells (using I3KO sgRNA targeting human and mouse genes and NT guide). (B) Western blot analysis showing IGF2BP3 expression in MI-503 treated MLL-Af4 Lin– cells depleted (I3KO) or nondepleted for IGF2BP3 (NT). Cells were treated with MI-503 (0.2 μM, 1.0 μM, or DMSO control) for 4 days. (C-E) Dose-response curves from cell viability assays, using CellTiterGlo, of human B-ALL cell lines, SEM, RS4;11, and NALM6, depleted (I3KO) vs nondepleted (NT) for IGF2BP3 treated with menin-MLL inhibitors (MI-503, MI-463, and MI-538) for 4 days. Viability has been normalized to DMSO control-treated cells not depleted for IGF2BP3 (NT DMSO); mean ± standard deviation (SD); n = 6. (F) Dose-response curves from cell viability assays, using CellTiterGlo, of MLL-Af4 Lin– cells depleted for IGF2BP3 (I3KO) vs nondepleted (NT) treated with menin-MLL inhibitors for 4 days. Viability has been normalized to DMSO control-treated cells not depleted for IGF2BP3 (NT DMSO); mean ± SD; n = 6. (G) Increased caspase 3/7 activity of MI-503 treated MLL-Af4 Lin– cells depleted for IGF2BP3 (I3KO) vs nondepleted (NT). Cells treated with MI-503 for 4 days at various concentrations for dose response. Caspase 3/7 activity measured using Caspase-Glo 3/7 and normalized to the activity of DMSO-treated control; mean ± SD, n = 3. (H) Increased annexin V positivity in MLL-Af4 Lin– I3KO cells (vs NT) and with MI-503 treatment (vs DMSO control); mean ± SD; n = 3 (1-way analysis of variance [ANOVA] with Bonferroni multiple comparisons test; ∗∗P < .01; ∗∗∗∗P < .0001). (I-J) Histograms from representative samples for annexin V staining, analyzed by flow cytometry. kDa, kilodalton.
IGF2BP3 knockdown increases sensitivity of MLL-r leukemia cells to menin-MLL inhibition. (A) Western blot analysis showing IGF2BP3 knockdown in RS4;11, NALM6, and SEM cell lines and MLL-Af4 Lin– cells (using I3KO sgRNA targeting human and mouse genes and NT guide). (B) Western blot analysis showing IGF2BP3 expression in MI-503 treated MLL-Af4 Lin– cells depleted (I3KO) or nondepleted for IGF2BP3 (NT). Cells were treated with MI-503 (0.2 μM, 1.0 μM, or DMSO control) for 4 days. (C-E) Dose-response curves from cell viability assays, using CellTiterGlo, of human B-ALL cell lines, SEM, RS4;11, and NALM6, depleted (I3KO) vs nondepleted (NT) for IGF2BP3 treated with menin-MLL inhibitors (MI-503, MI-463, and MI-538) for 4 days. Viability has been normalized to DMSO control-treated cells not depleted for IGF2BP3 (NT DMSO); mean ± standard deviation (SD); n = 6. (F) Dose-response curves from cell viability assays, using CellTiterGlo, of MLL-Af4 Lin– cells depleted for IGF2BP3 (I3KO) vs nondepleted (NT) treated with menin-MLL inhibitors for 4 days. Viability has been normalized to DMSO control-treated cells not depleted for IGF2BP3 (NT DMSO); mean ± SD; n = 6. (G) Increased caspase 3/7 activity of MI-503 treated MLL-Af4 Lin– cells depleted for IGF2BP3 (I3KO) vs nondepleted (NT). Cells treated with MI-503 for 4 days at various concentrations for dose response. Caspase 3/7 activity measured using Caspase-Glo 3/7 and normalized to the activity of DMSO-treated control; mean ± SD, n = 3. (H) Increased annexin V positivity in MLL-Af4 Lin– I3KO cells (vs NT) and with MI-503 treatment (vs DMSO control); mean ± SD; n = 3 (1-way analysis of variance [ANOVA] with Bonferroni multiple comparisons test; ∗∗P < .01; ∗∗∗∗P < .0001). (I-J) Histograms from representative samples for annexin V staining, analyzed by flow cytometry. kDa, kilodalton.
IGF2BP3 knockdown resulted in dramatically enhanced menin-MLL inhibition of cell growth in SEM and RS4;11 treated with MI-503, MI-463, and MI-538 for 4 days in luminescence-based cell viability assays (Figure 1C-D). In contrast, there was no difference in sensitivity to the effects of menin-MLL inhibition on cell growth in NALM6, a human B-ALL cell line without MLL-AF4 translocation, with IGF2BP3 knockdown (Figure 1E). This synergistic effect of IGF2BP3 knockdown and menin-MLL inhibition on cell growth was also seen in our murine MLL-Af4 Lin– cells across multiple menin-MLL inhibitors and 2 different guides targeting Igf2bp3 (Figure 1F; supplemental Figure 2D). MI-503 did not cause a significant increase in apoptosis of MLL-Af4 NT Lin– cells by annexin V staining or caspase 3/7 activity, except at the highest concentration of 1.0 μM, similar to the limited effect on cell growth (Figure 1F-I). MI-503 had a greater effect on apoptosis in MLL-Af4 Lin– I3KO cells by increased caspase 3/7 activity and annexin V positivity (Figure 1G-J). Together, these findings highlight the sensitization of MLL-Af4 leukemia cells to menin-MLL inhibition with IGF2BP3 knockdown, with enhanced inhibition of cell growth and increased apoptosis.
IGF2BP3 knockdown enhances effect of menin-MLL inhibition on LIC and differentiation
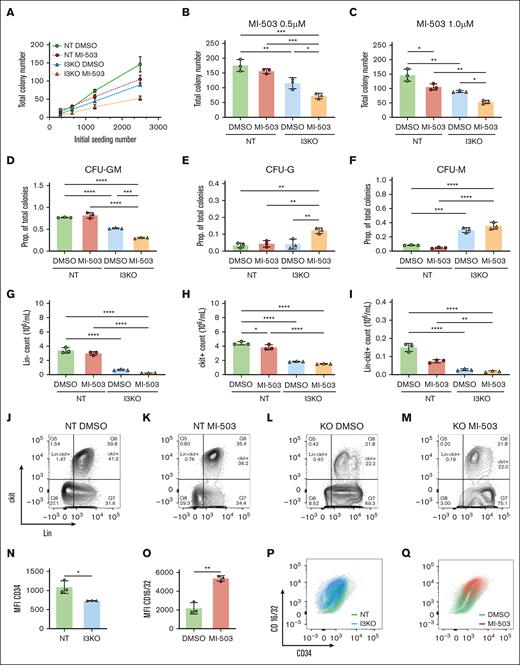
MLL-AF4 inhibition has been shown to decrease LICs and increase differentiation.7,22 To characterize the combined effects of IGF2BP3 knockdown and menin-MLL inhibition on LICs in vitro, we seeded MI-503–treated MLL-Af4 I3KO and NT cells into end point colony formation assays and performed flow cytometric analyses, including measurements of c-Kit expression, an LIC marker that has been reported in MLL model systems.6,13,18,23,24 IGF2BP3 knockdown more significantly reduced the total colony number than MI-503 treatment (Figure 2A). MI-503–treated MLL-Af4 Lin– I3KO cells showed further reduction in total colony formation, compared with IGF2BP3 knockdown alone, suggesting that the combined inhibitory effect on LICs is at least additive and is dose dependent (Figure 2A-C). Both MI-503 treatment and IGF2BP3 knockdown in MLL-Af4 Lin– cells showed a shift toward more differentiated colony morphologies in colony formation assays, decreased c-Kit expression, increased expression of maturation markers by flow cytometry, and morphologic changes consistent with increased differentiation. Decreased granulocyte-macrophage colony-forming unit (CFU-GM) and increased granulocyte colony-forming unit (CFU-G) and macrophage colony-forming unit (CFU-M) progenitor colonies were seen with MI-503 and in I3KO cells, with the maximal effect seen in the combination (Figure 2D-F). By flow cytometry, we found a marked reduction in the number of Lin– cells with I3KO, consistent with increased differentiation (Figure 2G,J-M). We also saw a decrease in c-Kit+ cells, more profoundly with I3KO than with MI-503 treatment, and with the maximal effect seen in combination (Figure 2H). Furthermore, expression of CD16/32, a marker for granulocyte-macrophage differentiation, was increased with MI-503 treatment (Figure 2O,Q), whereas expression of CD34, a marker that has been used to identify and enrich LICs, was decreased with I3KO (Figure 2N,P). This pattern of increased differentiation with MI-503 treatment and IGF2BP3 depletion was seen morphologically in cytospin preparations (supplemental Figure 3). Altogether, these findings confirm that both IGF2BP3 and menin inhibition affect the number and differentiation state of LICs and that combined inhibition appears to have an additive effect in our in vitro assay readouts.
Combined IGF2BP3 knockdown and menin inhibition increases differentiation of MLL-Af4 leukemia. (A) Total colony numbers of MI-503–treated MLL-Af4 Lin– cells, depleted (I3KO) or nondepleted (NT) for IGF2BP3. MLL-Af4 Lin– NT and I3KO cells were treated with MI-503 0.5 μM for 4 days and seeded in methylcellulose colony formation assays at various initial seeding densities and cultured for 10 days. (B,C) Total colony number was reduced with both I3KO and MI-503 treatment at 0.5 μM (B) and 0.5 μM (C) in methylcellulose colony formation assays at initial seeding density of 2500. (D) Proportion of CFU-GM colonies are decreased with both I3KO and MI-503 (at 0.5 μM). (E-F) Proportion of CFU-G and -M colonies are increased with both I3KO and MI-503 (at 0.5 μM). (G-I) Lin–, c-kit+, and Lin–c-kit+ cells are decreased with both I3KO and MI-503. MLL-Af4 Lin– NT and I3KO cells were treated with MI-503 0.5 mM for 7 days and then analyzed by flow cytometric immunophenotyping; mean ± SD, n = 3; 1-way ANOVA with Bonferroni multiple comparison’s test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). (J-M) Corresponding representative flow cytometry plots of MLL-Af4 Lin– NT and I3KO cells treated with MI-503 (or DMSO control), showing expression of c-kit and Lin markers. (N) Decreased CD34 expression by mean fluorescence intensity with I3KO; mean ± SD; n = 3; student t test, ∗P < .05. (O) Decreased CD16/32 expression by mean fluorescence intensity with MI-503 treatment; mean ± SD; n = 3; Student t test, ∗∗P < .01. (P-Q) Corresponding representative flow cytometry plots of MLL-Af4 Lin– NT and I3KO cells treated with MI-503 (or DMSO control), showing expression of CD16/32 and CD34. CFU-GM, granulocyte-macrophage colony-forming units; CFU-G, granulocyte colony-forming units; CFU-M, macrophage colony-forming units; MFI, mean fluorescence intensity; prop, proportion.
Combined IGF2BP3 knockdown and menin inhibition increases differentiation of MLL-Af4 leukemia. (A) Total colony numbers of MI-503–treated MLL-Af4 Lin– cells, depleted (I3KO) or nondepleted (NT) for IGF2BP3. MLL-Af4 Lin– NT and I3KO cells were treated with MI-503 0.5 μM for 4 days and seeded in methylcellulose colony formation assays at various initial seeding densities and cultured for 10 days. (B,C) Total colony number was reduced with both I3KO and MI-503 treatment at 0.5 μM (B) and 0.5 μM (C) in methylcellulose colony formation assays at initial seeding density of 2500. (D) Proportion of CFU-GM colonies are decreased with both I3KO and MI-503 (at 0.5 μM). (E-F) Proportion of CFU-G and -M colonies are increased with both I3KO and MI-503 (at 0.5 μM). (G-I) Lin–, c-kit+, and Lin–c-kit+ cells are decreased with both I3KO and MI-503. MLL-Af4 Lin– NT and I3KO cells were treated with MI-503 0.5 mM for 7 days and then analyzed by flow cytometric immunophenotyping; mean ± SD, n = 3; 1-way ANOVA with Bonferroni multiple comparison’s test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). (J-M) Corresponding representative flow cytometry plots of MLL-Af4 Lin– NT and I3KO cells treated with MI-503 (or DMSO control), showing expression of c-kit and Lin markers. (N) Decreased CD34 expression by mean fluorescence intensity with I3KO; mean ± SD; n = 3; student t test, ∗P < .05. (O) Decreased CD16/32 expression by mean fluorescence intensity with MI-503 treatment; mean ± SD; n = 3; Student t test, ∗∗P < .01. (P-Q) Corresponding representative flow cytometry plots of MLL-Af4 Lin– NT and I3KO cells treated with MI-503 (or DMSO control), showing expression of CD16/32 and CD34. CFU-GM, granulocyte-macrophage colony-forming units; CFU-G, granulocyte colony-forming units; CFU-M, macrophage colony-forming units; MFI, mean fluorescence intensity; prop, proportion.
IGF2BP3 knockdown in MLL-Af4 Lin– cells affects the global and MLL-AF4 transcriptome and leads to the upregulation of genes involved in differentiation
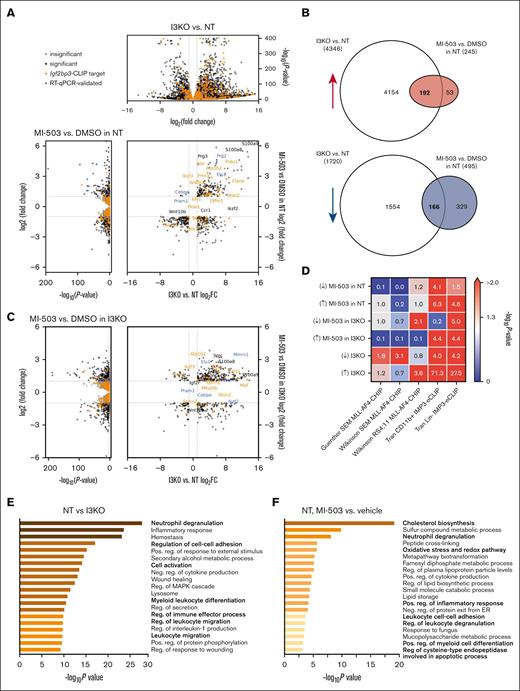
To gain insight into the phenotypic findings described, we next examined gene expression from cells with combined menin-MLL inhibition and IGF2BP3 knockdown. We performed RNA-sequencing analysis in MLL-Af4 Lin– cells with the following treatment conditions: (1) MLL-Af4 Lin– cells depleted for IGF2BP3 (I3KO) and treated with MI-503 (at 0.2 and 1.0 μM) or DMSO control and (2) MLL-Af4 Lin– cells nondepleted for IGF2BP3 (NT) and treated with MI-503 (at 0.2 and 1.0 μM) or DMSO control. Comparison of DMSO-treated MLL-Af4 Lin– I3KO vs NT cells showed dramatic changes in the global transcriptome with 4346 upregulated and 1720 downregulated genes by differential expression analysis with DESeq225,26 (Figure 3A; supplemental Figure 4A). In contrast, fewer genes are differentially expressed in MLL-Af4 Lin– NT with MI-503 than with DMSO (Figure 3A; supplemental Figure 4A). Remarkably, there is a highly significant overlap between genes that are differentially expressed with IGF2BP3 knockdown and MI-503 treatment (Hypergeometric test; P = 0). Seventy-eight percent of genes that are upregulated with MI-503 treatment are also upregulated with IGF2BP3 knockdown, and 34% of the genes that are downregulated with MI-503 treatment are downregulated with IGF2BP3 knockdown (Figure 3B).
Increased upregulation of genes involved in differentiation with IGF2BP3 knockdown and menin-MLL inhibition in MLL-Af4 leukemia. (A) Volcano plots of differentially expressed genes with IGF2BP3 knockdown (top) and with MI-503 treatment (left) in MLL-Af4 Lin– NT cells, using DESeq analysis on RNA-sequencing samples from MLL-Af4 Lin– NT or I3KO cells treated with MI-503 1.0 μM or DMSO vehicle. (B) Venn diagram of shared differentially expressed genes with IGF2BP3 knockdown and MI-503 treatment in MLL-Af4 Lin– NT cells. (C) Volcano plots of differentially expressed genes with IGF2BP3 knockdown (top) and with MI-503 treatment (left) in MLL-Af4 Lin– I3KO cells, using DESeq analysis on RNA-sequencing samples from MLL-Af4 Lin– NT or I3KO cells treated with MI-503 1.0 μM or DMSO vehicle. (D) Overlap between differentially expressed genes with MI-503 in MLL-Af4 Lin– NT or I3KO cells and with IGF2BP3 knockdown and known MLL-AF4 ChIP targets from previously published data sets in SEM and RS4;11 and known IGF2BP3 eCLIP targets from previously published data sets of MLL-Af4 CD11b+ and MLL-Af4 Lin– cells. P values calculated using Fisher exact test. (E) Pathway enrichment for upregulated genes with IGF2BP3 knockdown using Metascape analysis webtool on MLL-Af4 Lin− IGF2BP3 DESeq data set with an adjusted P < .05 cutoff. (F) Pathway enrichment for upregulated genes with MI-503 treatment using Metascape analysis webtool on MLL-Af4 Lin− NT MI-503 DESeq data set with an adjusted P < .05 cutoff. FC, fold change.
Increased upregulation of genes involved in differentiation with IGF2BP3 knockdown and menin-MLL inhibition in MLL-Af4 leukemia. (A) Volcano plots of differentially expressed genes with IGF2BP3 knockdown (top) and with MI-503 treatment (left) in MLL-Af4 Lin– NT cells, using DESeq analysis on RNA-sequencing samples from MLL-Af4 Lin– NT or I3KO cells treated with MI-503 1.0 μM or DMSO vehicle. (B) Venn diagram of shared differentially expressed genes with IGF2BP3 knockdown and MI-503 treatment in MLL-Af4 Lin– NT cells. (C) Volcano plots of differentially expressed genes with IGF2BP3 knockdown (top) and with MI-503 treatment (left) in MLL-Af4 Lin– I3KO cells, using DESeq analysis on RNA-sequencing samples from MLL-Af4 Lin– NT or I3KO cells treated with MI-503 1.0 μM or DMSO vehicle. (D) Overlap between differentially expressed genes with MI-503 in MLL-Af4 Lin– NT or I3KO cells and with IGF2BP3 knockdown and known MLL-AF4 ChIP targets from previously published data sets in SEM and RS4;11 and known IGF2BP3 eCLIP targets from previously published data sets of MLL-Af4 CD11b+ and MLL-Af4 Lin– cells. P values calculated using Fisher exact test. (E) Pathway enrichment for upregulated genes with IGF2BP3 knockdown using Metascape analysis webtool on MLL-Af4 Lin− IGF2BP3 DESeq data set with an adjusted P < .05 cutoff. (F) Pathway enrichment for upregulated genes with MI-503 treatment using Metascape analysis webtool on MLL-Af4 Lin− NT MI-503 DESeq data set with an adjusted P < .05 cutoff. FC, fold change.
Next, we looked at the overlap between differentially expressed genes with MI-503 and I3KO with MLL-AF4 ChIP targets and IGF2BP3 CLIP targets identified in CD11b+ and Lin– MLL-Af4 leukemic cells.13,27,28 We found significant overlap of differentially expressed genes with MI-503 treatment with IGF2BP3 CLIP targets (by Fisher exact test; Figure 3D), suggesting that IGF2BP3 directly regulates genes that are affected by menin-MLL inhibition. Conversely, there was an overlap seen between differentially expressed genes with IGF2BP3 knockdown and MLL-AF4 ChIP targets identified in SEM and RS4;11, human MLL-AF4 leukemia cell lines (Figure 3D). These overlap patterns highlight the interaction between the MLL-AF4 and IGF2BP3 transcriptomes and suggest a mechanism for the synergistic inhibition of leukemia by targeting both MLL-AF4 and IGF2BP3.
In comparisons of upregulated genes in NT and I3KO cells, we identified a significant enrichment by Metascape analysis29 in pathways involved in cell differentiation and activation, particularly in leukocytes: neutrophil degranulation, regulation of cell-cell adhesion, cell activation, myeloid leukocyte differentiation, and leukocyte migration (Figure 3E). Although fewer genes were differentially expressed with MI-503 treatment, partial pathway enrichment overlap was observed (eg, neutrophil degranulation), but additional distinct pathways were affected by MI-503 treatment (eg, oxidative stress and redox pathways and some metabolic pathways; Figure 3F). Pathway enrichment analyses of genes differentially expressed with MI-503 treatment in I3KO cells also revealed pathways important in hematopoietic differentiation (eg, leukocyte migration and neutrophil degranulation; Figure 3C; supplemental Figure 4D). Pathway enrichment analyses of downregulated genes in NT vs I3KO, NT with and without MI-503 treatment, and I3KO with and without MI-503 treatment revealed a variety of pathways involved in signaling, stem cell homeostasis, and immune-related pathways (supplemental Figure 4B-D).
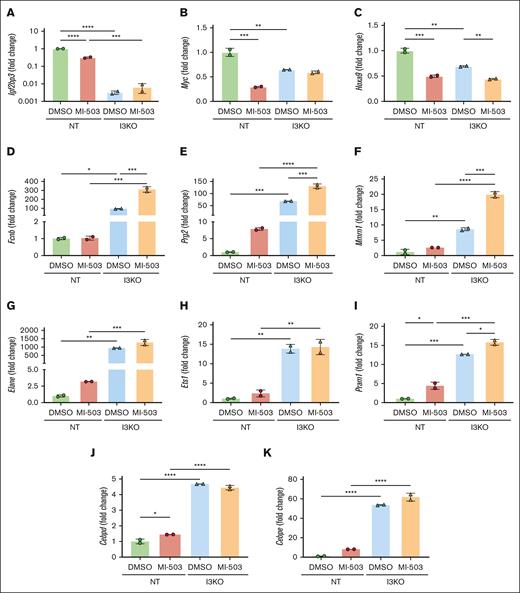
Given the phenotypic alterations to a more differentiated state and the strong enrichment for hematopoietic differentiation pathways among upregulated genes, we next looked to validate our findings using individual qRT-PCR for these genes of interest. As anticipated, we found a decrease in the expression of Igf2bp3 and its known target mRNAs, Myc and Hoxa9, in NT cells treated with MI-503 (Figure 4A-C). Next, we focused on the enriched differentially upregulated genes involved in leukocyte differentiation, Fcnb, Prg2, Mmrn1, Elane, Ets1, Pram1, Cebpd, and Cepbe. A significant increase was seen in all of these genes with Igf2bp3 depletion (Figure 4D-K). Fold change was significantly higher with I3KO than with MI-503 treatment (Figure 4D-K). For genes Fcnb, Prg2, Mmrn1, and Pram1, MI-503 treatment led to a significant increase in expression in MLL-Af4 Lin– I3KO, whereas a nonsignificant increase was seen with the other genes of interest, Elane, Ets1, Cebpd, and Cebpe, suggesting a saturating effect of Igf2bp3 depletion on upregulation of these genes. Together, our analyses demonstrate that similar, but not identical, impacts on gene expression are engendered by genetic inhibition of IGF2BP3 and menin-MLL inhibition with MI-503. We propose that these impacts on gene expression underlie the phenotypic effects seen upon the coinhibition of transcriptional and posttranscriptional gene expression regulation.
Increased upregulation of genes involved in differentiation with IGF2BP3 knockdown and menin-MLL inhibition in MLL-Af4 leukemia, validated by qRT-PCR. (A-C) MI-503 treatment at 1.0 μM leads to downregulation of Igf2bp3 and known IGF2BP3 targets, Myc and Hoxa9, in MLL-Af4 Lin– cells. (D-K) Upregulation of genes involved in differentiation with IGF2BP3 knockdown and MI-503 treatment. mRNA expression was measured by qRT-PCR in MLL-Af4 Lin– cells depleted (I3KO) or nondepleted (NT) for IGF2BP3 and treated with MI-503 1.0 μM or DMSO control. Expression shown as fold change from NT DMSO (mean ± SD; n = 2; 1-way ANOVA with Bonferroni multiple comparisons test, ∗P < .05; ∗∗ P < .01; ∗∗∗P < .001; ∗∗∗∗ P < .0001).
Increased upregulation of genes involved in differentiation with IGF2BP3 knockdown and menin-MLL inhibition in MLL-Af4 leukemia, validated by qRT-PCR. (A-C) MI-503 treatment at 1.0 μM leads to downregulation of Igf2bp3 and known IGF2BP3 targets, Myc and Hoxa9, in MLL-Af4 Lin– cells. (D-K) Upregulation of genes involved in differentiation with IGF2BP3 knockdown and MI-503 treatment. mRNA expression was measured by qRT-PCR in MLL-Af4 Lin– cells depleted (I3KO) or nondepleted (NT) for IGF2BP3 and treated with MI-503 1.0 μM or DMSO control. Expression shown as fold change from NT DMSO (mean ± SD; n = 2; 1-way ANOVA with Bonferroni multiple comparisons test, ∗P < .05; ∗∗ P < .01; ∗∗∗P < .001; ∗∗∗∗ P < .0001).
IGF2BP3 knockdown and MI-503 treatment decrease engraftment of MLL-Af4 leukemia cells in vivo
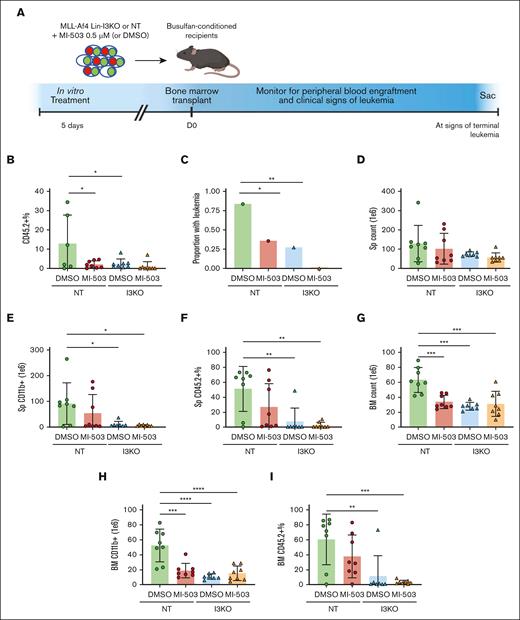
To further characterize our findings in vivo, we developed and validated a congenic CD45.1/CD45.2 transplantation model for the murine MLL-Af4 leukemia, using the earlier-described cellular systems (MLL-Af4 Lin– NT and I3KO) derived from murine BM. In timed experiments terminated at 8 to 10 weeks, I3KO demonstrated a clear reduction in engraftment (assessed using mCherry+ and GFP+ cells; supplemental Figure 5B), gross leukemia or histologic preleukemia (supplemental Figure 5C-D), and spleen weights (supplemental Figure 5E). At the cellular level, I3KO led to reductions in cell counts, CD11b+ cells, and engraftment in the spleen (supplemental Figure 5F-G) and BM (supplemental Figure 5I-K). Next, we transplanted either NT or I3KO Lin– cells (derived from CD45.2+ mice) into CD45.1+ mice after 5 days of treatment with MI-503 in vitro (Figure 5A). Six weeks after transplantation, evaluation of peripheral blood showed that both treatment with MI-503 and IGF2BP3 depletion led to decreased CD45.2+ cells in the peripheral blood, reflective of decreased leukemic engraftment (Figure 5B). In end point experiments at 8.5 weeks, IGF2BP3 depletion led to a significantly decreased proportion of mice with gross leukemia at necropsy, decreased BM counts, and decreased CD11b+ count and CD45.2+ percentage in the spleen and BM (Figure 5C,D-I). MI-503 treatment resulted in a modest decrease in the proportion of mice with gross leukemia, total cellular and CD11b+ counts, and CD45.2+ percentage in the spleen and BM (Figure 5D).
Combinatorial inhibition of menin-MLL and IGF2BP3 decreases leukemic engraftment and burden and increases survival in vivo. (A) Schematic representation of BM transplantation of MI-503–treated MLL-Af4 Lin– cells, depleted (I3KO) or nondepleted (NT) for IGF2BP3. Cells treated with MI-503 0.5 μM (or DMSO control) for 5 days in vitro were transplanted into CD45.1 recipients after busulfan conditioning. (B) Decreased peripheral blood engraftment of leukemic cells by CD45.2+ percentage with MI-503 treatment and I3KO at D42 (mean ± SD; n = 8 mice per group; 1-way ANOVA with Bonferroni multiple comparison’s test, ∗ P < .05). (C) Decreased proportion of mice with leukemia with MI-503 treatment and IGF2BP3 knockdown, (8 mice per group; Fisher exact test; ∗ P < .05; ∗∗ P < .01). Mice were all evaluated at necropsy at 8.5 weeks after the first mouse developed signs of terminal leukemia. (D-I) Decreased leukemic burden seen with IGF2BP3 knockdown and MI-503 treatment in NT groups, based on total counts, CD11b+ counts, and CD45.2+ percentage by flow cytometry in the spleen (D-F) and BM (G-I) (mean ± SD; n = 7-8 mice per group; 1-way ANOVA with Bonferroni multiple comparison’s test, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). Mice were all evaluated at necropsy at 8.5 weeks after the first mouse developed signs of terminal leukemia.
Combinatorial inhibition of menin-MLL and IGF2BP3 decreases leukemic engraftment and burden and increases survival in vivo. (A) Schematic representation of BM transplantation of MI-503–treated MLL-Af4 Lin– cells, depleted (I3KO) or nondepleted (NT) for IGF2BP3. Cells treated with MI-503 0.5 μM (or DMSO control) for 5 days in vitro were transplanted into CD45.1 recipients after busulfan conditioning. (B) Decreased peripheral blood engraftment of leukemic cells by CD45.2+ percentage with MI-503 treatment and I3KO at D42 (mean ± SD; n = 8 mice per group; 1-way ANOVA with Bonferroni multiple comparison’s test, ∗ P < .05). (C) Decreased proportion of mice with leukemia with MI-503 treatment and IGF2BP3 knockdown, (8 mice per group; Fisher exact test; ∗ P < .05; ∗∗ P < .01). Mice were all evaluated at necropsy at 8.5 weeks after the first mouse developed signs of terminal leukemia. (D-I) Decreased leukemic burden seen with IGF2BP3 knockdown and MI-503 treatment in NT groups, based on total counts, CD11b+ counts, and CD45.2+ percentage by flow cytometry in the spleen (D-F) and BM (G-I) (mean ± SD; n = 7-8 mice per group; 1-way ANOVA with Bonferroni multiple comparison’s test, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). Mice were all evaluated at necropsy at 8.5 weeks after the first mouse developed signs of terminal leukemia.
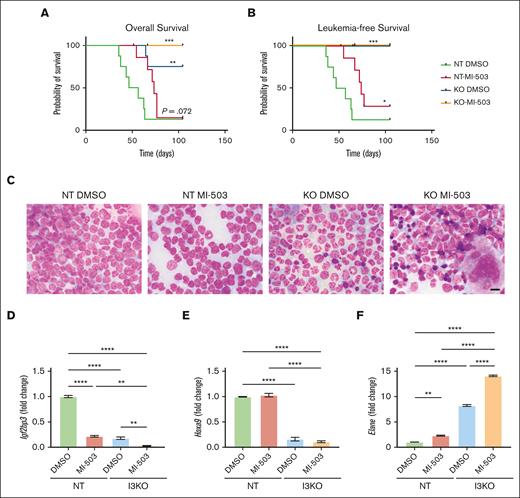
In addition to the aforementioned end point experiments, we evaluated mice for overall survival and leukemia-free survival in a separate experiment. MI-503 treatment showed a modest effect with a delay in leukemia progression but without improvement in overall survival in mice that received transplantation with MLL-Af4 leukemia (NT; comparison of MI-503 vs DMSO; Figure 6A-B). In contrast, IGF2BP3 depletion significantly increased overall and leukemia-free survival in mice that received transplantation with MLL-Af4 leukemia (NT DMSO vs I3KO DMSO; Figure 6A-B). This increase in overall and leukemia-free survival was also seen in mice that received transplantation with MI-503-treated MLL-Af4 leukemia cells (comparison NT MI-503 vs I3KO MI-503). Interestingly, histopathologic analysis showed morphologic changes consistent with increased differentiation in both the BM and spleens of mice that received transplantation with MI-503–treated or IGF2BP3-depleted MLL-Af4 Lin– cells, with the cells appearing most differentiated in mice that received transplantation with MI-503 treated I3KO cells (Figure 6C; supplemental Figure 6A). Furthermore, in BM samples from these mice, we saw an upregulation in granulocytic differentiation-related genes, Cebpe, Elane, Fcnb, Prg1, Mmrn1, and Ets1, previously identified in our evaluation of the Lin– cells in vitro (Figure 6D-F; supplemental Figure 6C-G). Together, the data confirm that MI-503 and I3KO both have impacts on leukemogenesis, with an additive effect on leukemic engraftment and cellular differentiation.
Combinatorial inhibition of menin-MLL and IGF2BP3 increases survival in vivo and is accompanied by concordant changes in gene expression. (A-B) Increased overall survival and leukemia survival with IGF2BP3 knockdown and MI-503 treatment in NT groups (n = 8 mice per group; Kaplan-Meier with log-rank test, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001). Comparisons made vs NT DMSO. Follow-up to 15 weeks with terminal sac of remaining mice. (C) Photomicrographs of Wright-stained BM smears from mice as above; original magnification, ×1000; scale bar, 10 μm. (D-F) Expression of genes of interest in the BM of mice that received transplantation with MI-503–treated MLL-Af4 Lin– NT or I3KO cells was measured by qRT-PCR. MLL-Af4 Lin– cells depleted (I3KO) or nondepleted (NT) for IGF2BP3 were treated with MI-503 0.5 mM (MI-503) or carrier control (DMSO) for 5 days in vitro before transplantation. Mice were euthanized at 8.5 weeks at first signs of the first mouse developing terminal leukemia. Gene expression data shown from selected mice in each group. Shown as fold change from NT DMSO (mean ± SD; n = 2; 1-way ANOVA with Bonferroni multiple comparisons test, ∗P < .05; ∗∗ P < .01; ∗∗∗P < .001; ∗∗∗∗ P < .0001).
Combinatorial inhibition of menin-MLL and IGF2BP3 increases survival in vivo and is accompanied by concordant changes in gene expression. (A-B) Increased overall survival and leukemia survival with IGF2BP3 knockdown and MI-503 treatment in NT groups (n = 8 mice per group; Kaplan-Meier with log-rank test, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001). Comparisons made vs NT DMSO. Follow-up to 15 weeks with terminal sac of remaining mice. (C) Photomicrographs of Wright-stained BM smears from mice as above; original magnification, ×1000; scale bar, 10 μm. (D-F) Expression of genes of interest in the BM of mice that received transplantation with MI-503–treated MLL-Af4 Lin– NT or I3KO cells was measured by qRT-PCR. MLL-Af4 Lin– cells depleted (I3KO) or nondepleted (NT) for IGF2BP3 were treated with MI-503 0.5 mM (MI-503) or carrier control (DMSO) for 5 days in vitro before transplantation. Mice were euthanized at 8.5 weeks at first signs of the first mouse developing terminal leukemia. Gene expression data shown from selected mice in each group. Shown as fold change from NT DMSO (mean ± SD; n = 2; 1-way ANOVA with Bonferroni multiple comparisons test, ∗P < .05; ∗∗ P < .01; ∗∗∗P < .001; ∗∗∗∗ P < .0001).
Discussion
In this study, we tested a novel combinatorial strategy of targeting leukemia at the transcriptional and posttranscriptional level using commercially available inhibitors of menin-MLL and genetic inhibition of IGF2BP3, an RBP critical to MLL-Af4–mediated leukemogenesis.13 We found that IGF2BP3 knockdown enhanced the therapeutic effect of pharmacologic menin-MLL inhibition in MLL-Af4–driven leukemia in our in vitro and in vivo studies. Both IGF2BP3 knockdown and the menin-MLL inhibition negatively affect the number and function of LICs, as shown by functional readouts in end point colony formation assays and decreased c-Kit+ cells by flow cytometry. Furthermore, detailed evaluation of colony morphologies, flow cytometry, histopathology, and RNA-sequencing data show a consistent shift toward increased differentiation with IGF2BP3 knockdown and menin-MLL inhibition, highlighting a mechanism of their combined antileukemic effects.
RBPs are proving to be viable therapeutic targets in cancer. Small molecule inhibitors have been developed against RBPs, including Musashi-2, HuR, LIN28B, MBNL1, and IGF2BP2, and studied as single agents but have not been combined with therapies directed toward upstream transcriptional regulators.33-38 Here, we have shown a potent effect of combining genetic inhibition of the RBP IGF2BP3 and pharmacologic inhibition of the menin-MLL interaction, resulting in decreased cell growth in vitro and leukemic burden in vivo. These results highlight an important interaction between MLL and IGF2BP3 in the gene regulation of MLL-Af4–driven leukemia and support the combined targeting of menin-MLL and IGF2BP3 for the treatment of MLL-rearranged leukemia. Our finding demonstrates the feasibility of a novel treatment paradigm, concomitantly targeting transcriptional and posttranscriptional gene regulation.
Regarding underlying mechanisms of IGF2BP3 function in leukemogenesis, we have previously shown that deletion of Igf2bp3 led to decreased number and function of MLL-Af4 LICs.13 Borkin et al showed that treatment with menin-MLL inhibitors, MI-503 and MI-563, led to decreased c-Kit expression in MLL-r leukemia cells.18 In this study, we demonstrated a decrease in the number of Lin– and c-Kit+ cells with IGF2BP3 knockdown and MI-503 treatment, consistent with prior observations showing decreased CD11b+c-kit+ cells in I3KO/MLL-Af4 mice and decreased c-Kit+ expression in MI-503–treated MLL-AF9–transformed BM13,18 and the prior characterization of menin-MLL inhibitors in promoting differentiation of MLL-rearranged leukemia.18,19 We observed a decrease in total colony numbers and a shift toward more differentiated colony morphologies in end point colony formation assays, consistent with the prior characterization of these menin-MLL inhibitors. Notably, IGF2BP3 knockdown enhanced the effect of MI-503 in these readouts of LIC function and number, suggesting that concomitant inhibition of IGF2BP3 and menin-MLL additively target the leukemic stem cell, a known critical driver of refractory and relapsed disease.39-42
Furthermore, we tested the combined inhibition of IGF2BP3 and the menin-MLL interaction on the ability to initiate MLL-Af4 leukemia in vivo. We demonstrated that transformed MLL-Af4 HSPCs can efficiently engraft and initiate leukemia in syngeneic mice, after limited time in culture and CRISPR/Cas9-mediated deletion of Igf2bp3. Treatment of these MLL-Af4–transformed HSPCs with menin-MLL inhibitor in vitro and CRISPR/Cas9-mediated deletion of Igf2bp3 effectively reduced leukemic engraftment in vivo, showing that IGF2BP3 and menin-MLL inhibition reduce the number and function of LICs and is consistent with the results from our in vitro colony formation assays. Hence, this represents a single integrated system that allows for both in vitro and in vivo analyses of leukemogenesis.
Our RNA-sequencing data in drug-treated and knockout cells provide some mechanistic insight into the enhanced antileukemic effects seen with combinatorial inhibition. We demonstrated that both menin-MLL inhibition and IGF2BP3 upregulated genes involved in leukocyte/granulocyte differentiation, with a stronger effect seen with Igf2bp3 depletion than pharmacologic menin-MLL inhibition alone and an additive effect in combination with menin-MLL inhibition. These results help provide a molecular explanation for the increased differentiation seen with menin-MLL inhibitor treatment, previously reported in studies of MI-503, MI-463, and VTP-50469.16,18,19 Furthermore, the pattern of upregulation of genes involved in granulocyte differentiation, seen with MI-503 treatment alone, IGF2BP3 knockdown alone, and the combination, mirror functional and phenotypic outputs of antileukemic effects on cell growth, differentiation, and LIC number and function.
We demonstrated that menin-MLL inhibition reduces IGF2BP3 expression and the expression of known IGF2BP3 targets that are important in leukemogenesis, such as Hoxa9 and Myc, and that the addition of IGF2BP3 knockdown further reduced the expression of these important oncogenic transcripts. This effect supports our understanding of IGF2BP3 as an oncogenic amplifier of MLL-Af4–mediated leukemogenesis. Additionally, this study provides possible targets of interest that can be studied to further understand the interaction between MLL-Af4 and IGF2BP3. For example, we identified Elane as a differentially upregulated gene with MI-503 treatment and IGF2BP3 knockdown, which is interestingly also a direct target of IGF2BP3, in MLL-Af4 Lin– cells.13 Additional studies using functional genomic analyses to knock out direct targets of IGF2BP3 would help define the contribution of these transcripts to MLL-Af4 leukemogenesis and elucidate the mechanisms by which IGF2BP3 contributes to leukemogenesis. Our model system of MLL-Af4 leukemia using Cas9-expressing MLL-Af4–transformed HSPCs lends itself readily to these functional genomic analyses for accelerated screening of important functional targets, using CRISPR sgRNA libraries. The myeloid lineage of the MLL-Af4 leukemia generated in this system in vitro and in vivo is a potential limitation to the mouse model in modeling human disease. However, human MLL-AF4 leukemia is marked by lineage infidelity, particularly after treatment.43,44 Furthermore, Lin et al demonstrated important similarities between the phenotypic and molecular aspects of MLL-Af4–induced pro–B-ALL and acute myeloid leukemia in mice.6
The molecular mechanisms of IGF2BP3 as an RBP remain an area of active investigation. Prior work from our group and others has shown the ability of IGF2BP3 and other RBPs to affect RNA splicing and RNA stability through interaction with microRNAs, the RNA-induced silencing complex, as potential molecular mechanisms underlying the role and function of RBP in oncogenesis.12,13,45-48 Whether these functions, particularly the proposed role in mRNA splicing, interact with the aberrant transcriptional regulation mediated by menin-MLL fusion proteins remains unknown. In this regard, it is interesting to note that METTL3, an RNA N6 methyl adenosine (m6A) methyltransferase, binds to promoters of genes whose mRNA is modified.49 Given the overlap between MLL- and IGF2BP3-regulated gene expression, it is interesting to speculate that a similar mechanism connecting transcriptional and posttranscriptional regulation may exist.
Despite the strong evidence for IGF2BP3 in leukemogenesis, there may be alternative models to consider. A recent publication has suggested that IGF2BP3, among several other RBPs, may be important in negatively regulating the stability of the human MLL-AF4 transcript.50 Although some of the findings in this study are in agreement with ours, there are significant differences in other observations, perhaps because of significantly different models and readouts and differential induction of IGF2BP3 by chimeric fusion proteins that were tested. Nonetheless, this work does bring up new possibilities that IGF2BP3 may directly regulate MLL-AF4: a possibility that requires further work to fully explore.
Our data have demonstrated that IGF2BP3 is a high value therapeutic target as we look toward methods of inhibiting IGF2BP3 in combination with existing experimental therapeutics, including menin-MLL inhibitors. One limitation of our study is that Igf2bp3 deletion occurred before the functional readouts that we pursued. Therefore, the role of IGF2BP3 in leukemia maintenance remains an important and unanswered question. Future studies, using a conditional knockout system and/or protein degradation systems, will help us further assess the therapeutic potential of targeting IGF2BP3. Our initial attempts at developing an inducible knockdown using cyclization recombinase-locus of cross-over, P1 (Cre-LoxP) system resulted in a significantly leaky system that demonstrated the antileukemic effects of IGF2BP3 deletion, but deletion of Ifg2bp3 occurred concurrent with leukemia induction (supplemental Figure 7).51 Nonetheless, in this study, we have shown that targeting IGF2BP3 had potent antileukemic effects against MLL-Af4 leukemia in vitro and in vivo and that this results in additional antileukemic effects with menin-MLL inhibitors. Our studies confirm a role of IGF2BP3 as an oncogenic amplifier of MLL-AF4–driven leukemia and suggest a promising and novel combinatorial approach to targeting leukemia at the transcriptional and posttranscriptional level.
Acknowledgments
The authors thank members of the Rao Lab for helpful discussions regarding the research.
This work was supported by R01CA264986 from the National Institutes of Health (D.S.R. and J.R.S.) and R03CA251854 (D.S.R.); Hematology Training Grant (T32HL066992), Tumor Immunology Training Grant (NIH T32CA009120), a training grant from the California Institute of Regenerative Medicine, the Broad Stem Cell Research Center, and the UCLA Specialty Training and Advanced Research Program (T.L.L.); and the Tumor Cell Biology Training Grant (NIH T32CA009056) (T.M.T.). Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility that is supported by National Institutes of Health awards AI-28697, and award number P30CA016042, the UCLA Jonsson Comprehensive Cancer Center, the UCLA AIDS Institute, and the David Geffen School of Medicine at UCLA.
Authorship
Contribution: T.L.L., A.K.J., A.J.R., J.R., T.M.T., Z.T.N., S.K., M.L.T., and A.C. performed experiments; T.L.L., A.K.J., A.J.R., S.K., and D.S.R. analyzed results and made the figures; T.L.L., J.R.S., and D.S.R. designed the research and wrote the manuscript; and T.L.L., A.K.J., A.J.R., J.R., T.M.T., Z.T.N., S.K., M.L.T., A.C., J.R.S., and D.S.R. reviewed and edited the manuscript.
Conflict-of-interest disclosure: D.S.R. serves on advisory boards and has consulted for AbbVie, Inc, a pharmaceutical company that markets drugs for acute leukemia. The remaining authors declare no competing financial interests.
Correspondence: Dinesh S. Rao, Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at UCLA, 650 Charles E Young Dr, 12-272 Factor, Los Angeles, CA 90095; email: drao@mednet.ucla.edu.
References
Author notes
RNA-sequencing data are shared via deposit to the NCBI Short Read Archive repository (accession number PRJNA918326).
Plasmids, vectors, and other renewable resources included in the article are available on request from the corresponding author, Dinesh S. Rao (drao@mednet.ucla.edu).
The full-text version of this article contains a data supplement.

![IGF2BP3 knockdown increases sensitivity of MLL-r leukemia cells to menin-MLL inhibition. (A) Western blot analysis showing IGF2BP3 knockdown in RS4;11, NALM6, and SEM cell lines and MLL-Af4 Lin– cells (using I3KO sgRNA targeting human and mouse genes and NT guide). (B) Western blot analysis showing IGF2BP3 expression in MI-503 treated MLL-Af4 Lin– cells depleted (I3KO) or nondepleted for IGF2BP3 (NT). Cells were treated with MI-503 (0.2 μM, 1.0 μM, or DMSO control) for 4 days. (C-E) Dose-response curves from cell viability assays, using CellTiterGlo, of human B-ALL cell lines, SEM, RS4;11, and NALM6, depleted (I3KO) vs nondepleted (NT) for IGF2BP3 treated with menin-MLL inhibitors (MI-503, MI-463, and MI-538) for 4 days. Viability has been normalized to DMSO control-treated cells not depleted for IGF2BP3 (NT DMSO); mean ± standard deviation (SD); n = 6. (F) Dose-response curves from cell viability assays, using CellTiterGlo, of MLL-Af4 Lin– cells depleted for IGF2BP3 (I3KO) vs nondepleted (NT) treated with menin-MLL inhibitors for 4 days. Viability has been normalized to DMSO control-treated cells not depleted for IGF2BP3 (NT DMSO); mean ± SD; n = 6. (G) Increased caspase 3/7 activity of MI-503 treated MLL-Af4 Lin– cells depleted for IGF2BP3 (I3KO) vs nondepleted (NT). Cells treated with MI-503 for 4 days at various concentrations for dose response. Caspase 3/7 activity measured using Caspase-Glo 3/7 and normalized to the activity of DMSO-treated control; mean ± SD, n = 3. (H) Increased annexin V positivity in MLL-Af4 Lin– I3KO cells (vs NT) and with MI-503 treatment (vs DMSO control); mean ± SD; n = 3 (1-way analysis of variance [ANOVA] with Bonferroni multiple comparisons test; ∗∗P < .01; ∗∗∗∗P < .0001). (I-J) Histograms from representative samples for annexin V staining, analyzed by flow cytometry. kDa, kilodalton.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/2/10.1182_bloodadvances.2023011132/2/m_blooda_adv-2023-011132-gr1.jpeg?Expires=1769106329&Signature=wUQWvYmHb5knxetDVDLzgAiJd6XsUCZb0UbxcGcAaVPV9RPd-1F8w1U6K72hinm5crmhX1l2fpFIHP4VGlX9xOe7pAl1TukdX1U~bnA2fBzFXiHqFZ69RZh~fuPUkpJIfKYzrv5uDPiYx7Myuz6iEyQKb96sLCFwaLLTM4-jDrp0qnwiPCZeME34tmYCx6vTEVVNjrwOSKySD1q~f3kHXGpNgrHLEDJpOi09NZrVg5QwZHQZGcPlpcb47vAHsTzhwnVLav4rGPvKXvjdsWTRcK9o4X9H6Tw9VQfkckkCehYotmDbp0Lb3kaSBNoambkOcaa-V1JkNRWhxM9IfN~FNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)