TO THE EDITOR:
Donor-specific anti-human leukocyte antigen (HLA) antibodies (DSA) are a major cause of allograft failure and delayed engraftment,1,2 and guidelines recommend against the selection of grafts targeted by DSA.3,4 However, the relationship between allograft patient demographics and HLA-antibody burden, and the degree to which HLA-antibody burden impacts donor type received in the current era of “donors for all,” are not established. Given that HLA antibodies are more prevalent in parous females,5,6 we hypothesized that multiparous females have a greater HLA-antibody burden, potentially limiting the provision of mismatched donors, especially given the risk of graft rejection with haploidentical donors.
We examined the associations between patient sex/parity, ancestry, and HLA-antibody burden in consecutive adult allograft recipients (excluding HLA-identical siblings) who underwent transplantation January 2016 to December 2022 for acute leukemia, myelodysplastic syndrome, or myeloproliferative neoplasm. We also assessed the impact of HLA-antibody burden on donor type received. We classified the HLA-antibody burden according to the number and intensity of class I (HLA-A, -B, and -C) and II (HLA-DR, -DQ, and -DP) HLA antibodies. HLA antibodies were assayed using solid-phase immunoassays, including single-antigen bead assays for antibody identification. A mean fluorescence intensity (MFI) >1000 defined a positive HLA-antibody. Patients were classified as broadly sensitized (more than the median number of class I/II HLA antibodies among patients who had an antibody-positive screen result) and/or highly sensitized (MFI >10,000 for at least 1 HLA-antibody). For patients with >1 screen, the screen closest to transplant was used. Pearson's chi-square tests were used to compare the HLA-antibody burden between the groups. During the study period, in the absence of an HLA-identical sibling donor, an 8/8 HLA allele–matched unrelated donor (URD) was prioritized, followed by either a double-unit cord blood (CB) or haploidentical grafts, as previously described.7 Five to seven/eight mismatched URDs (mmURD) have been used as an additional alternative more recently. The selection of grafts against which the patient had DSA was avoided where possible, especially in the haploidentical setting, by prioritizing alternative graft types. Ancestry was classified as previously described8 based on a detailed kinship history performed by the transplant staff during the pretransplant evaluation. This study was approved by the institutional review board of the Memorial Sloan Kettering Cancer Center and conducted in accordance with the Declaration of Helsinki.
Of the 672 patients [median age 60 years (range 22-80), 419/672 (62%) with acute leukemia, 472/672 (70%) with European ancestry], 278/672 (41%) were female, of whom 54/278 (19%) were nulliparous, 52/278 (19%) were uniparous and 172/278 (62%) were multiparous. Overall, 367/672 (55%) received 8/8 URD and 305/672 (45%) received HLA-disparate grafts [137 CB, 88 haploidentical, and 80 mmURD]. As expected,9-11 compared with Europeans, non-European patients received twice the proportion of HLA-disparate grafts [165/472 (35%) vs 140/200 (70%), P < .001].
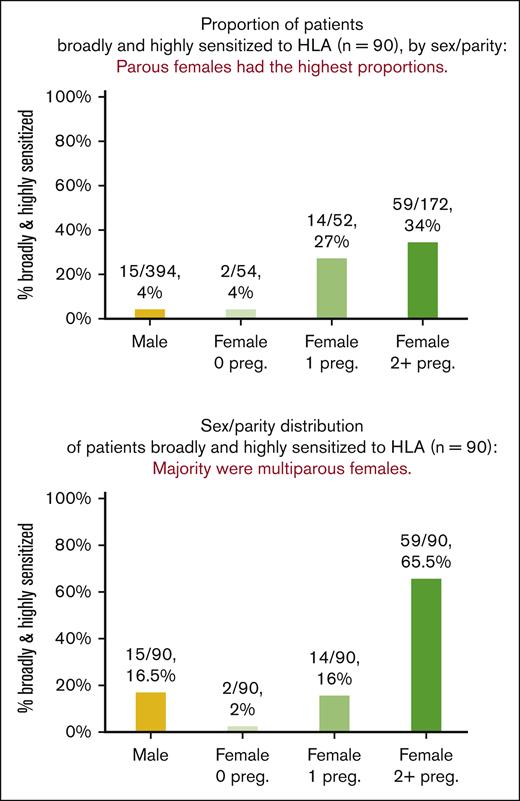
Patient ancestry was not associated with the HLA-antibody burden. Patient sex/parity and HLA-antibody burden associations are shown in Table 1 and Figure 1. More than one-third (254/672 [38%]) of the patients had a positive screen for class I/II HLA antibodies (median of 8 HLA antibodies among patients who had a positive screen result; range, 1-113). Among the 254 patients who had a positive screen result, 3,563 HLA antibodies were detected with an MFI distribution of 1,001-4,000 in 1,340/3,563 (38%), 4,001-10,000 in 874/3,563 (25%) and >10,000 in 1,349/3,563 (38%). Of the total patient cohort, 140/672 (21%) were broadly and/or highly sensitized, and 90/672 (13%) were both broadly and highly sensitized. Compared with male and nulliparous female patients, uniparous and multiparous female patients had more than twice the proportion of positive screens [117/448 (26%) vs 137/224 (61%), P < .001]. Only 17/448 (4%) males and nulliparous females were broadly and highly sensitized. In comparison, one-third (73/224, 33%) of uniparous/multiparous females were broadly and highly sensitized (P < .001).
HLA-Ab burden by sex/parity and donor type in 8/8 URD and HLA–disparate graft recipients (n = 672∗)
| HLA-antibody burden by sex/parity . | ||||
|---|---|---|---|---|
| Class I or II HLA-Abs . | ||||
| . | Male (n = 394) . | Female (n = 278) . | ||
| Nulliparous (n = 54) . | Uniparous (n = 52) . | Multiparous (n = 172) . | ||
| HLA-Ab screen | ||||
| Positive (n = 254)† | 105/394, 27% | 12/54, 22% | 27/52, 52% | 110/172, 64% |
| Broadly and/or highly sensitized (n = 140)‡ | 37/394, 9% | 5/54, 9% | 21/52, 40% | 77/172, 45% |
| Broadly and highly sensitized (n = 90)§ | 15/394, 4% | 2/54, 4% | 14/52, 27% | 59/172, 34% |
| HLA-antibody burden by sex/parity . | ||||
|---|---|---|---|---|
| Class I or II HLA-Abs . | ||||
| . | Male (n = 394) . | Female (n = 278) . | ||
| Nulliparous (n = 54) . | Uniparous (n = 52) . | Multiparous (n = 172) . | ||
| HLA-Ab screen | ||||
| Positive (n = 254)† | 105/394, 27% | 12/54, 22% | 27/52, 52% | 110/172, 64% |
| Broadly and/or highly sensitized (n = 140)‡ | 37/394, 9% | 5/54, 9% | 21/52, 40% | 77/172, 45% |
| Broadly and highly sensitized (n = 90)§ | 15/394, 4% | 2/54, 4% | 14/52, 27% | 59/172, 34% |
| Class I HLA-Abs only . | ||||
|---|---|---|---|---|
| . | Male (n = 394) . | Female (n = 278) . | ||
| Nulliparous (n = 54) . | Uniparous (n = 52) . | Multiparous (n = 172) . | ||
| HLA-Ab screen | ||||
| Positive (n = 211) | 84/394, 21% | 10/54, 19% | 25/52, 48% | 92/172, 53% |
| Broadly and/or highly sensitized (n = 128) | 31/394, 8% | 5/54, 9% | 21/52, 40% | 71/172, 41% |
| Broadly and highly sensitized (n = 89) | 14/394, 4% | 2/54, 4% | 14/52, 27% | 59/172, 34% |
| Class I HLA-Abs only . | ||||
|---|---|---|---|---|
| . | Male (n = 394) . | Female (n = 278) . | ||
| Nulliparous (n = 54) . | Uniparous (n = 52) . | Multiparous (n = 172) . | ||
| HLA-Ab screen | ||||
| Positive (n = 211) | 84/394, 21% | 10/54, 19% | 25/52, 48% | 92/172, 53% |
| Broadly and/or highly sensitized (n = 128) | 31/394, 8% | 5/54, 9% | 21/52, 40% | 71/172, 41% |
| Broadly and highly sensitized (n = 89) | 14/394, 4% | 2/54, 4% | 14/52, 27% | 59/172, 34% |
| HLA-antibody burden by donor type . | ||||
|---|---|---|---|---|
| Class I or II HLA-Abs . | ||||
| . | 8/8 URD (n = 367)‖ . | HLA-disparate (n = 306) . | ||
| CB (n = 137)¶ . | Haplo (n = 88)# . | 5-7/8 URD (n = 80)∗∗ . | ||
| HLA-Ab screen | ||||
| Positive (n = 254)† | 131/367, 36% | 58/137, 42% | 27/88, 31% | 38/80, 48% |
| Broadly and/or highly sensitized (n = 140)‡ | 73/367, 20% | 31/137, 23% | 13/88, 15% | 23/80, 29% |
| Broadly and highly sensitized (n = 90)§ | 46/367, 13% | 20/137, 15% | 7/88, 8% | 17/80, 21% |
| HLA-antibody burden by donor type . | ||||
|---|---|---|---|---|
| Class I or II HLA-Abs . | ||||
| . | 8/8 URD (n = 367)‖ . | HLA-disparate (n = 306) . | ||
| CB (n = 137)¶ . | Haplo (n = 88)# . | 5-7/8 URD (n = 80)∗∗ . | ||
| HLA-Ab screen | ||||
| Positive (n = 254)† | 131/367, 36% | 58/137, 42% | 27/88, 31% | 38/80, 48% |
| Broadly and/or highly sensitized (n = 140)‡ | 73/367, 20% | 31/137, 23% | 13/88, 15% | 23/80, 29% |
| Broadly and highly sensitized (n = 90)§ | 46/367, 13% | 20/137, 15% | 7/88, 8% | 17/80, 21% |
Bolded values emphasize comparisons of most interest.
Median age 60 years (range 22-80); 419/672 (62%) with acute leukemia; 472/672 (70%) with European ancestry.
211/254 (83%) patients who had a positive screen result were positive for class I HLA antibodies, 121/254 (48%) for class II HLA antibodies, and 78/254 (31%) for both.
128/140 (91%) broadly and/or highly sensitized patients were broadly and/or highly sensitized against class I HLA, 81/140 (58%) against class II HLA, and 69/140 (49%) against both.
89/90 (99%) broadly and highly sensitized patients were broadly and highly sensitized against class I HLA, 60/90 (67%) against class II HLA, and 59/90 (66%) against both.
153/367 (42%) female (98/367 [27%] multiparous).
67/137 (49%) female (36/137 [26%] multiparous).
29/88 (33%) female (16/88 [18%] multiparous).
2980 (36%) female (22/80 [28%] multiparous).
Proportion and distribution of patients broadly and highly sensitized to class I/II HLA (n = 90), by sex/parity. Parous females had the highest proportions of broadly and highly sensitized patients (top panel), with multiparous females making up the majority of those broadly and highly sensitized (bottom panel).
Proportion and distribution of patients broadly and highly sensitized to class I/II HLA (n = 90), by sex/parity. Parous females had the highest proportions of broadly and highly sensitized patients (top panel), with multiparous females making up the majority of those broadly and highly sensitized (bottom panel).
Given that class I HLA antibodies are associated with platelet refractoriness,12 we also examined the distribution of class I HLA antibodies in our cohort by patient sex/parity. Overall, among patients with a positive screen, 211/254 (83%) were positive for class I HLA antibodies, of whom 128/211 (61%) were broadly and/or highly sensitized, and 89/211 (42%) were both broadly and highly sensitized against class I HLA. Compared with males and nulliparous females, uni- and multiparous females had more than double the proportion of class I HLA antibodies (94/448 [21%] vs 117/224 [52%]; P < .001) and more than 8 times the proportion broadly and highly sensitized against class I antibodies (16/448 [4%] vs 73/224 [33%]; P < .001).
The HLA-antibody burden according to donor type is shown in Table 1. Among HLA-disparate graft recipients, compared with recipients of CB or mmURD, fewer haploidentical recipients had positive screens (96/217 [44%] CB/mmURD vs 27/88 [31%] haploidentical; P = .029]. Additionally, less than half the proportion of haploidentical recipients were broadly and highly sensitized (37/217 [17%] CB/mmURD vs 7/88 [8%] haploidentical; P = .041). In addition, haploidentical recipients included the fewest multiparous females (16/88 [18%]) compared with making up greater than one-quarter of 8/8 URD (98/367 [27%]), CB (36/137 [26%]), or mmURD (22/80 [28%]) transplants.
In our study, >10% of the patients who underwent transplantation were broadly and highly sensitized to HLA. The HLA-antibody burden disproportionately impacted parous females (33% of our patient cohort), most of whom had HLA antibodies, with one-third of this group being both broadly and highly sensitized. Moreover, patients with a high HLA-antibody burden were least likely to receive haploidentical grafts, and thus these transplants were less common in multiparous females.
These data have immediate implications for alternative donor transplant evaluations of female parous patients: as HLA antibodies should be expected, an antibody assay should be performed, and patient ancestry should be determined7 at the outset of evaluation. If the patient does not have an HLA-identical sibling and has haploidentical DSA, URD may be the best option. Thus, an early search formalization is warranted. If the patient has non-European ancestry, they are much less likely to have a matched URD,9-11 the speed to transplant is slower,7 and URDs are less likely to be available regardless of match grade.13 In this setting, URD search prognosis should be evaluated14-16 and the presence of DSA could further compound the difficulty of identifying a suitable URD such that many more URDs may need to be pursued concurrently to secure an optimal donor at the time required. Additionally, our data are highly relevant to the transplant management of parous women, given that the burden of class I HLA antibodies renders them at a higher risk of platelet refractoriness. These challenges are underappreciated in the transplant field.
Finally, large prospective, multicenter analyses are urgently needed to examine the impact of DSA on transplant candidacy, donor selection, speed to transplant,7 and transplant outcomes (including platelet transfusion refractoriness12), taking into account the number/severity of DSA as well as multiple other recipient demographics including degree of T-cell immunosuppression, conditioning intensity, and graft variables. A study of the effectiveness of novel HLA desensitization strategies17 and their impact on the time to transplantation is also needed. Further investigation is also warranted to better define the role of DSA in relation to each HLA locus, as well as to complement proteins.18 These efforts are critical to address gender disparities in allograft access, advance equity for parous female patients (especially those of non-European ancestry), and optimize the provision of allogeneic transplantation.
Acknowledgment: This work was supported in part by the National Cancer Institute P30 CA008748.
Contribution: W.B.F., E.D., and J.N.B. designed and performed the research; W.B.F., E.D., A.A., S.C., and A.G.B. collected the data; W.B.F., E.D., S.B., S.D., and J.N.B. analyzed the data; W.B.F., E.D., and J.N.B. wrote the manuscript; and all authors interpreted the data, reviewed and edited the manuscript, and approved the submitted version of the manuscript.
Conflict-of-interest disclosure: I.P. has received research funding from Merck and serves as a member of the Data and Safety Monitoring Board for ExCellThera. B.C.S. received consultancy payments from Gamida Cell and Hansa Biopharma. The remaining authors declare no competing financial interests.
Correspondence: Warren B. Fingrut, Department of Medicine, Adult Bone Marrow Transplantation Service, Memorial Sloan Kettering Cancer Center, New York, NY, 10065; email: wfingrut@gmail.com; and Juliet N. Barker, Department of Medicine, Bone Marrow Transplant and Cellular Therapy Program, Weill Cornell Medicine, New York, NY; email: jub2029@med.cornell.edu.
References
Author notes
∗W.B.F. and E.D. contributed equally to this study.
Data are available upon reasonable request from coauthor, Eric Davis (davise@mskcc.org).