TO THE EDITOR:
Long before the discovery of the JAK2, CALR, and MPL mutations, chronic inflammation was an established characteristic of BCR-ABL–negative myeloproliferative neoplasms (MPNs). Numerous studies showed that various conditions of chronic inflammation could precede and facilitate the development of all subtypes of MPNs. A nonexhaustive list of such conditions includes inflammatory bowel diseases, inflammatory rheumatic or autoimmune diseases, obesity, diabetes mellitus and not least, cigarette smoking.1-7 Cellular sources of production of proinflammatory cytokines include hematopoietic progenitors, especially megakaryocytes, typically mutated in MPNs, but also T-lymphocytes and endothelial cells, rarely mutated in MPNs, and stromal cells, which remain wild-type. The extent to which MPN-driver mutations contribute to the high level of inflammation characteristic of MPNs remains debated.2,3,8
In a recent and elegant study, Rai et al extensively studied the role of a major inflammatory cytokine, interleukin-1β (IL-1β), in murine models combining conditional JAK2V617F-induced MPN and knockout of either IL-1β or IL-1 receptor 1 (IL-1R1).9 Rai et al demonstrated that both IL-1β and IL-1R1 are required by JAK2V617F-mutated hematopoietic cells for optimal MPN disease initiation and expansion. Importantly, they also showed that in vivo inhibition of IL-1β (using a neutralizing anti–IL-1β antibody), reduces disease initiation of JAK2V617F-induced MPN in mice. Their parallel studies of patients with MPN show that 2 IL-1β gene polymorphisms associated with increased IL-1β levels are more frequent in patients with JAK2V617F-mutated MPN than in healthy controls.9 Rai et al also studied cellular sources of production of IL-1β in mice and show that megakaryocytes and monocytes produce more IL-1β in JAK2V617F-expressing mice than in wild-type JAK2 mice.9 Altogether, the data imply that the expansion of clonal hematopoiesis of indeterminate potential (CHIP) with JAK2V617F mutation is facilitated by and dependent on IL-1β. Rai et al concluded that IL-1β produced by JAK2V617F-mutated cells induces an inflammatory bone marrow environment that promotes the expansion of JAK2V617F-mutated cells and the conversion to MPN, and that consequently, IL-1β inhibition could be beneficial to individuals with JAK2V617F CHIP.9
IL-1β is indeed an interesting target in MPNs, not only to prevent or slow the expansion of JAK2V617F-mutated clones when they are still minor, but also to reduce myelofibrosis in established MPNs, as recently reported in mice with JAK2V617F-driven MPN.10-12 Yet Rai et al provide new and important information about just 1 arm of the complex story of chronic immune stimulation and inflammation in MPNs. In the pathogenic model they propose, the principal inflammatory culprit is IL-1β, and the JAK2V617F mutation is responsible for the excess of IL-1β.9 In fact, the association between inflammation and the MPNs is more complex.1-3,8,13,14 IL-1 is clearly a major contributor to chronic inflammation, capable of stimulating the production of numerous cytokines and chemokines, by various cell types, both wild-type cells and eventually mutated cells in individuals with an MPN. A major inhibitor of IL-1 production, acting on different cell types, is interferon alpha (IFN-α). There is renewed interest in IFN-α treatment, which we have known for over 40 years to be effective in both solid and blood malignancies, including MPNs, both JAK2- or CALR-mutated.15-18 Thus, owing to its many anticancer effects and being an important regulator of inflammation, angiogenesis and fibrosis, IFN-α has been used with efficacy for the treatment of hairy cell leukemia, chronic myelogenous leukemia, lymphoma, multiple myeloma, and BCR-ABL–negative MPNs. Decades ago, several groups reported the potential impact of IL-1β inhibition in blood malignancies. For instance, IL-1β could be antagonized with the IL-1 soluble receptor or IL-1 receptor antagonists, shown to be additive with IFN-α in suppressing chronic myelogenous leukemia precursor cells.19,20
In the different pathological contexts described above, obviously the chronic excess of IL-1β cannot be explained solely by the JAK2V617F mutation, because IL-1β is also elevated in patients with CALR- or MPL-mutated MPNs.21 Rather, IL-1β overproduction is likely caused by a combination of chronic, pathological proinflammatory mechanisms that precede CHIP and the development of blood malignancy and, directly or indirectly, various genetic alterations associated with blood malignancy, including the JAK2V617F and CALR mutations in patients with MPN. Thus, in addition to JAK2V617F-dependent IL-1β, we would like to underscore the importance of JAK2V617F-independent and pre-JAK2V617F production of IL-1β, and that IL-1β is also found elevated in patients with CALR- or MPL-mutated MPNs, which was not discussed in the study by Rai et al9
Various chronic inflammatory conditions, congenital or acquired, have been reported by many groups as frequent in the history of patients diagnosed with all subtypes of MPNs. This supports a model where inflammation and subsequent IL-1β overproduction precedes the acquisition of MPN-associated mutations in hematopoietic progenitor cells for subsets, if not all, patients with MPN. The data by Rai et al on certain IL-1β gene polymorphisms being over-represented in patients with MPN support this model. Polymorphisms in other genes, including JAK2 and NF-κB1, have been shown to be associated with chronic inflammation, elevated IL-1β levels, and an increased risk of MPN.22 Inversely, loss-of-function polymorphism in IL6R reduces the risk of JAK2V617F mutation and MPN.23 The efficacy of the pharmaceutical inhibition of the NF-κB cascade, critical in inflammation and notably for the production of IL-1β, is demonstrated in MPNs.24 Importantly, when cytokine production associated with the different MPN subtypes and mutations were analyzed and compared, in patients and in vitro, distinct cytokine profiles were reported depending on the JAK2V617F or CALR mutants but also depending on MPN subtype, that is, polycythemia vera, essential thrombythemia (ET), primary myelofibrosis.21,25 For patients with JAK2V617F-mutated MPN, blood levels of IL-1β and IL-1R1 (IL-1Rα) correlated with the JAK2V617F allelic burden, and in vitro expression of JAK2V617F clearly induced the production of IL-1β, IFN-γ-inducible protein 10 (IP-10, or CXCL10), and RANTES.21 In contrast, the cytokine profiles observed for patients with CALR-mutated ET were characterized by overproduction of tumor growth factor b and several interleukins produced by nonmutated cells (IL-4, IL-9, IL-26, IL-33).21 Notably, in vitro expression of CALR mutants did not induce the production of these cytokines.21 In addition, GRO-α and epidermal growth factor (produced by nonhematopoietic cells) were reported as elevated and markers of disease progression in patients with ET with either JAK2V617F or CALR mutations.26
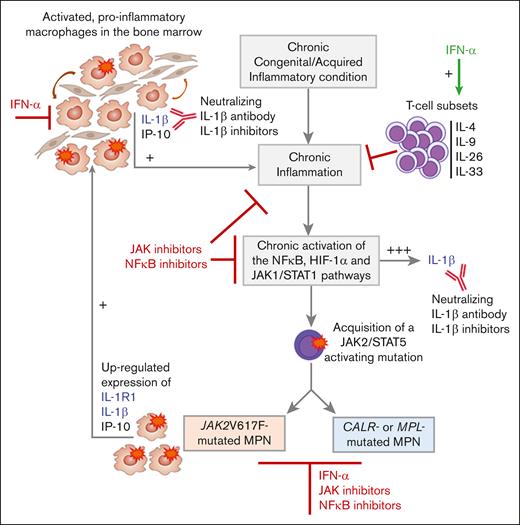
To summarize, numerous cytokines are overproduced in all subsets of MPNs. CALR mutants do not seem to directly alter cytokine production whereas JAK2V617F clearly increases the expression of IL-1β and IL-1R1. Several cytokines overexpressed in MPNs are produced by nonmutated cells, and it is established that inflammation can precede the acquisition of JAK2/STAT5-activating mutations. For instance, autoimmune disease can cause chronic inflammation and excess of IL-1β, eventually creating a fertile ground for the acquisition of JAK2 or CALR mutations and the development of MPN. In JAK2V617F-mutated MPNs, strongly inflammatory IL-1β induced by JAK2V617F further stimulates cytokine production by both mutated and nonmutated cells. The model of MPN pathogenesis represented in Figure 1 takes into account both mutation-dependent and mutation-independent inflammation and IL-1β production.3,8
JAK2V617F-dependent vs mutation-independent IL-1β and inflammation in MPNs. Blood levels of numerous cytokines, including strongly inflammatory IL-1β, are elevated in all subtypes of JAK2-, CALR- or MPL-mutated MPNs. Several cytokines over-expressed in MPNs, including IL-1β, are produced by nonmutated cells, and inflammation may precede the acquisition of JAK2/STAT5-activating mutations. For instance, autoimmune disease can cause chronic inflammation and IL-1β production, eventually leading to the acquisition of JAK2 CALR or MPL mutations and MPN. In vitro studies showed that expression of CALR mutants did not induce cytokine production. In contrast, JAKV617F clearly increases the expression of IL-1R1, IL-1β and IP-10. In JAK2V617F-mutated MPNs, JAK2V617F-induced expression of IL-1β and IL-1R1 further stimulates cytokine production and inflammation, by both the mutated and nonmutated cells in the bone marrow. Inflammation and JAK1- and JAK2V617F-dependent IL-1β production are sensitive to most JAK inhibitors. In contrast, JAK inhibitors have no effect on IL-1β action, since IL-1β does not signal via JAK/STAT. However, IL-1β can be inhibited by IL-1β antagonists, neutralizing anti-IL-1β antibodies, and NF-κB inhibitors. Importantly, IFN-α represses IL-1β and stimulates T-lymphocyte subsets that secrete anti-inflammatory IL-4, and IL-9, a prothrombopoiesis and prothrombosis cytokine.
JAK2V617F-dependent vs mutation-independent IL-1β and inflammation in MPNs. Blood levels of numerous cytokines, including strongly inflammatory IL-1β, are elevated in all subtypes of JAK2-, CALR- or MPL-mutated MPNs. Several cytokines over-expressed in MPNs, including IL-1β, are produced by nonmutated cells, and inflammation may precede the acquisition of JAK2/STAT5-activating mutations. For instance, autoimmune disease can cause chronic inflammation and IL-1β production, eventually leading to the acquisition of JAK2 CALR or MPL mutations and MPN. In vitro studies showed that expression of CALR mutants did not induce cytokine production. In contrast, JAKV617F clearly increases the expression of IL-1R1, IL-1β and IP-10. In JAK2V617F-mutated MPNs, JAK2V617F-induced expression of IL-1β and IL-1R1 further stimulates cytokine production and inflammation, by both the mutated and nonmutated cells in the bone marrow. Inflammation and JAK1- and JAK2V617F-dependent IL-1β production are sensitive to most JAK inhibitors. In contrast, JAK inhibitors have no effect on IL-1β action, since IL-1β does not signal via JAK/STAT. However, IL-1β can be inhibited by IL-1β antagonists, neutralizing anti-IL-1β antibodies, and NF-κB inhibitors. Importantly, IFN-α represses IL-1β and stimulates T-lymphocyte subsets that secrete anti-inflammatory IL-4, and IL-9, a prothrombopoiesis and prothrombosis cytokine.
Hence, given the complexity of inflammation in MPNs, only the integrated analysis of multiple experimental models, both in vitro and in vivo, human and murine, will allow full understanding of its biology and various mechanisms. For instance, mouse models cannot recapitulate the genetic predisposition of patients to acquire MPN, but they can be manipulated experimentally to observe the impact of different stimuli on the selection and expansion of MPN-mutated clones. Patient samples studied at different stages of MPN disease provide unique information, but they also have limitations of their own, particularly a high interindividual variability.
Such an approach is also essential for the therapy of MPNs. To improve treatments, it is important to remember that 3 main pathways are involved in inflammation: the NF-κB, hypoxia inducible factor (HIF ), and JAK1/STAT1 pathways.3 Most JAK inhibitors reduce both JAK1- and JAK2V617F-dependent IL-1β production but have no effect on the action of IL-1β, since this cytokine does not signal via JAK/STAT. The action of IL-1β can be inhibited by IL-1β antagonists, neutralizing anti–IL-1β antibodies and NF-κB inhibitors. Importantly, IFN-α not only represses IL-1β but also stimulates T-cell subsets that secrete anti-inflammatory cytokines.27,28 Ultimately, optimal therapy for MPNs would combine drugs that target JAK2/STAT5-activating mutations (JAK2V617F, CALR, MPL) and inflammation in general, not just IL-1β, as reported for IFN-α2 and ruxolitinib in both clinical and experimental studies.29-31 The results from these studies together with those of Rai et al call for new studies in patients with MPN on the safety and efficacy of combination therapy with IFN-α2 and neutralizing anti–IL-1β monoclonal antibody therapy.
Contribution: S.H. wrote the first version of the manuscript; and H.C.H. contributed to the final version of the manuscript.
Conflict-of-interest disclosure: H.C.H. has received a research grant from Novartis A/S; and serves on the advisory board for AOPOrphan and Incyte. S.H. declares no competing financial interests.
Correspondence: Sylvie Hermouet, Immunology and New Concepts in ImmunoTherapy, INCIT, UMR 1302, Institut de Recherche en Santé 2, Université de Nantes, 22 Blvd Benoni Goullin, F-44000 Nantes, France; email: sylvie.hermouet@univ-nantes.fr.