Key Points
Cerebral organoids mimic critical structures of the human CNS tissue and can be using to investigate CNS leukemia.
Using cerebral organoids concomitant to PDX mouse models, we identify the AP-1 pathway as a critical driver of CNS leukemia.
Visual Abstract
Central nervous system (CNS) involvement remains a clinical hurdle in treating childhood B-cell precursor acute lymphoblastic leukemia (BCP-ALL). The disease mechanisms of CNS leukemia are primarily investigated using 2-dimensional cell culture and mouse models. Given the variations in cellular identity and architecture between the human and murine CNS, it becomes imperative to seek complementary models to study CNS leukemia. Here, we present a first-of-its-kind 3-dimensional coculture model combining human brain organoids and BCP-ALL cells. We noticed significantly higher engraftment of BCP-ALL cell lines and patient-derived xenograft (PDX) cells in cerebral organoids than non-ALL cells. To validate translatability between organoid coculture and in vivo murine models, we confirmed that targeting CNS leukemia–relevant pathways such as CD79a/Igα or C-X-C motif chemokine receptor 4–stromal cell-derived factor 1 reduced the invasion of BCP-ALL cells into organoids. RNA sequencing and functional validations of organoid-invading leukemia cells compared with the noninvaded fraction revealed significant upregulation of activator protein 1 (AP-1) transcription factor–complex members in organoid-invading cells. Moreover, we detected a significant enrichment of AP-1 pathway genes in PDX ALL cells recovered from the CNS compared with spleen blasts of mice that had received transplantation with TCF3::PBX1+ PDX cells, substantiating the role of AP-1 signaling in CNS disease. Accordingly, we found significantly higher levels of the AP-1 gene, jun proto-oncogene, in patients initially diagnosed as CNS-positive BCP-ALL compared with CNS-negative cases as well as CNS-relapse vs non–CNS-relapse cases in a cohort of 100 patients with BCP-ALL. Our results suggest CNS organoids as a novel model to investigate CNS involvement and identify the AP-1 pathway as a critical driver of CNS disease in BCP-ALL.
Introduction
Despite the progress being made in the treatment of B-cell precursor acute lymphoblastic leukemia (BCP-ALL), therapy optimization is necessary to improve patient quality of life. Central nervous system (CNS) involvement at initial diagnosis is seen in ∼2% to 5% cases of pediatric ALL (classified as CNS3).1,2 However, a third of relapse cases in pediatric ALL involve the CNS compartment.2-5 CNS-directed therapy is standard in clinical treatment of ALL, with patients with ALL receiving risk-adapted therapy, albeit relying on imprecise diagnostic measures such as liquor cytospin analysis.3 Prophylactic radiation therapy in the early stages has been replaced by the administration of intensive systemic and intrathecal chemotherapeutic agents, such as methotrexate and cytarabine.4 Despite concerted efforts, a persisting detrimental burden on the CNS remains evident.1,5 In BCP-ALL, cytogenetic alterations resulting in the t(1;19) TCF3::PBX1, or the t(9;22) BCR::ABL1 (or Philadelphia-chromosome) fusion genes, are associated with enhanced risk of CNS leukemia.6-8 Several molecules and pathways have been linked to CNS infiltration of ALL cells,9-11 including interleukin-17,12 integrin subunit alpha 6,12 and adaptation to hypoxia.9 Moreover, targeting the SDF-1–C-X-C motif chemokine receptor 4 (CXCR4) pathway with plerixafor has been shown to prevent dissemination of BCP-ALL into the murine CNS.13,14 In addition, a crucial role of the pre–B-cell receptor (preBCR) and its signaling units CD79a and CD79b for CNS involvement have been demonstrated.15,16 Regardless of dedicated efforts, our current understanding of the factors that govern the survival and proliferation of BCP-ALL cells within the CNS is limited.
Current 2-dimensional in vitro models for investigating CNS-ALL are disadvantaged because of their inability to replicate the intricate interactions within a complex tumor microenvironment. Although xenograft models play a crucial role in ALL research, their use often entails considerable ethical, financial, and feasibility considerations.17,18 Furthermore, differences exist in cellular identity and architecture between the human and murine CNS, which can impair faithful reproduction. Brain organoids are stem cell–based 3-dimensional (3D) in vitro models, which partially resemble the donor host brain concerning regional organization and cellular identity.19 Organoid technologies have become a valuable tool in biomedical research, to model organ function and for pharmacological screenings.20-22 The generation of CNS organoids can be tailored to model specific regions of the brain (ie, cerebrum, midbrain, frontal lobe, etc) and even the cerebral cortex, whereas meningeal models are in the early stages of development.23-25 However, importantly in the deeper layer of cerebral organoids, hypoxic conditions are prevalent similar to what ALL cells encounter when invading the CNS.
Recent studies cocultivating patient-derived glioblastoma stem cells with cerebral organoids exposed those as models, faithfully recapitulating the disease and suggesting their application for the investigation of selective CNS invasion by cancer cells.22,26 Similarly, bone marrow organoids have revealed potential for clinically relevant investigation of hematological conditions, providing favorable engraftment of sensitive myeloid and lymphoid patient grafts.27 Although current biomimetic models are progressing in achieving substantial cellular and organizational complexity, as of present, organoid models have not been used in the context of CNS-ALL.28
Here, we show that human induced pluripotent stem cell (iPSC)–derived cerebral organoids mimic critical structures of human CNS tissue and can be used as a tool to investigate CNS-ALL. Using CNS organoid/BCP-ALL coculture models, we show that BCP-ALL cell lines and patient-derived xenograft (PDX) cells invade robustly in organoids as compared with cord blood–derived CD34+ hematopoietic stem and progenitor cells (HSPCs) and chronic myeloid leukemia (CML) cells, which can be reversed by CD79a/Igα knockdown or CXCR4–SDF-1 blockade. Pairing results of our CNS organoid models with data from PDX mouse models and an cohort of patients with ALL, we revealed the activator protein 1 (AP-1) pathway as critically involved in CNS-ALL. Our data suggest that CNS organoid models as feasible to study CNS-ALL and unveil new targets for better diagnostics and treatment options of this clinical challenge.
Methods:
Generation of cerebral organoids from human iPSCs
A detailed protocol of the generation of CNS organoid/BCP-ALL coculture models is provided in supplemental Material (section A-O) and see Figure 1A-C for the workflow. Different human iPSC lines were used to account for the heterogeneity among iPSCs and for genetic diversity (see supplemental Tables 1-4).
Establishment of cerebral organoids to model CNS invasion of leukemia cells. (A-C) A graphical illustration of the workflow showing differentiation of the mature cerebral organoids from (low passage) human iPSCs, and cocultured with leukemic or HSPCs controls carrying a fluorescent (CFSE) dye.
Establishment of cerebral organoids to model CNS invasion of leukemia cells. (A-C) A graphical illustration of the workflow showing differentiation of the mature cerebral organoids from (low passage) human iPSCs, and cocultured with leukemic or HSPCs controls carrying a fluorescent (CFSE) dye.
Patient samples/human cell lines and healthy controls
One hundred patients with BCP-ALL of different cytogenetics were treated according to ALL-Berlin-Frankfurt-Münster 2000 or 2009 protocols.29 Informed consent was obtained in accordance with the Declaration of Helsinki. The study was approved by the ethical committee of the Christian-Albrechts University Kiel (D 437/17). Kasumi 2, 697 (TCF3::PBX1), HAL01 (TCF3::HLF1), KCL22, K562, and SUPB15 (BCR::ABL1) leukemia cell lines (Deutsche Sammlung von Mikroorganismen und Zellkulturen) were cultured in RPMI 1640 GlutaMAX (ThermoFisher Scientific) supplemented with 10% to 15% fetal calf serum (Sigma-Aldrich) and penicillin/streptomycin (Invitrogen). The cell line authentication was regularly performed by short tandem repeat profiling. Fresh cord blood–derived CD34+ HSPCs were enriched using the EasySep isolation kit (StemCell Technologies).
Coculture assay leukemia cells/healthy controls and cerebral organoids
Cerebral organoids were grown from an iPSC line according to a protocol adopted from Gabriel et al,23 and grown until reaching 60 days of age. See supplemental Data, section E through G for detailed description.
Image processing and analysis (Python, ProPlot Package)
We stored and exported images, as well as created figures, using the Omero software.30,31 Carboxyfluorescein succinimidyl ester (CFSE)-positive cells in each coculture image were counted using Imaris (research resource identifiers: SCR_007370) software (Oxford Instruments). All cartoon illustrations were created using BioRender.com.
BCP-ALL xenografts
RNA sequencing and expression assay
After coculture with leukemia cells, organoids were pooled and mashed through a 40-μm strain using the plunger end of a syringe. Workflow of the setup is depicted in supplemental Figure 5. Five patient-derived TCF3::PBX1+ ALL samples were injected into NSG mice and upon leukemia development blasts were recovered from the spleen or CNS as described previously,33,34 and subjected to RNA sequencing.
Flow cytometry
Organoids and suspension cells were recovered from coculture assays to process for fluorescence staining and subsequent fluorescence-activated cell sorting analysis. See supplemental Data (section M) for a detailed description.
Statistical analysis
Statistical analysis was performed using GraphPad PRISM 9.00 and SPSS 22. Gaussian distribution was tested using the Shapiro-Wilk test. In case of normal distribution, statistical significance was assessed using an unpaired t test, otherwise the Mann-Whitney U test was applied. In case of normal distribution and multiple groups, analysis of variance was used. A P value of < .05 was considered significant. Bivariate correlation analysis was performed as described previously.32
Results
Establishment of cultivation and tissue processing protocol for imaging mature 3D cerebral organoids
First, to establish a new model system for studying CNS involvement of BCP-ALL cells, we established cerebral organoid model for coculture assays (Figure 1A-C). We devised a protocol for cultivating cerebral organoids by drawing upon previous studies.19,22,23,36 In short, organoid precursors were formed by neural induction of human iPSC aggregates (supplemental Figure 1A-C). A common hallmark is the pseudoneuroepithelial layer surrounding a lumen, also known as neural rosettes.24,36 Accordingly, our median sections showed the distinct pattern of numerous neural rosettes composed up of a dense, circular- or oval-shaped arrangement of nuclei surrounding a lumen (supplemental Figure 1B). Because organoids are large tissues, optimal imaging conditions are required to observe events in detail. Therefore, we developed a tissue “clearing” protocol using ethyl cinnamate,37 leading to transparent organoids (supplemental Figure 1D-E).
Overall, we successfully established cerebral organoids mimicking the critical structures of the human CNS tissue. Additionally, we developed an image processing protocol essential for capturing precise images of the organoids after coculturing them with ALL cells.
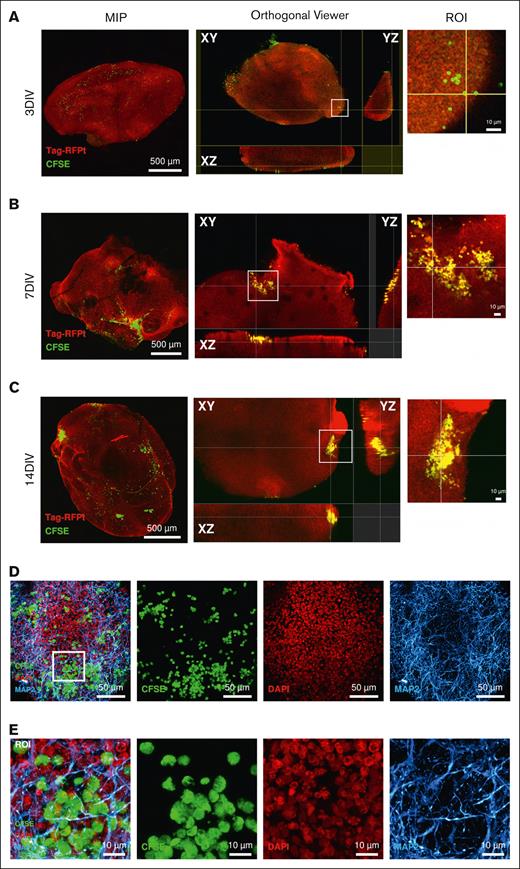
Leukemia cells invade robustly and deep into cerebral organoids after 14 days of coculture
Next, we asked whether BCP-ALL cells invade cerebral organoids. We first used TCF3::PBX1+ ALL cells (PDX1, supplemental Table 5) for coculture experiments and tested their engraftment in CNS organoids. During 3-day coculture, PDX cells attached to organoids, however, the cells were not found in the inner regions of the cerebral organoid (Figure 2A). The duration of the coculture assay was therefore extended up to 7 or 14 days, which revealed a deeper migration of many leukemia cells after 14 days of coculture (Figure 2B-E). Moreover, cerebral organoids of differing maturation stages showed comparable extents of invading leukemia cells (supplemental Figure 2A). We then aimed to determine the minimum number of leukemic cells required to engraft in the cerebral organoids. Coculture experiments were carried out with a limiting dilution of PDX cells (50, 100, 5000, and 10 000 cells of PDX1) for 14 days. Invasion was detected with initial cell numbers as low as 50 leukemia cells in a concentration-dependent manner (supplemental Figure 2B). In summary, leukemia cells invaded deeper and formed larger cell aggregates after 14 days of coculture with cerebral organoids, and only a few cells are needed to study engraftment behavior.
Deep invasion of TCF3::PBX1+ BCP-ALL cells into cerebral organoids occurs after 14 days of coculture. Cerebral organoids were cultured with (10 000) CFSE-stained TCF3::PBX1+ PDX cells. (A-C) Maximum intensity projections (MIP) are shown on the left-hand column, showing the DAPI (4’,6-diamidino-2-phenylindole) and monomeric tag red fluorescent protein tandem colocalizing with the cells’ CFSE signal. Orthogonal views on the right-hand column indicate the position of cells (green dots) by visualizing the organoid in 3 dimensions (XY, ZY, and ZX). Areas of leukemia cells found on the surface or within the organoid itself are shown in the orthogonal viewer and enhanced by the region of interest (ROI) image. (D) Subsurface CFSE-stained leukemia cells localize with other neuronal cell types. (D-E) Collapsed z-stack and merge of CFSE, DAPI, and anti-MAP2 staining are shown. Magnified images provide enhanced details of the leukemia cells colocalizing with the organoid volume (ROI), indicating that the leukemic cells sit in a complex network of neurons and their axonal/dendritic connections. Scale bars: 10 μm, 50 μm, and 500 μm.
Deep invasion of TCF3::PBX1+ BCP-ALL cells into cerebral organoids occurs after 14 days of coculture. Cerebral organoids were cultured with (10 000) CFSE-stained TCF3::PBX1+ PDX cells. (A-C) Maximum intensity projections (MIP) are shown on the left-hand column, showing the DAPI (4’,6-diamidino-2-phenylindole) and monomeric tag red fluorescent protein tandem colocalizing with the cells’ CFSE signal. Orthogonal views on the right-hand column indicate the position of cells (green dots) by visualizing the organoid in 3 dimensions (XY, ZY, and ZX). Areas of leukemia cells found on the surface or within the organoid itself are shown in the orthogonal viewer and enhanced by the region of interest (ROI) image. (D) Subsurface CFSE-stained leukemia cells localize with other neuronal cell types. (D-E) Collapsed z-stack and merge of CFSE, DAPI, and anti-MAP2 staining are shown. Magnified images provide enhanced details of the leukemia cells colocalizing with the organoid volume (ROI), indicating that the leukemic cells sit in a complex network of neurons and their axonal/dendritic connections. Scale bars: 10 μm, 50 μm, and 500 μm.
ALL cell invasion into CNS organoids is driven by directed migration and proliferation
Next, we aimed to investigate the cellular processes ALL cells undergo when being exposed to CNS organoids. First, to examine the role of cellular migration, we conducted coculture experiments involving CD34+ HSPCs sourced from cord blood as compared with TCF3::PBX1+ 697 leukemia cells with cerebral organoids. Using live-cell time-lapse imaging using the Incucyte platform, we monitored the cell migration patterns in the first hours after coculture initiation and observed that 697 cells displayed directed movement of 697 cells toward CNS organoids (supplemental Figure 2C-E). This was not seen in HSPCs, which exposed random motility (Brownian motion) when cocultured with CNS organoids and not in 697 cells cultured without organoids. These results indicate that cellular migration plays a significant role during the infiltration of CNS organoids.
Next, to investigate the role of ALL cell proliferation during CNS organoid infiltration, we examined the effect of mitosis inhibition in ALL cells within our model. We exposed 697 cells to a noncytotoxic concentration of mitomycin-C (30 nM) and subsequently cocultivated the cells with brain organoids; 697 cells pretreated with mitomycin-C still engrafted in CNS organoids (supplemental Figure 2F). However, we observed a notable reduction in the number of cells within the cerebral organoids relative to the corresponding vehicle control. These results support that CNS organoid invasion by ALL cells is driven by migration, however, the ability to proliferate is crucial for ALL cells to successfully colonize the organoid.
BCP-ALL cells invade robustly into cerebral organoids in comparison with non-ALL cells
To assess the impact of leukemia cell engraftment on organoid biology, confocal imaging was conducted, examining 2 distinct areas: 1 with leukemia cell engraftment, and 1 without. It was determined that leukemia cell engraftment did not influence the expression of key neuronal markers MAP2 and Nestin (supplemental Figure 2G). Subsequently, to evaluate potential loss of CFSE staining by leukemia cells after a 14-day coculture with organoids, a minimal reduction in CFSE signals was observed; however, this reduction did not reach a threshold rendering them indistinguishable in immunofluorescence images (supplemental Figure 2H).
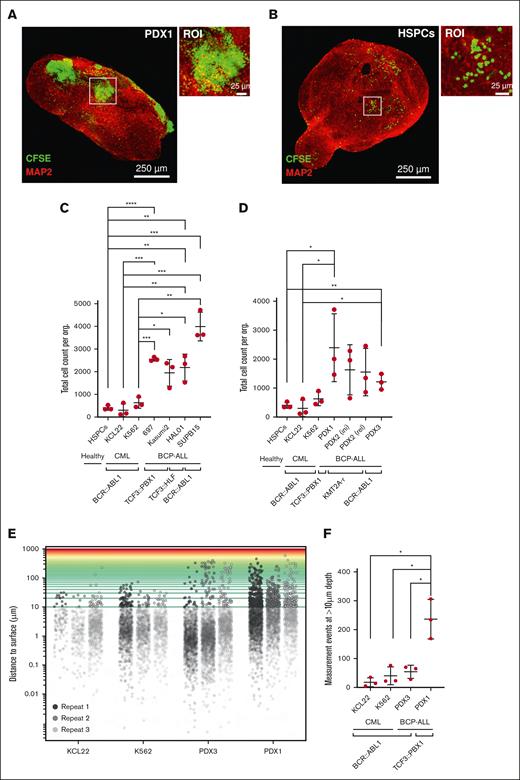
Additionally, to elucidate the distinctions in engraftment propensity among different leukemia samples, we counted individual leukemia cells within each 3D organoid image. The counting revealed a significantly (P < .05) higher engraftment of TCF3::PBX1+ (PDX1, 697, and Kasumi 2 cells) leukemia cells than CD34+ HSPCs (Figure 3A-C). The HSPCs did not form large aggregates in the organoids after 14 days in contrast to PDX ALL cells (Figure 3A-B). Importantly, leukemia cell lines, PDX cells, or HSPCs retrieved from the suspension after coculture with cerebral organoids exhibited high viability (≥80%) after 14 days of the coculture (supplemental Figure 2D). Furthermore, as additional controls, we included 2 BCR::ABL1+ CML cell lines, K562 and KCL22, into the coculture assay as a non–BCP-ALL subtype, which is not typically associated with CNS leukemia. However, despite comparable growth rates, K562 and KCL22 cells exhibited low engraftment in the cerebral organoids, similar to HSPCs. Moreover, we extended our analysis to further PDX ALL samples representing high-risk ALL subgroups including TCF3::HLF+, KMT2A-rearranged, and BCR::ABL1+ ALL (Figure 3D; supplemental Table 5). We observed differential organoid engraftment kinetics in different PDX ALL samples, all of which significantly exceeded the organoid engraftment of HSPCs and K562 and KCL22 cells (Figure 3D).
BCP-ALL cells robustly engraft into cerebral organoids as compared with healthy CD34+ HSPCs. (A-B) Cerebral organoids were cocultured with 10 000 CFSE-stained cells, either cord blood–isolated CD34+ HSPCs or leukemia cells, for 14 days. Enlarged images of cell clusters are shown by white squares (ROI). Scale bars: 25 μm and 250 μm. (C-D) Cerebral organoids were cocultured with 10 000 CFSE-stained leukemia cells or CD34+ HSPCs. After 14 days of coculture period, the total cell counts within the invaded organoids were quantitatively assessed by enumerating CFSE-positive cells in each organoid, using Imaris image processing software. Statistical analysis was conducted to compare the HSPCs, K562, and KCL22 controls to every other condition using the unpaired 2-tailed t test (n = 3 replicates). (E) Visualization of leukemia cell distribution within organoids using Matplotlib-based image analysis. 3D representation illustrates the relative distribution of leukemia cells within the organoid structure. Distances exceeding 10 μm are considered boundaries for deep invasion. (F) Depicted are number of cells that have invaded beyond 10-μm depth in the organoid 3D space (n = 3 replicates, unpaired 2-tailed t test).
BCP-ALL cells robustly engraft into cerebral organoids as compared with healthy CD34+ HSPCs. (A-B) Cerebral organoids were cocultured with 10 000 CFSE-stained cells, either cord blood–isolated CD34+ HSPCs or leukemia cells, for 14 days. Enlarged images of cell clusters are shown by white squares (ROI). Scale bars: 25 μm and 250 μm. (C-D) Cerebral organoids were cocultured with 10 000 CFSE-stained leukemia cells or CD34+ HSPCs. After 14 days of coculture period, the total cell counts within the invaded organoids were quantitatively assessed by enumerating CFSE-positive cells in each organoid, using Imaris image processing software. Statistical analysis was conducted to compare the HSPCs, K562, and KCL22 controls to every other condition using the unpaired 2-tailed t test (n = 3 replicates). (E) Visualization of leukemia cell distribution within organoids using Matplotlib-based image analysis. 3D representation illustrates the relative distribution of leukemia cells within the organoid structure. Distances exceeding 10 μm are considered boundaries for deep invasion. (F) Depicted are number of cells that have invaded beyond 10-μm depth in the organoid 3D space (n = 3 replicates, unpaired 2-tailed t test).
Because deeper layers of CNS organoids harbor hypoxic conditions, we hypothesize that depth of organoid infiltration by ALL cells correlates with their ability to adapt to unfavorable conditions. To be able to assess variations in the depth of ALL cell invasion within cerebral organoids, we used the 3D data processing tool Open3D,38 allowing us to accurately measure the penetration distance of leukemia or control cells into the organoids (supplemental Figure 3A-E). Using spatial measurements we found that TCF3::PBX1+ PDX1 cells demonstrated significantly higher propensity to migrate deeper into the organoids than respective K562 and KCL22 CML control cells (Figure 3E-F; supplemental Table 5). Also, we detected differences in infiltration depth between different PDX ALL (TCF3::PBX1+ PDX1 vs BCR::ABL1+ PDX3) cells, which were in line with their CNS-engraftment features in xenotransplant mice (supplemental Table 5).
Altogether, coculture assays revealed contrasting infiltration patterns among leukemia cells in cerebral organoids.
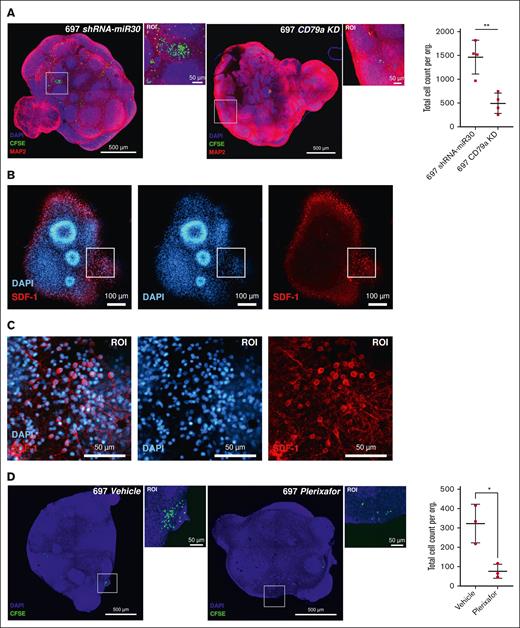
Knock down of CD79a or inhibition of CXCR4–SDF-1 signaling ablates engraftment of TCF3::PBX1+ leukemia cells into cerebral organoids
Next, to validate our model as a vector to investigate targets with relevance in CNS-ALL, we tested, using our organoid system, targets previously shown to be involved in CNS leukemia. We had previously shown a crucial role of preBCR signaling, particularly the preBCR signaling units CD79a/Igα and CD79b/Igβ as crucial mediators of CNS infiltration in BCP-ALL xenografts and patients.16,29 Therefore, we performed short-hairpin RNA (shRNA)-mediated knock down of CD79a in the TCF3::PBX1+ cell line 697 and tested the CNS organoid infiltrating features of these cells in coculture experiments (supplemental Figure 4A-B). Indeed, CD79a knockdown (shCD79a) impeded the engraftment of 697 cells into cerebral organoids in comparison with shRNA control (shRNA-miR30) cells (Figure 4A). Furthermore, both CD79a knockdown and control cells displayed high viability, which remained comparable after 14 days of the coculture experiment (supplemental Figure 2I).
Inhibition of CD79a/Igα or CXCR4–SDF-1 interaction ablates TCF3::PBX1+ leukemia cells’ engraftment into cerebral organoids. (A) shRNA-miR30 (control) or shRNA-mediated CD79a knockdown (KD) 697 cells (10 000 cells) were seeded in a coculture assay with cerebral organoids for 14 days. Both conditions were analyzed using the unpaired 2-tailed t test, 697 CD79a vs 697 shRNA-miR30 (P = .0034). (B-C) To demonstrate the expression of stromal cell-derived factor in our cerebral organoids, we stained cerebral organoids with anti-SDF-1 antibody. A single z-slice of a complete 3D stack is shown above, including a portion of the image in the ROI. Immunofluorescence staining revealed colocalization with anti-MAP2 signal in cerebral organoids and the expression of SDF-1 significantly increased in neurons. (D) 697 wild-type (10 000) cells pretreated for 12 hours with (44 nM) plerixafor or with vehicle control in a coculture assay with cerebral organoids for 14 days. Both conditions were analyzed using the unpaired 2-tailed, vehicle treated vs plerixafor treated 697 cells (P = .0155). Scale bars: 50 μm, 100 μm, and 500 μm.
Inhibition of CD79a/Igα or CXCR4–SDF-1 interaction ablates TCF3::PBX1+ leukemia cells’ engraftment into cerebral organoids. (A) shRNA-miR30 (control) or shRNA-mediated CD79a knockdown (KD) 697 cells (10 000 cells) were seeded in a coculture assay with cerebral organoids for 14 days. Both conditions were analyzed using the unpaired 2-tailed t test, 697 CD79a vs 697 shRNA-miR30 (P = .0034). (B-C) To demonstrate the expression of stromal cell-derived factor in our cerebral organoids, we stained cerebral organoids with anti-SDF-1 antibody. A single z-slice of a complete 3D stack is shown above, including a portion of the image in the ROI. Immunofluorescence staining revealed colocalization with anti-MAP2 signal in cerebral organoids and the expression of SDF-1 significantly increased in neurons. (D) 697 wild-type (10 000) cells pretreated for 12 hours with (44 nM) plerixafor or with vehicle control in a coculture assay with cerebral organoids for 14 days. Both conditions were analyzed using the unpaired 2-tailed, vehicle treated vs plerixafor treated 697 cells (P = .0155). Scale bars: 50 μm, 100 μm, and 500 μm.
Considering prior evidence suggesting the role of CXCR4–SDF-1 inhibition in averting CNS involvement of BCP-ALL cells, we proceeded to examine whether the CXCR inhibitor plerixafor could also hinder the engraftment of leukemia cells in CNS organoids.13 First, immunostainings were performed, which revealed high expression of SDF-1 on the surface of mature organoids (Figure 4B-C). Moreover, the expression pattern of SDF-1 closely coincided to that of mitogen-associated protein 2 (MAP2), a well-established marker for neuronal cells (supplemental Figure 4C). Subsequently, cerebral organoids were cocultured with 697 cells pretreated with either plerixafor (at its sublethal concentration, 44 nM) or vehicle control for 12 hours (supplemental Figure 4D). The visual burden and absolute quantification of leukemic cells into the organoids identified a significant reduction in the engraftment of leukemia cells as compared with vehicle-treated controls (Figure 4D).
Cerebral organoid infiltrating TCF3::PBX1+ leukemia cells show upregulation of AP-1–related genes
Next, we employed our CNS organoid model to identify novel molecules and pathways with implication in CNS-ALL. It has been reported that BCP-ALL cells undergo various adaptations when colonizing the CNS niche.10,11,39 Thus, we postulated that BCP-ALL cells invading brain organoids differ from noninvaded at the transcriptomic level. To this end, cerebral organoids were cocultured with TCF3::PBX1+ cell lines (697 and Kasumi 2). Infiltrating vs noninfiltrating cells were collected after dissociation and sorting of coculture-organoids, and then subjected to RNA sequencing (detailed workflow depicted in supplemental Figure 5). Differential RNA expression analysis identified a significant upregulation of AP-1 pathway-related genes FOS and FosB proto-oncogene (FOSB) in the invaded leukemia cells as compared with the noninvading fraction (Figure 5A-B). To validate these findings on the protein level, intracellular fluorescence-activated cell sorting and IF staining were performed, which also revealed a consistent upregulation of AP-1 signaling-related FOS, FOSB and jun proto-oncogene (JUN) expression in organoid invading leukemia cells as compared with the noninvaded fraction (Figure 5C-D; supplemental Figure 5B). AP-1 signaling deregulation was shown to be associated with hematological cancers, including ALL.40,41 Thus, we hypothesized a central role of AP-1 genes for CNS-colonization by BCP-ALL cells.
TCF3::PBX1+ leukemia cells upregulate AP-1 transcription factor upon infiltration into cerebral organoids. (A-B) Volcano plot showing significantly (false discovery rate [FDR] ˂ 0.05; log2(FC) < −1 or log2(FC) ˃ 1) upregulated or downregulated genes from RNA sequencing data of (A) cerebral organoid–infiltrated 697 and (B) Kasumi 2 (K2) cells compared with the noninfiltrated fraction. (C-D) 697 and K2 cell lines isolated from organoid cocultures were stained for FOSB and FOS and measured using flow cytometry. The mean fluorescence intensity (MFI) is plotted for both infiltrating (leukemia cells inside organoids) and suspension (noninfiltrated) cells. Statistical analysis was performed using the unpaired 2-tailed t test (FOSB, n = 3; FOS, n = 4). (E) 697 cells transduced with either the AP-1 construct (697-AP1) or a corresponding negative control (697-Neg) were cocultured in the presence (+ORG) or absence (−ORG) of organoids, respectively. Cells (10 000) were seeded from each condition in triplicate. Live cell imaging revealed an early and consistent rise in green fluorescent protein (GFP) fluorescence exclusively within the 697–AP-1 cells when cocultured with organoids. 697 wild-type cells (697 WT culture) and normal culturing media were included as nonfluorescent controls. Significance between 697–AP-1 + ORG vs 697–AP-1 − ORG was calculated using 2-way analysis of variance (697 AP1 + ORG vs 697 AP1 − ORG, n = 3, P < .0001. (F) The total track length covered by 697 cells, with or without T-5224 treatment was measured by time-lapse imaging for 20 hours, and analysis was performed via TrackMate plugin. The results depict the total track length covered by the cells. Statistical analysis was performed using the unpaired 2-tailed t test (dimethyl sulfoxide [DMSO] vs 5 μM, n = 3, P < .0001). FC, fold change.
TCF3::PBX1+ leukemia cells upregulate AP-1 transcription factor upon infiltration into cerebral organoids. (A-B) Volcano plot showing significantly (false discovery rate [FDR] ˂ 0.05; log2(FC) < −1 or log2(FC) ˃ 1) upregulated or downregulated genes from RNA sequencing data of (A) cerebral organoid–infiltrated 697 and (B) Kasumi 2 (K2) cells compared with the noninfiltrated fraction. (C-D) 697 and K2 cell lines isolated from organoid cocultures were stained for FOSB and FOS and measured using flow cytometry. The mean fluorescence intensity (MFI) is plotted for both infiltrating (leukemia cells inside organoids) and suspension (noninfiltrated) cells. Statistical analysis was performed using the unpaired 2-tailed t test (FOSB, n = 3; FOS, n = 4). (E) 697 cells transduced with either the AP-1 construct (697-AP1) or a corresponding negative control (697-Neg) were cocultured in the presence (+ORG) or absence (−ORG) of organoids, respectively. Cells (10 000) were seeded from each condition in triplicate. Live cell imaging revealed an early and consistent rise in green fluorescent protein (GFP) fluorescence exclusively within the 697–AP-1 cells when cocultured with organoids. 697 wild-type cells (697 WT culture) and normal culturing media were included as nonfluorescent controls. Significance between 697–AP-1 + ORG vs 697–AP-1 − ORG was calculated using 2-way analysis of variance (697 AP1 + ORG vs 697 AP1 − ORG, n = 3, P < .0001. (F) The total track length covered by 697 cells, with or without T-5224 treatment was measured by time-lapse imaging for 20 hours, and analysis was performed via TrackMate plugin. The results depict the total track length covered by the cells. Statistical analysis was performed using the unpaired 2-tailed t test (dimethyl sulfoxide [DMSO] vs 5 μM, n = 3, P < .0001). FC, fold change.
To establish a functional link between and AP-1 signaling and CNS-involvement in BCP-ALL, we stably transduced TCF3::PBX1+ 697 cells with an AP-1-GFP reporter construct. Live cell imaging revealed an early and consistent rise in GFP fluorescence exclusively within the 697-AP1 cells when cocultured with CNS organoids (Figure 5E). This effect was not observed in suspension 697 negative control cells and in the absence of organoids. To further elucidate the functional role of AP-1 signaling in the engraftment of leukemia cells within the organoids, we conducted experiments employing T-5224, an inhibitor specifically targeting the transcription factors c-Fos/activator protein AP-1, while exhibiting minimal impact on other transcription factors.41,42 697 cells were incubated with 5 μM T-5224 and cocultivated with CNS organoids and their migration toward organoids was recorded applying live-cell imaging. Indeed, treatment of 697 cells with T-5224 significantly attenuated the migration of 697 leukemia cells toward cerebral organoids (Figure 5F; supplemental Figure 5C-D). Collectively, these findings further confirm the involvement of the AP-1 pathway in CNS leukemia and support the potential targetability of the AP-1 pathway in leukemia via pharmacological inhibition.
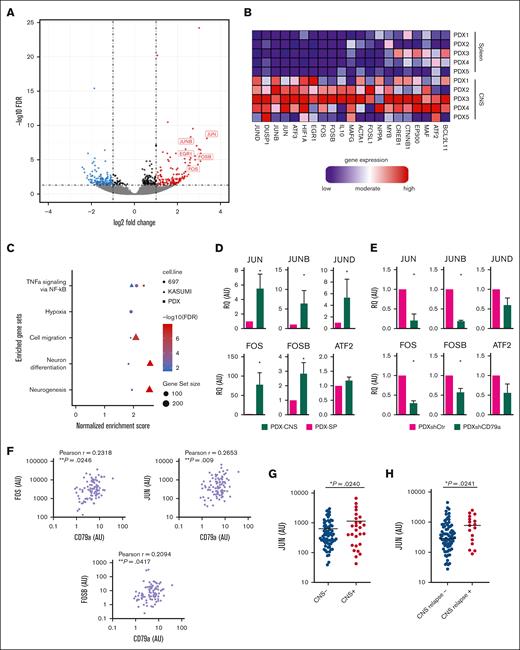
AP-1 genes are selectively upregulated in the CNS invading TCF3::PBX1+ leukemia cells
To further substantiate the role of the AP-1 signaling pathway in CNS-ALL and to validate our findings from organoid cocultures in vivo, we conducted ALL-xenograft mouse experiments. PDX cells from 5 patients with TCF3::PBX1+ BCP-ALL were transplanted into NSG-mice. and comparative RNA sequencing of leukemic cells recovered from the CNS vs the spleen was performed (Figure 6A; supplemental Table 5, PDX 1-5). Indeed, we found a significant enrichment of genes of the AP-1 pathway (FOS, FOSB, and JUN) in PDX cells isolated from the CNS compared with PDX cells isolated from the spleen (Figure 6A-B; supplemental Table 5). Notably, gene-set enrichment analysis of both, CNS PDX cells and CNS organoid–invading cells (697 and Kasumi 2) identified an upregulation of gene sets regulated by NF-κB in response to tumor necrosis factor (Figure 6C), a signaling pathway involved in cancer cell migration and invasion.43 Moreover, validating previous reports,9,44 hypoxia- and cell migration–related gene sets were found to be upregulated in both comparisons. Quantitative reverse transcription polymerase chain reaction analysis (qRT-PCR) was used to validate RNA sequencing findings and showed significantly higher messenger RNA (mRNA) levels of JUN, JUNB, JUND, FOS, and FOSB, and mildly increased ATF2-mRNA levels in CNS leukemia cells than PDX cells isolated from the spleen (Figure 6D).
AP-1 family members act downstream CD79a and are selectively upregulated in CNS leukemia. (A) Volcano plot showing significantly (FDR ˂0.05; log2(FC) < −1 or log2(FC) ˃ 1) regulated genes from RNA sequencing data of BCP-ALL PDX cells recovered from the CNS compared with PDX cells isolated from the spleen (SP). (B) Heat map representation of the top differentially regulated AP-1 pathway genes in CNS relative to SP (blue: downregulated; red: upregulated; samples are represented in columns whereas rows show genes. (C) Fast gene set enrichment analysis on the RNA sequencing data of CNS-isolated PDX cells vs spleen and cerebral organoid–infiltrated 697 and K2 cells vs respective noninfiltrated fraction, displaying significantly regulated gene set signatures. (D) Validation of upregulation of AP-1 genes via qRT-PCR in 7 PDX ALL samples isolated from the SP vs CNS, Mann-Whitney U test, graphs show mean with standard error; ∗P ≤ .05, ∗∗P ≤ .01, and ∗∗∗P ≤ .001. (E) The effect of shRNA-mediated knock down of CD79a (shCD79a) on the expression of AP-1 genes compared with control shRNA (shCtr) in an TCF3::PBX1+ PDX sample as determined via qRT-PCR, Mann-Whitney U test, graphs show mean with standard error. ∗P ≤ .05, ∗∗P ≤ .01, and ∗∗∗P ≤ .001. (F-G) CD79a, JUN, and other genes mRNA levels (all normalized to mRNA levels in the 697 cell line) were measured in diagnostic bone marrow (BM) samples in a selected cohort of 100 pediatric patients with BCP-ALL of mixed cytogenetics, which contained 28 patients with CNS-positive disease matched to 72 patients with CNS-negative disease of corresponding sex and age. (F) Bivariate correlation analysis between CD79a expression and JUN, FOS, and FOSB in patients with BCP-ALL diagnosed as CNS positive (CNS+) vs CNS-negative (CNS−), 2-sided t test. (G) JUN-expression levels in patients with BCP-ALL diagnosed as CNS+ vs CNS−, 2-sided t test. (H) mRNA levels of JUN were detected within patients with BCP-ALL in a cohort enriched for patients who are CNS+ and analyzed for association with the occurrence of relapse. Depicted are mRNA levels normalized to calibrator of n = 83 patients without CNS relapse vs n = 17 patients with CNS relapse, 2-tailed Mann-Whitney U test, ∗P < .05. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels were used for relative quantification of the mRNA transcript. AU, arbitrary units; FC, fold change.
AP-1 family members act downstream CD79a and are selectively upregulated in CNS leukemia. (A) Volcano plot showing significantly (FDR ˂0.05; log2(FC) < −1 or log2(FC) ˃ 1) regulated genes from RNA sequencing data of BCP-ALL PDX cells recovered from the CNS compared with PDX cells isolated from the spleen (SP). (B) Heat map representation of the top differentially regulated AP-1 pathway genes in CNS relative to SP (blue: downregulated; red: upregulated; samples are represented in columns whereas rows show genes. (C) Fast gene set enrichment analysis on the RNA sequencing data of CNS-isolated PDX cells vs spleen and cerebral organoid–infiltrated 697 and K2 cells vs respective noninfiltrated fraction, displaying significantly regulated gene set signatures. (D) Validation of upregulation of AP-1 genes via qRT-PCR in 7 PDX ALL samples isolated from the SP vs CNS, Mann-Whitney U test, graphs show mean with standard error; ∗P ≤ .05, ∗∗P ≤ .01, and ∗∗∗P ≤ .001. (E) The effect of shRNA-mediated knock down of CD79a (shCD79a) on the expression of AP-1 genes compared with control shRNA (shCtr) in an TCF3::PBX1+ PDX sample as determined via qRT-PCR, Mann-Whitney U test, graphs show mean with standard error. ∗P ≤ .05, ∗∗P ≤ .01, and ∗∗∗P ≤ .001. (F-G) CD79a, JUN, and other genes mRNA levels (all normalized to mRNA levels in the 697 cell line) were measured in diagnostic bone marrow (BM) samples in a selected cohort of 100 pediatric patients with BCP-ALL of mixed cytogenetics, which contained 28 patients with CNS-positive disease matched to 72 patients with CNS-negative disease of corresponding sex and age. (F) Bivariate correlation analysis between CD79a expression and JUN, FOS, and FOSB in patients with BCP-ALL diagnosed as CNS positive (CNS+) vs CNS-negative (CNS−), 2-sided t test. (G) JUN-expression levels in patients with BCP-ALL diagnosed as CNS+ vs CNS−, 2-sided t test. (H) mRNA levels of JUN were detected within patients with BCP-ALL in a cohort enriched for patients who are CNS+ and analyzed for association with the occurrence of relapse. Depicted are mRNA levels normalized to calibrator of n = 83 patients without CNS relapse vs n = 17 patients with CNS relapse, 2-tailed Mann-Whitney U test, ∗P < .05. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels were used for relative quantification of the mRNA transcript. AU, arbitrary units; FC, fold change.
AP-1 molecules are upregulated in patients with CNS-positive disease and regulated by the preBCR signaling complex
Because we showed a critical role of CD79a/CD79b16,29 in CNS involvement and a lack of CNS organoid infiltration upon CD79a knockdown, we hypothesized that a direct interplay of the preBCR signaling pathway and AP-1 genes is crucial for CNS disease in BCP-ALL. To investigate this, we examined the expression of AP-1 genes in TCF3::PBX1+ PDX and 697 cells harboring the shRNA-mediated knock down of CD79a. Indeed, we found a significant downregulation of JUN, JUNB, FOS, and FOSB and a visible decrease of JUND and ATF2 mRNA levels in TCF3::PBX1+ PDX cells bearing the CD79a shRNA (Figure 6E). Similarly, downregulation of JUN, JUNB, FOS, and FOSB was detected in 697 cells with knock down of CD79a expression (supplemental Figure 6). To further investigate the association of preBCR and AP-1 signaling on the protein level, 697 and SUP-B15 cells were stimulated with an anti-μ–heavy chain (anti-μHC) antibody that binds the μHC of the preBCR and leads to the activation of the downstream preBCR signaling machinery (supplemental Figure 7).45 Indeed, both cell lines showed an activation of extracellular signal-regulated kinases 1 and 2 and the AP-1 factor activating transcription factor 2 upon preBCR stimulation. Importantly, we also observed enhanced expression of phosphorylated c-Jun N-terminal kinase, the key mediator of JUN activity in both cell lines after anti-μHC treatment. These data further support the view that the preBCR may also promote signals via AP-1 factors.
Next, we investigated whether AP-1 genes may serve as diagnostic markers for CNS infiltration in patients. Therefore, FOS, FOSB, and JUN mRNA levels were measured in diagnostic bone marrow samples in a selected cohort of 100 pediatric patients with BCP-ALL, which contained 28 patients with CNS-positive disease matched to 72 patients with CNS-negative disease of corresponding sex and age.32 The cohort was designed to contain representative numbers of patients with different cytogenetics including 4 patients with BCR::ABL1+ subtype and 4 patients with TCF3::PBX1+ subtype,32 which matches the frequency of these cytogenetic subtypes in pediatric patients with BCP-ALL. To validate the relevance of the CD79a/CD79b/AP-1 axis in patients with BCP-ALL, we measured mRNA levels of different AP-1 genes in our patient cohort and performed correlation analyses. We found that CD79a expression levels correlated significantly with the expression levels of FOS, FOSB, and JUN (Figure 6F; supplemental Figure 8). Similarly, significant correlations between CD79b and FOS as well as JUN levels were detected (supplemental Table 6), further substantiating the association of preBCR signaling and AP-1 genes in BCP-ALL. We did not find a significant association of FOS and FOSB with initial CNS status. However, patients initially diagnosed as CNS-positive showed significantly higher levels of JUN than those with CNS-negative disease (mean JUN expression: 1.8-fold upregulation in CNS-positive cases, P = .0240; Figure 6G). Importantly, patients with BCP-ALL who developed CNS relapses showed significantly higher levels of JUN than non–CNS-relapse cases (1.65-fold upregulation in CNS-relapse cases, P = .0241; Figure 6H; supplemental Figure 9). Altogether, our data support the notion that AP-1 molecules may be regulated by the preBCR signaling complex and that the CD79a/CD79b/AP-1 axis is involved in CNS infiltration in BCP-ALL.
Discussion
The infiltration of the CNS at initial diagnosis or relapse of ALL remains a major clinical challenge to this day. Efforts are being undertaken to elucidate the underlying biological mechanisms of CNS invasion to more reliably diagnose CNS disease and to develop less-toxic yet highly efficacious treatment strategies for CNS leukemia.1,2 Here, we propose a new CNS organoid/ALL coculture system to investigate mechanisms and molecules underlying CNS-ALL. We showed higher engraftment of TCF3::PBX1+ leukemia cells into cerebral organoids within 14 days of coculture than non-ALL cells. This also held true for further ALL cell lines and PDX samples, which showed differential engraftment properties but all significantly exceeding organoid engraftment of HSPCs and CML cell lines. Future studies will show if the ability of ALL cells to invade CNS organoids (either in absolute number or depth) correlates with their propensity to invade the murine or human CNS as suggested by our data.
Our data obtained from cerebral organoid cocultures and PDX mouse models identify the AP-1 pathway as a driver of CNS colonization by leukemia cells. Previous reports promote the view that leukemic cells in the CNS acquire a state of quiescence for which continuous survival signals provided by the microenvironment are required.7,46 AP-1 transcription factors can control cell survival, proliferation, apoptosis, and chemoresistance based on the composition of their subunits.40,47 Increased amounts of AP-1 genes have been shown to negatively regulate cell proliferation, which could explain the low proliferation phenotype associated with ALL cells in the CNS.7 Moreover, AP-1 signaling is frequently associated with adaptation to hypoxia,48 reported as a crucial pathway used by BCP-ALL cells when interacting with the CNS microenvironment.9,44
The hypothesis that AP-1 targets are associated with CNS relapse is further supported by data from a T-cell ALL (T-ALL) study. AP-1 candidate genes were found overexpressed in T-ALL cells recovered from the CNS as compared with bone marrow of PDX mice and enhanced expression of AP-1 genes was associated with a higher occurrence of CNS relapses.49 A further independent recent report identified the c-Jun N-terminal kinase/JUN axis as a critical factor for steroid resistance in T-ALL,50 which underlines the role of AP-1 as a driver of therapy resistance and relapse. Interestingly, in the T-ALL cohort, not JUN but FOSB was detected as the AP-1 candidate with the most prominent association with CNS involvement in patients. Thus, it appears that different means of AP-1 regulation and transcription networks drive CNS involvement in patients with BCP-ALL and those with T-ALL, probably in dependence of different upstream players. One of these players could be the preBCR. Our findings lead us to speculate that the activation of preBCR signaling instigates the expression of AP-1 genes, thus conferring survival advantages to BCP-ALL cells and enabling their survival in the CNS niche. Yet, the microenvironmental factors that promote enhanced preBCR signaling in the CNS niche remain to be identified. Of note, various parts of the proposed signaling axes are targetable: CD79b antibodies showed robust antileukemia efficacy and reduced CNS-ALL burden in ALL models,16 and AP-1 signaling can be potentially blocked with substances such as T-5224.41 Future studies have to show whether such approaches can be safely translated into clinical practice.
New CNS organoid systems are constantly being developed and improved. It will be interesting to see whether similar results as obtained in our organoid model will be observed when adding additional layers of complexity. To that end, Pellegrini et al generated CNS organoids that produced a cerebrospinal fluid (CSF)-like fluid and exposed barrier-forming features.51 This is an interesting model, taking into account that crossing of the blood-CSF barrier is considered a central entry route of ALL cells into the leptomeninges and the CSF. Moreover, Jalilian et al grew meningeal cells on top of parenchymal CNS organoids.25 Those “assembloids” could be used to model the impact of the leptomeningeal barrier/microenvironment on ALL cells in a 3D fashion. Yet, using our model, we observed similar phenotypical and transcriptional characteristics in ALL cells that invaded CNS organoids as in those engrafting in the mouse CNS in vivo. Hence, it will be interesting to see whether adaptation and survival mechanisms of ALL cells such as adaptation to hypoxic conditions9 are more important for successful CNS colonization than migration and homing properties. Those questions can also be addressed by comparing ALL engraftment in CNS organoid models with organoids of other leukemia-relevant organs such as the bone marrow27 or less leukemia-relevant organs such as the colon.52 However, until full validation of those 3D in vitro models, animal models will still represent the gold standard for investigating niche tropism of ALL. Overall, we propose CNS organoid/ALL coculture models as a new tool to investigate CNS leukemia and propose the AP-1 signaling pathway as a new target in this context, warranting further preclinical and clinical validation.
Acknowledgments
The authors thank the patients and physicians who contributed samples and data for this study. The authors thank Gabriele Riesen, Katrin Timm-Richert, Katrin Neumann, Birthe Fedders, and Steffi Spielberg for the excellent technical assistance. The authors thank Christiane Knobbe-Thomsen for providing a microscope for color images. The authors also thank the Center for Advanced Imaging at the Heinrich Heine University for providing access to a confocal microscope, image analysis software, and guidance throughout this project. The authors thank Valentina Bazyleva for helping set up our GitHub repository and their guidance for Python; and the colleagues from Biological and Medical Research Center, Medical Faculty, Heinrich-Heine-University Düsseldorf, especially Thorsten Wachtmeister and Karl Köhrer for RNA sequencing. Computational infrastructure and support were provided by the Centre for Information and Media Technology at Heinrich Heine University Düsseldorf. The authors thank Dennis Das Gupta and Malwine J. Barz for insightful scientific discussions.
This study was funded, in part, by the Christiane und Claudia Hempel-foundation (A.B.). S.B. acknowledges the financial support by Elterninitiative Kinderkrebsklinik e.V. A.B. additionally acknowledges the financial support from Löwenstern e.V. and Katharina-Hardt Foundation. D.M.S. is funded by the Deutsche Krebshilfe e.V. (111963), the Wilhelm Sander-Stiftung (2016.110.1 and 2019.119.1), the Deutsche José Carreras Leukämie-Stiftung (17 R/2017), and the Deutsche Forschungsgemeinschaft (CRU5010; P6). L.L. and L.S. have been supported by the Faculty of Medicine, University of Kiel, Germany.
Authorship
Contribution: P.G., A.B., A.A., D.M.S., L.L., U.F., and S.B. designed the research; P.G., S.L., and S.H. developed and applied the methodology; P.G., S.L., S.H., J.S.-D., L.S., P.S., V.J., M.V., A.P., A.A., U.F., J.Z., L.L., and S.B. analyzed the data; H.W. and Z.Z. provided HW8 iPSC line; M.S. and G.C. provided patient-derived ALL samples and data; P.G., L.L., and S.B. wrote the manuscript; and A.B., L.L., and S.B. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arndt Borkhardt, Department of Pediatric Oncology, Hematology and Clinical Immunology, Heinrich Heine University Düsseldorf, Moorenstraße 5, 40225 Düsseldorf, Germany; email: arndt.borkhardt@med.uni-duesseldorf.de.
References
Author notes
L.L., U.F., and S.B. are joint last authors.
The code used for distance analysis of the leukemia cells in the organoids and for generating plots can be found at https://github.com/BIOGOAT/organoid_leukemia_analysis.git. The imaging data used for plotting are available at https://figshare.com/s/4835d0f62aabc61a8c64. Accession numbers are GSE271750 and GSE271998.
The full-text version of this article contains a data supplement.


![TCF3::PBX1+ leukemia cells upregulate AP-1 transcription factor upon infiltration into cerebral organoids. (A-B) Volcano plot showing significantly (false discovery rate [FDR] ˂ 0.05; log2(FC) < −1 or log2(FC) ˃ 1) upregulated or downregulated genes from RNA sequencing data of (A) cerebral organoid–infiltrated 697 and (B) Kasumi 2 (K2) cells compared with the noninfiltrated fraction. (C-D) 697 and K2 cell lines isolated from organoid cocultures were stained for FOSB and FOS and measured using flow cytometry. The mean fluorescence intensity (MFI) is plotted for both infiltrating (leukemia cells inside organoids) and suspension (noninfiltrated) cells. Statistical analysis was performed using the unpaired 2-tailed t test (FOSB, n = 3; FOS, n = 4). (E) 697 cells transduced with either the AP-1 construct (697-AP1) or a corresponding negative control (697-Neg) were cocultured in the presence (+ORG) or absence (−ORG) of organoids, respectively. Cells (10 000) were seeded from each condition in triplicate. Live cell imaging revealed an early and consistent rise in green fluorescent protein (GFP) fluorescence exclusively within the 697–AP-1 cells when cocultured with organoids. 697 wild-type cells (697 WT culture) and normal culturing media were included as nonfluorescent controls. Significance between 697–AP-1 + ORG vs 697–AP-1 − ORG was calculated using 2-way analysis of variance (697 AP1 + ORG vs 697 AP1 − ORG, n = 3, P < .0001. (F) The total track length covered by 697 cells, with or without T-5224 treatment was measured by time-lapse imaging for 20 hours, and analysis was performed via TrackMate plugin. The results depict the total track length covered by the cells. Statistical analysis was performed using the unpaired 2-tailed t test (dimethyl sulfoxide [DMSO] vs 5 μM, n = 3, P < .0001). FC, fold change.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/19/10.1182_bloodadvances.2023011145/2/m_blooda_adv-2023-011145-gr5.jpeg?Expires=1769086503&Signature=3PZgmocw83mOxTvAC7-Vz-wqIZlkj4YaCJ8svIok61ozwvZUObWQmiQmfeATPDPPCYh8W5z~fyMxJiijozxl8lH7YGHzg~lBmLot09GlKpJjoPyQN55t~AgAS6ffGo9Q9i~uvYFicptF15up24q7GjvCKeRlwiZTGc3GB6lAxLmEu7WYwZrXCRvgXYE3~ChuA6DyzJ5YWBLxRfeF3xSBAUuurbtH9Jdwn4t45jX84~YX~oxKHi0iWSfzG2S~4cJoJLW8Ux0uimuKl6iADXJRidc4Txc5GGvc9~8FqcsYRIByVzF5NLu5~OZzHXPH-OomF0038YS~aKiAReemxtK7eg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)