TO THE EDITOR:
Intensive chemotherapy followed by allogeneic stem cell transplantation (ASCT) remains the mainstay for the treatment of fit patients with de novo acute myeloid leukemia (AML) aged <60 years.1,2 For patients who achieve remission with induction, appropriate selection of postremission therapy (including ASCT) using tools such as the European LeukemiaNet (ELN) risk stratification is essential to determine the patients’ risk of relapse and ASCT candidacy,1 to balance treatment-related mortality (primarily associated with ASCT), and the risk of relapse itself. Patients with a favorable genetic/cytogenetic profile are generally offered postconsolidation chemotherapy, whereas patients with adverse characteristics are offered ASCT. For those in the intermediate category, no consensus exists about the optimal postremission therapy. Still, there is agreement that measurable residual disease (MRD) assessment can be a reliable tool to assist decision-making in these patients.1,3
The Gruppo Italiano Malattie EMatologiche dell'Adulto (GIMEMA) AML1310 trial aimed to determine whether delivering a postremission therapy with risk-driven intensity, integrating upfront genetics and postconsolidation MRD status, improved antileukemic efficacy and reduced therapy-related toxicity. These trials were registered at ClinicalTrials.gov (identifier: NCT01452646) and EudraCT (identifier: 2010-023809-36). The patients’ risk assignment incorporated pretreatment cytogenetic/genetic features (in this study, National Comprehensive Cancer Network [NCCN] 2009)4 and postconsolidation assessment of MRD assessed by multiparameter flow cytometry (MFC). The results showed that a risk-stratified therapeutic approach was feasible, with a 2-year overall survival (OS) and disease-free survival (DFS) of 56% and 54%, respectively. It also showed that in selected situations (favorable-risk [FR] category and intermediate-risk [IR] category MRD negative), autologous stem cell transplantation (AuSCT) might still have a role in the postremission treatment of patients with AML, and using all the available sources of stem cells, ASCT was delivered to 67% of patients in the high-risk categories (adverse-risk category and intermediate MRD-positive risk category).5 Here, we report the results of this study after a prolonged follow-up of 6 years.
GIMEMA AML 1310 was a multicenter, prospective, risk-adapted, MRD-oriented phase 2 study that relied on pretreatment cytogenetic/genetic features and postconsolidation assessment of MRD to establish the final risk assignment and treatment of younger patients (aged ≤60 years) with AML. This clinical trial aimed to verify whether delivering a postremission therapy whose intensity was risk driven would improve the outcome in terms of both increased antileukemic efficacy and reduced therapy-related toxicity.5
To participate in this study, among other eligibility criteria, the patients had to give informed consent and have previously untreated, de novo AML. The study was approved by the ethics committees of the participating hospitals/academic institutions and was conducted following the Declaration of Helsinki.6
The primary objective of this study was the percentage of OS at 2 years. The initial sample size of 213 participants to accomplish this objective was expanded to 515 participants via a protocol amendment to reach the target of 150 participants in the IR category. The efficacy analysis was performed per treatment received, including individuals who commenced induction therapy and censored patients when they received a nonassigned treatment. OS and DFS were calculated using the Kaplan-Meier product limit estimator. Differences in OS and DFS were evaluated by log-rank test in univariate analysis and by Cox regression model in multivariate analysis, after assessment of proportionality of hazards. MRD was assessed by MFC, adopting a threshold of positivity of ≥0.035% residual leukemic cells (supplemental Materials).
Between January 2012 and June 2015, a total of 515 patients with untreated AML, aged 18 to 60 years, were enrolled in the study. The patients’ demographics and clinical characteristics are shown in supplemental Table 1, and their disposition is depicted in supplemental Figure 1. All patients received induction and consolidation chemotherapy according to the GIMEMA AML1310 trial.5 Postconsolidation therapy was based on risk allocation, in general: FR patients (NPM1+ FLT3-ITD– or CBF+ without c-Kit mutations) received AuSCT; poor-risk (PR) or adverse-risk patients (adverse karyotype or FLT3-ITD+) received ASCT; IR patients (intermediate karyotype or FLT3-TKD+ or c-kit mutated CBF+) received AuSCT or ASCT depending on the levels of postconsolidation MRD, as measured by MFC; lastly, the IR patients with no leukemia-associated immunophenotype (IR-no-LAIP) received AuSCT. Five-hundred patients were evaluable. High-risk patients were offered allogeneic transplantation options (HLA identical sibling, matched unrelated donor from international registries, unrelated cord blood, and haploidentical family donor).
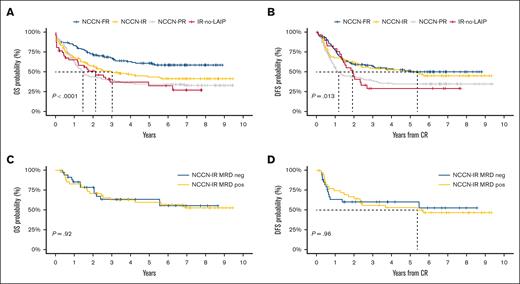
As shown in Table 1, the 2-year OS and DFS for the whole cohort were 56% and 54%, respectively. Two-year OS and DFS, by category, were 74% and 61% for FR, 42% and 45% for PR, 58% and 61% for IR (IR MRD negative, 79% and 61%; IR MRD positive, 70% and 67%), 50% and 48% for IR-no-LAIP, respectively. After 6 years, the OS and DFS for the whole cohort were 42.7% and 41.9%, respectively. Six-year OS and DFS, by category, were FR, 58.5% and 50.1%; PR, 34.1% and 34.8%; IR, 41.4% and 45%; and IR-no-LAIP, 32.5% and 29.1%, respectively (Figure 1 A-B). Remarkably, IR MRD-positive and MRD-negative patients, whose transplant allocation was decided according to the MRD status, showed an identical 6-year OS (57.8% vs 57%; P = NS) and DFS (52.7% vs 46.6%; P = NS; Figure 1 C-D).
2- and 6-year OS and DFS
| . | OS (95% CI) . | DFS (95% CI) . | ||
|---|---|---|---|---|
| 2-y . | 6-y . | 2-y . | 6-y . | |
| Overall | 56% (52-61) | 42.7% (38.3-47.6) | 54% (49-60) | 41.9% (36.8-47.6) |
| NCCN-FR | 74% (67-82) | 58.5% (50.3-68.2) | 61% (52-71) | 50.1% (41.4-60.6) |
| NCCN-IR | 58% (50-68) | 41.4% (33-51.8) | 61% (52-73) | 45% (34.6-58.6) |
| NCCN-IR MRD negative | 79% (66-94) | 57.8% (39.8-83.8) | 61% (47-80) | 52.7% (35.9-77.3) |
| NCCN-IR MRD positive | 70% (57-86) | 57% (42.9-75.6) | 67% (53-83) | 46.6% (32.8-66.1) |
| NCCN-PR | 42% (36-50) | 34.1% (27.8-41.9) | 45% (37-55) | 34.8% (27.3-44.4) |
| IR-No-LAIP | 50% (37-67) | 32.5% (20.1-52.7) | 48% (33-70) | 29.1% (16.2-52.3) |
| . | OS (95% CI) . | DFS (95% CI) . | ||
|---|---|---|---|---|
| 2-y . | 6-y . | 2-y . | 6-y . | |
| Overall | 56% (52-61) | 42.7% (38.3-47.6) | 54% (49-60) | 41.9% (36.8-47.6) |
| NCCN-FR | 74% (67-82) | 58.5% (50.3-68.2) | 61% (52-71) | 50.1% (41.4-60.6) |
| NCCN-IR | 58% (50-68) | 41.4% (33-51.8) | 61% (52-73) | 45% (34.6-58.6) |
| NCCN-IR MRD negative | 79% (66-94) | 57.8% (39.8-83.8) | 61% (47-80) | 52.7% (35.9-77.3) |
| NCCN-IR MRD positive | 70% (57-86) | 57% (42.9-75.6) | 67% (53-83) | 46.6% (32.8-66.1) |
| NCCN-PR | 42% (36-50) | 34.1% (27.8-41.9) | 45% (37-55) | 34.8% (27.3-44.4) |
| IR-No-LAIP | 50% (37-67) | 32.5% (20.1-52.7) | 48% (33-70) | 29.1% (16.2-52.3) |
95% CI, 95% confidence interval.
OS and DFS of the study population and of IR patients. (A-B) Six-year OS (A) and DFS (B) by risk category. (C-D) 6-year OS (C) and DFS (D) in IR patients MRD-negative (<0.035%) for the blue curve and MRD-positive (>0.035%) for the yellow one.
OS and DFS of the study population and of IR patients. (A-B) Six-year OS (A) and DFS (B) by risk category. (C-D) 6-year OS (C) and DFS (D) in IR patients MRD-negative (<0.035%) for the blue curve and MRD-positive (>0.035%) for the yellow one.
The 6-year OS and DFS results show that a modern approach to treating AML should consider both pretreatment (genetics/cytogenetics) and posttreatment factors (MRD).6 This integrated approach appears to have a powerful prognostic role across all the genetic/cytogenetic NCCN categories. For patients belonging to the FR or IR MRD-negative category, excess of toxicity was prevented by delivering an AuSCT. On the contrary, most of the PR and IR MRD-positive patients received ASCT and had remarkable 6-year OS and DFS.7 However, the 6-year OS and DFS of the IR-no-LAIP patients demonstrated that the choice of AuSCT was suboptimal for them, and in this situation, ASCT should have been preferred.
In this study, risk categorization was originally conducted according to the 2009 NCCN recommendations. However, we conducted a post hoc analysis of AML 1310 to determine the applicability of the ELN 2017 risk stratification to the study population and to re-evaluate the study results.6,8 However, 55 of 500 patients were not classifiable due to lack of molecular data (eg, FLT3 allelic ratio). Furthermore, next-generation sequencing for high-risk mutated genes (eg, RUNX1 and ASXL1) was not available. Nevertheless, we found an overall concordance of 65.4% between ELN 2017 and the per-protocol NCCN 2009 categorization. The overlap was more evident for the NCCN-FR/ELN2017-FR and NCCN-IR/ELN2017-IR groups, with less concordance between NCCN-PR/ELN2017-AR cases. We also found that postconsolidation MRD assessment remained critical in the IR category to inform transplant allocation decision-making.8
The 6-year update of this post hoc analysis confirms the capability of ELN 2017 risk classification in predicting outcome in the context of a risk-based, MRD-driven strategy (supplemental Figure 4; supplemental Table 2). By category, OS and DFS were 56.7% and 51.6% ELN2017-FR; 36.6% and 36.1% for ELN2017-IR; and 35.3% and 37.8% for ELN2017-AR, respectively (P < .001 and .031). Furthermore, when analyzing the prognostic impact of MRD status (supplemental Figure 5) in the ELN2017-IR category, we confirmed a superimposable 6-year outcome both for OS (57.2% and 53.4%) and DFS (52.7% and 46.6%) in MRD negative and MRD positive, respectively (supplemental Figure 5).
In conclusion, the extended 6-year analysis confirms the long-term advantage of a risk-adapted, MRD-driven strategy to determine postremission therapeutic decisions. For FR or IR MRD-negative categories, an excess of mortality was prevented by delivering an AuSCT (supplemental Figures 2 and 3). The vast majority of PR and IR MRD-positive patients received an ASCT, with a remarkable 6-year OS and DFS benefit. Based on the present knowledge, an MRD-directed approach is being prospectively explored also in FR patients in the GIMEMA AML1819 trial.
Acknowledgments: The authors thank the patients, their families, and caregivers for participating in this study, along with all investigators and site personnel. The study was sponsored and designed by Gruppo Italiano Malattie EMatologiche dell'Adulto (GIMEMA) Foundation. Medical writing assistance was provided by Maria Carolina Rojido (medical writing consultant), and proofing assistance was provided by Laura Carolina Collada Ali (medical writing consultant).
GIMEMA Foundation funded this assistance.
Contribution: A.V., A.P., E.l.S., L.M., M.I.D.P., M.T.V., P.F., M.V., and F.B. conceived and designed the study; A.V., A.P., A. Candoni., C.F., C.A.M, V.C., R.C., P.d.F., G.S., P.S., F.L., G.M., A. Cuneo., M.L., C.I., M.P.M., A. Curti., F.A., A.M., A. Tafuri., L.C., A. Tieghi., N.S.F., D.C., S.M.T., C.A., R.C., and F.B. were responsible for provision of study materials or patients; A.V., A.P., V.A., R.P., M.I.-C., T.O., and F.B. collected and assembled the data; A.V., A.P., V.A., P.F., M.V., and F.B. analyzed and interpreted the data; A.V., A.P., V.A., R.P., E.l.S., L.M., M.I.D.P., M.T.V., P.F., M.V., and F.B. wrote the manuscript; A.V., A.P., and F.B. are accountable for all aspects of the work; and all authors provided final approval of the manuscript.
Conflict-of-interest disclosure: A.V. reports consultancy fee from Novartis, Pfizer, Jazz Pharmaceuticals, Amgen, AbbVie, Gilead, Astellas, Incyte, Medac, AstraZeneca, Servier, Daiichi Sankyo, Janssen-Cilag, Menarini-StemLine, and Laboratories Delbert; research funding from Sandoz and Jazz Pharmaceuticals; speaker bureau fees from Pfizer; and invited speaker fees from Bristol Myers Squibb, Pfizer, AbbVie, Novartis, AstraZeneca, Janssen-Cilag, Astellas, and Daiichi Sankyo. A. Candoni reports consultancy fees from Pfizer, Jazz Pharmaceuticals, Amgen, AbbVie, Gilead, Astellas, Incyte, Servier, Janssen-Cilag, and Laboratories Delbert; and invited speaker fees from Bristol Myers Squibb, Pfizer, AbbVie, Incyte, Janssen-Cilag, and Astellas. R.C. reports consultancy fees from Novartis, Pfizer, Jazz Pharmaceuticals, AbbVie, Gilead, Astellas, Servier, Janssen-Cilag, and BeiGene; and research funding from Novartis. F.L. reports research grant from Pfizer; and advisory board fees from Jazz. G.M. reports consultant/advisor/speaker bureau fees from Ariad/Incyte, Pfizer, Celgene/Bristol Myers Squibb, Amgen, Roche, AbbVie, GlaxoSmithKline, Astellas, Daiichi Sankyo, Takeda, and Janssen; and research support from Pfizer, AbbVie, AstraZeneca, Daiichi Sankyo, Takeda, and Ariad/Incyte. A. Curti reports advisory board fees and meeting with honoraria from AbbVie, Pfizer, Novartis, and Jazz Pharma. M.L. reports advisory board fees from AbbVie, Gilead sci, Jazz Pharma, Novartis, Merck Sharp & Dohme, Grifols, Sanofi, and Daiichi Sankyo. M.P.M. reports honoraria from Rasna Therapeutics, Inc for scientific adviser activities and honoraria/consultancy at scientific advisory board for AbbVie, Amgen, Celgene, Janssen, Novartis, Pfizer, and Jazz Pharmaceuticals. D.C. reports research funding from Seattle Genetics, Pfizer, Daiichi Sankyo, and AstraZeneca. F.B. reports consultancy fees from Jazz Pharmaceuticals, Novartis, and Laboratories Delbert; and research funding from Becton Dickinson; invited speaker fees from AbbVie, Jazz Pharmaceuticals, Bristol Myers Squibb, Janssen-Cilag, and Astellas. The remaining authors declare no competing financial interests.
Correspondence: Adriano Venditti, Hematology, Department of Biomedicine and Prevention, University Tor Vergata, Viale Oxford 81, Rome 00133 Italy; email: adriano.venditti@uniroma2.it.
References
Author notes
Presented at the 22nd Congress of the European of Hematology Association, Madrid, Spain, 23 June 2017; 62nd Annual Meeting of the American Society of Hematology, Atlanta, GA, 11 December 2021; and 49th Italian Society of Hematology, Rome, Italy, 27 September 2022.
Two-year follow-up data are available at https://doi.org/10.1182/blood.2018886960.
The full-text version of this article contains a data supplement.