TO THE EDITOR:
Acquired aplastic anemia (AA) represents a rare bone marrow failure syndrome characterized by pancytopenia in peripheral blood and empty bone marrow.1 The increased interferon-gamma (IFN-γ) levels, contributed principally by circulating T cells in patients with AA, have a central role in the immune destruction of hematopoietic progenitors, leading to the clinical presentation of this rare and difficult to manage disease. We and others have previously shown that the transcription factor T-bet, a key regulator of Th1 development and function, is overexpressed in T cells from patients with AA.2-4 It has also been shown that age-associated B cells (ABC), a subpopulation of peripheral CD11c+CD19+CD21- CD38-B cells, also express T-bet.5-7 Such T-bet+ ABC are reportedly increased in patients with autoimmune diseases (ie, systemic lupus erythematosus, rheumatoid arthritis, and multiple sclerosis).6,8-11 Stimulation of B cells with antigens, Toll-like receptor ligands and IFN-γ leads to the expansion of ABC; ABC in turn “talk” with T cells and stimulate them in order to produce IFN-γ. In a paracrine manner, IFN-γ may lead to the further expansion of T-bet+ ABC. It has been proposed that the elimination of B-cell-intrinsic T-bet results in diminished T-cell activation and suppressed IFN-γ production.11 The molecular mechanisms controlling the expansion and function of ABC are not completely understood; IFN-γ and interleukin-21 (IL-21) promote B-cell T-bet expression, whereas IL-4 suppresses it. It has been shown that Ets-1 is an essential cofactor for STAT1 regulating of T-bet expression in ABC.12 A small subset of CD4+ helper T cells circulating in the blood component of T follicular helper cells (cTfh) is also implicated in T-bet expression in ABC.11,13 cTfh are characterized by the surface expression of CXCR5, ICOS, and PD1, the transcription factor Bcl-6, and produce mainly IL-21, as well as IL-17, IL-4, and IFN-γ. Tfh represents the major population that helps B cells turn into plasma cells and is implicated in the pathogenesis of different diseases through excessive or insufficient antibody production. In patients with autoimmune diseases, cTfh cells are usually increased.13-15
The expression of ABC and cTfh cells in patients with AA is not known. We enrolled 21 patients with AA (median age, 26 years; range, 8-50 years; 7 patients were children/adolescents; Table 1) and 20 healthy volunteers (median age, 29 years; range, 13-60 years). The diagnosis of AA was based on the criteria of the International Agranulocytosis and Aplastic Anemia Study. No patient underwent transfusion immediately before blood sampling. All blood samples were obtained after written informed consent was obtained in accordance with the Declaration of Helsinki and using protocols approved by the institutional review board of the University Hospital in Patras and the University of Patras Medical School, Rion, Greece. Peripheral blood mononuclear cells (PBMC) were separated by density gradient centrifugation immediately after blood sampling as previously described.16 At the time of sampling, 19 of the 21 patients included in the study were diagnosed but untreated, and 2 patients were in complete response and off any immunosuppressive treatment (Table 1). ABC (CD11c+CD19+CD21-CD38-T-bet+), cTfh (CD4+CXCR5+ICOS+PD1+), and regulatory T cell (Tregs, CD4+CD25hiCD127lo) subpopulation percentages were evaluated using flow cytometry as previously described (supplemental Data).17,18
Patient demographics
| Patient # . | Age (y) . | Sex . | Hgb (g/dL) . | ANC (/uL) . | PLT (∗103/μL) . | Severity . |
|---|---|---|---|---|---|---|
| 1 | 26 | F | 8.5 | 100 | 13 | VSAA |
| 2 | 31 | M | 8.3 | 400 | 15 | SAA |
| 3 | 30 | M | 7.7 | 185 | 2 | VSAA |
| 4 | 18 | F | 12.2 | 1750 | 120 | Remission- SAA (at diagnosis) |
| 5 | 25 | M | 7.0 | 230 | 7 | SAA |
| 6 | 14 | M | 7.5 | 390 | 9 | SAA |
| 7 | 50 | F | 12.7 | 4700 | 145 | Remission- SAA (at diagnosis) |
| 8 | 13 | F | 8.1 | 280 | 5 | SAA |
| 9 | 11 | M | 6.5 | 450 | 12 | SAA |
| 10 | 27 | M | 9 | 370 | 11 | SAA |
| 11 | 23 | F | 8.7 | 150 | 3 | VSAA |
| 12 | 37 | F | 8.0 | 320 | 5 | SAA |
| 13 | 23 | F | 7.3 | 490 | 15 | SAA |
| 14 | 19 | M | 6.3 | 100 | 9 | VSAA |
| 15 | 33 | M | 5.5 | 190 | 2 | VSAA |
| 16 | 38 | F | 8.2 | 340 | 14 | SAA |
| 17 | 41 | F | 7.0 | 400 | 13 | SAA |
| 18 | 38 | F | 4.9 | 290 | 7 | SAA |
| 19 | 31 | F | 7.9 | 410 | 17 | SAA |
| 20 | 9 | F | 7.2 | 380 | 13 | SAA |
| 21 | 8 | M | 6.1 | 135 | 5 | VSAA |
| Patient # . | Age (y) . | Sex . | Hgb (g/dL) . | ANC (/uL) . | PLT (∗103/μL) . | Severity . |
|---|---|---|---|---|---|---|
| 1 | 26 | F | 8.5 | 100 | 13 | VSAA |
| 2 | 31 | M | 8.3 | 400 | 15 | SAA |
| 3 | 30 | M | 7.7 | 185 | 2 | VSAA |
| 4 | 18 | F | 12.2 | 1750 | 120 | Remission- SAA (at diagnosis) |
| 5 | 25 | M | 7.0 | 230 | 7 | SAA |
| 6 | 14 | M | 7.5 | 390 | 9 | SAA |
| 7 | 50 | F | 12.7 | 4700 | 145 | Remission- SAA (at diagnosis) |
| 8 | 13 | F | 8.1 | 280 | 5 | SAA |
| 9 | 11 | M | 6.5 | 450 | 12 | SAA |
| 10 | 27 | M | 9 | 370 | 11 | SAA |
| 11 | 23 | F | 8.7 | 150 | 3 | VSAA |
| 12 | 37 | F | 8.0 | 320 | 5 | SAA |
| 13 | 23 | F | 7.3 | 490 | 15 | SAA |
| 14 | 19 | M | 6.3 | 100 | 9 | VSAA |
| 15 | 33 | M | 5.5 | 190 | 2 | VSAA |
| 16 | 38 | F | 8.2 | 340 | 14 | SAA |
| 17 | 41 | F | 7.0 | 400 | 13 | SAA |
| 18 | 38 | F | 4.9 | 290 | 7 | SAA |
| 19 | 31 | F | 7.9 | 410 | 17 | SAA |
| 20 | 9 | F | 7.2 | 380 | 13 | SAA |
| 21 | 8 | M | 6.1 | 135 | 5 | VSAA |
SAA, severe aplastic anemia; VSAA, very severe aplastic anemia.
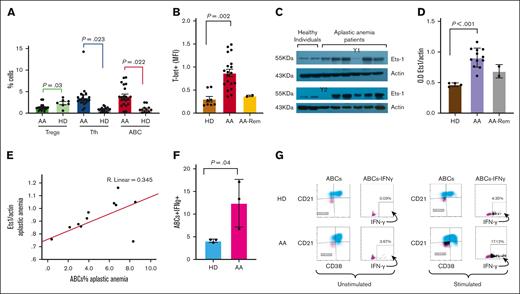
We first measured the frequency of ABC in patients with AA (results are presented as percentage ± SEM). Patients with AA significantly increased the frequency of ABC compared with healthy individuals (3.68% ± 0.82% vs 0.94% ± 0.34%; P = .022; Figure 1A). In the 2 patients with AA in remission, the percentages of ABC were lower than those of the patients at the time of diagnosis (Figure 1B). We further examined whether there were any differences in the percentages of cTfh cells. Patients with AA had greater percentages of cTfh cells than healthy individuals (4.02% ± 1.01% vs 1.11% ± 0.10%; P = .023; Figure 1A).
Increased age-associated B cells in acquired AA. (A) PBMC from patients and healthy individuals were stained with anti-CD38, anti-CD11c, anti-CD19, and anti-CD21 monoclonal antibodies followed by intracellular anti–T-bet monoclonal antibody for the ABCs, with anti-CD4, anti-CXCR5, anti-ICOS, and anti-PD1 monoclonal antibodies for the cTfh and with anti-CD4, anti-CD25, anti-CD127 monoclonal antibodies for the Tregs. Patients with AA (n = 21) displayed an increased frequency of ABCs and increased cTfh compared with HDs (n = 20). Patients with AA showed a lower percentage of CD4+CD25hiCD127lo T cells than healthy donors. The relative numbers from all study subjects are shown. The 2 patients in remission showed a comparable percentage of ABC to that observed in healthy individuals. All samples from patients and healthy donors were run in parallel during the same experiments. (B) Patients with AA showed increased T-bet expression in the ABC population compared with the T-bet expression in healthy individuals. The mean fluorescence intensity of T-bet expression is shown. The 2 patients in remission showed comparable levels of T-bet expression in ABC to those observed in healthy individuals. (y-axis; MFI T-bet in the ABC population). (C) Ets-1 protein levels were examined in protein extracts from the PBMC of patients and healthy controls. Western blot analysis revealed that patients with AA (n = 12) had increased Ets-1 protein levels compared with controls (n = 4). Two patients in remission (Y1 and Y2) showed comparable Ets-1 protein levels to those of healthy individuals. Increased Ets-1 protein levels are associated with increased T-bet expression in ABC in patients with AA. (D) Densitometry analysis of the western blots (Ets1/actin) showed statistically significant increased levels of Ets-1 protein in patients with AA compared with those of controls. The 2 patients in remission had comparable Ets-1 protein levels to those of healthy individuals. (E) The increased percentage of ABC (x-axis: % of ABC) in patients with AA correlates with increased Ets-1 protein levels (y-axis: Ets-1/actin protein levels) (R2 = 0.345). (F-G) PBMCs from patients with AA at diagnosis before any treatment (n = 3) and healthy individuals (n = 3) were left overnight in media or stimulated with phorbol myristate acetate and ionomycin. The cells were collected and analyzed using flow cytometry for the intracellular expression of IFN-γ. A representative experiment is shown from 1 patient and 1 healthy subject. The cells were stained with anti-CD38, anti-CD11c, anti-CD19, and anti-CD21 monoclonal antibodies, followed by intracellular staining for IFN-γ. The gating of IFN-γ expression in the ABC population is shown. Patients with AA showed increased intracellular levels of IFN-γ in the ABC compared with those in healthy controls. Stimulated cells showed a statistically significant increased IFN-γ levels compared with healthy subjects. Data from all patients and controls analyzed are shown in Panel F. AA, aplastic anemia; AA Rem, patients with AA in remission; ABC, age-associated B cells; ABCs+IFN-γ, percentage of intracellular IFN-γ levels in age-associated B cells; cTfh, circulating T follicular helper cells; HD, healthy donor; OD Ets1/Actin, densitometry analysis of Ets1 protein levels to actin levels; Tregs, regulatory T cells.
Increased age-associated B cells in acquired AA. (A) PBMC from patients and healthy individuals were stained with anti-CD38, anti-CD11c, anti-CD19, and anti-CD21 monoclonal antibodies followed by intracellular anti–T-bet monoclonal antibody for the ABCs, with anti-CD4, anti-CXCR5, anti-ICOS, and anti-PD1 monoclonal antibodies for the cTfh and with anti-CD4, anti-CD25, anti-CD127 monoclonal antibodies for the Tregs. Patients with AA (n = 21) displayed an increased frequency of ABCs and increased cTfh compared with HDs (n = 20). Patients with AA showed a lower percentage of CD4+CD25hiCD127lo T cells than healthy donors. The relative numbers from all study subjects are shown. The 2 patients in remission showed a comparable percentage of ABC to that observed in healthy individuals. All samples from patients and healthy donors were run in parallel during the same experiments. (B) Patients with AA showed increased T-bet expression in the ABC population compared with the T-bet expression in healthy individuals. The mean fluorescence intensity of T-bet expression is shown. The 2 patients in remission showed comparable levels of T-bet expression in ABC to those observed in healthy individuals. (y-axis; MFI T-bet in the ABC population). (C) Ets-1 protein levels were examined in protein extracts from the PBMC of patients and healthy controls. Western blot analysis revealed that patients with AA (n = 12) had increased Ets-1 protein levels compared with controls (n = 4). Two patients in remission (Y1 and Y2) showed comparable Ets-1 protein levels to those of healthy individuals. Increased Ets-1 protein levels are associated with increased T-bet expression in ABC in patients with AA. (D) Densitometry analysis of the western blots (Ets1/actin) showed statistically significant increased levels of Ets-1 protein in patients with AA compared with those of controls. The 2 patients in remission had comparable Ets-1 protein levels to those of healthy individuals. (E) The increased percentage of ABC (x-axis: % of ABC) in patients with AA correlates with increased Ets-1 protein levels (y-axis: Ets-1/actin protein levels) (R2 = 0.345). (F-G) PBMCs from patients with AA at diagnosis before any treatment (n = 3) and healthy individuals (n = 3) were left overnight in media or stimulated with phorbol myristate acetate and ionomycin. The cells were collected and analyzed using flow cytometry for the intracellular expression of IFN-γ. A representative experiment is shown from 1 patient and 1 healthy subject. The cells were stained with anti-CD38, anti-CD11c, anti-CD19, and anti-CD21 monoclonal antibodies, followed by intracellular staining for IFN-γ. The gating of IFN-γ expression in the ABC population is shown. Patients with AA showed increased intracellular levels of IFN-γ in the ABC compared with those in healthy controls. Stimulated cells showed a statistically significant increased IFN-γ levels compared with healthy subjects. Data from all patients and controls analyzed are shown in Panel F. AA, aplastic anemia; AA Rem, patients with AA in remission; ABC, age-associated B cells; ABCs+IFN-γ, percentage of intracellular IFN-γ levels in age-associated B cells; cTfh, circulating T follicular helper cells; HD, healthy donor; OD Ets1/Actin, densitometry analysis of Ets1 protein levels to actin levels; Tregs, regulatory T cells.
Patients with increased ABC and cTfh percentages were also analyzed for Tregs. Patients with AA displayed decreased percentages of Tregs compared with healthy volunteers, in agreement with previously published data (1.31% ± 0.26% vs 2.33% ± 0.36%; P = .03; Figure 1A).17,19 T-bet expression in the ABC population from the patients with AA was significantly higher than that in healthy individuals (Figure 1B).
Because the transcription factor Ets-1 is essential for T-bet activation,12 we examined Ets-1 protein levels in PBMC from patients and healthy individuals. Protein extracts prepared (2, supplemental data) from patients showed statistically significantly increased levels of Ets-1 protein compared with healthy individuals (Ets-1/actin OD, 0.91 ± 0.04 vs 0.46 ± 0.01, respectively; P < .001, Figure 1C-D). Ets-1 protein levels correlated with increased ABC counts (R2 = 0.345; Figure 1E). Stat-1 protein levels were comparable (data not shown) between patients and healthy individuals, as previously described.2
To explore whether the increased ABC percentages in patients contributed to the increased IFN-γ levels, we examined the levels of intracellular IFN-γ in the cytoplasm of ABC before and after stimulation. Stimulation was induced when PBMC from patients and healthy individuals were incubated overnight with phorbol myristate acetate and ionomycin and intracellular levels of IFN-γ were evaluated (2, supplemental data). ABC from patients with AA displayed increased IFN-γ levels compared with healthy individuals (Figure 1F-G).
In this study, we aimed to explore the role of ABC, T-bet-expressing B cells, in the immune pathogenesis of acquired AA. Herein, to our knowledge we report for the first time that the ABC subpopulation frequency is increased along with increased percentages of cTfh cells, which are required for ABC generation.9,10 The transcription factor Ets-1, which is essential for ABC ontogenesis, is increased in patients with AA. Ruxolitinib can block cTfh20 and could be a potential treatment option in the future.21 Increased numbers of ABC augment autoimmune diseases in animal models22 and are also correlated with autoantibody production.7 Autoantibodies are frequently detected in AA.23 Two of our patients were in complete remission, and the ABC percentages and T-bet levels in these patients were comparable to those in healthy individuals, suggesting that ABC plays a role in disease status.
The ABC from patients have increased intracellular IFN-γ levels, especially after stimulation. Whether these alterations are a result of increased IFN-γ in polarized Th1 cells is not fully understood. One possible explanation could be that the IFN-γ produced by activated T cells might lead to increased numbers of circulating ABC that are already increased because of the changes in the cTfh population; these ABC induce further IFN-γ production and T-cell activation, leading to a vicious cycle of IFN-γ production and hematopoietic stem cell destruction leading to the clinical presentation of AA. Gene expression analysis of ABC and cTfh in future studies can provide insights into the molecular mechanisms of these cell interactions. Based on our results, it is proposed that T-bet has a role not only in T but also in a small B-cell subpopulation in patients with AA. The limitations of our study include the small number of patients analyzed and we did not evaluate possible alterations in other transcription factors involved in ABC generation. The development of agents that specifically target T-bet+ cells might potentially represent a novel therapeutic approach not only for the treatment of AA but also for other autoimmune diseases.
Acknowledgments: This work was supported in part by a grant (#104) from the Hellenic Society of Hematology (E.E.S.).
Contribution: E.E.S. designed and performed the research, analyzed the data, and wrote the manuscript; C. Sirinian, M.T., E.P., and C. Salamaliki performed the research and analyzed the data; A.K., A.S., P.D., M.P., N.G., N.K., P.K., N-A.V., and A.G. collected and analyzed the data; S-N.L. and G.V. analyzed the data and revised the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elena E. Solomou, University of Patras Medical School, Rion 26500, Greece;.
References
Author notes
Part of this work was presented in oral abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 11 December 2022.
Data are available upon reasonable request from the corresponding author, Elena E. Solomou (esolomou@upatras.gr).
The full-text version of this article contains a data supplement.