Key Points
Tumor histology and number of sites of extranodal involvement are prominent risk factors for CNS relapse.
The CITI score is a validated risk model to predict patients with MTNKN at the highest risk of CNS relapse.
Visual Abstract
Little is known about risk factors for central nervous system (CNS) relapse in mature T-cell and natural killer cell neoplasms (MTNKNs). We aimed to describe the clinical epidemiology of CNS relapse in patients with MTNKN and developed the CNS relapse In T-cell lymphoma Index (CITI) to predict patients at the highest risk of CNS relapse. We reviewed data from 135 patients with MTNKN and CNS relapse from 19 North American institutions. After exclusion of leukemic and most cutaneous forms of MTNKNs, patients were pooled with non-CNS relapse control patients from a single institution to create a CNS relapse–enriched training set. Using a complete case analysis (n = 182), including 91 with CNS relapse, we applied a least absolute shrinkage and selection operator Cox regression model to select weighted clinicopathologic variables for the CITI score, which we validated in an external cohort from the Swedish Lymphoma Registry (n = 566). CNS relapse was most frequently observed in patients with peripheral T-cell lymphoma, not otherwise specified (25%). Median time to CNS relapse and median overall survival after CNS relapse were 8.0 and 4.7 months, respectively. We calculated unique CITI risk scores for individual training set patients and stratified them into risk terciles. Validation set patients with low-risk (n = 158) and high-risk (n = 188) CITI scores had a 10-year cumulative risk of CNS relapse of 2.2% and 13.4%, respectively (hazard ratio, 5.24; 95% confidence interval, 1.50-18.26; P = .018). We developed an open-access web-based CITI calculator (https://redcap.link/citicalc) to provide an easy tool for clinical practice. The CITI score is a validated model to predict patients with MTNKN at the highest risk of developing CNS relapse.
Introduction
Mature T-cell and natural killer (NK) cell neoplasms (MTNKNs) are a heterogeneous group of disease entities, comprising <15% of all non-Hodgkin lymphomas (NHLs) in Western countries. More than 70 subtypes of NHL have been defined, of which >25 are categorized as MTNKNs.1,2 Although >70% of patients in the United States with NHL will survive beyond 5 years,3 the prognosis for patients diagnosed with MTNKNs remains poor, with 5-year overall survival (OS) reported <50%,3,4 except for a few more favorable subtypes. Moreover, relapse after frontline therapy occurs frequently, often in those with more advanced disease, and is associated with a high mortality rate.5-7
Central nervous system (CNS) relapse may be especially prone to debilitating symptoms and is associated with increased mortality. Previous retrospective studies report secondary CNS involvement in ∼2% to 11% of patients.7-19 In these small series of <30 patients, CNS relapse/progression typically occurred within 6 months of MTNKN diagnosis and was associated with a median OS under 3 months from the time of CNS involvement.12-19 Certain histologic subtypes, such as extranodal NK/T-cell lymphomas (ENKTL) and adult T-cell leukemia/lymphoma (ATLL), have a well-described risk of CNS involvement, but the incidence of these diseases varies geographically, and they are excluded from many of these retrospective studies.18
To better describe the epidemiology and outcomes of these patients, we compiled, to our knowledge, the largest retrospective case series to date of CNS relapse in MTNKN using a multi-institutional cohort of patients. We then created a training set enriched for patients with CNS relapse to develop a predictive risk model for CNS relapse, termed the CNS relapse In T-cell lymphoma Index (CITI), which was able to accurately discriminate patients at the highest risk of CNS relapse in an independent, population-based validation set.
Methods
Data sources and study population
Data from 141 adult patients diagnosed with MTNKN between 2009 and 2019 who experienced CNS relapse were submitted from 19 North American institutions (supplemental Table 1). CNS relapse had to be confirmed radiographically, by tissue sampling (flow cytometry, cytology, and/or biopsy), or a combination of both. We excluded 6 patients with CNS involvement at diagnosis or before the initiation of frontline therapy as well as patients with T-cell lymphoblastic lymphoma. We compiled summary statistics for these 135 patients to provide a clinical and epidemiologic description of CNS relapse in North American patients with MTNKNs.
For model fitting to predict CNS relapse, we further excluded patients with leukemic and cutaneous forms of MTNKN as defined by the fifth World Health Organization classification,2 except subcutaneous panniculitis-like T-cell lymphoma (SPTCL) and primary cutaneous gamma-delta T-cell lymphoma (PCGD-TCL). We combined these patients with a control cohort of patients from the University of Pennsylvania with MTNKN and without CNS relapse using the same inclusion and exclusion criteria listed above; by combining data from patients with CNS relapse from multiple institutions with a single-center control, we created a training set (n = 226) enriched for patients with CNS relapse, allowing for us to better determine risk factors associated with this uncommon event. Leukemic and cutaneous forms of MTNKNs were excluded for model fitting in the training set because the external validation set from the Swedish Lymphoma Registry13 (n = 745) did not have data for patients with these diagnoses. For the final model fitting, a complete case analysis was performed, so patients with missing data were excluded. A diagram for patient selection and allocation is shown in Figure 1.
Flowchart of patient selection for study. Contributing sites1 are shown in supplemental Table 1.
Flowchart of patient selection for study. Contributing sites1 are shown in supplemental Table 1.
Outcomes
The primary outcome assessed was the incidence of CNS relapse. Time was defined as days from the initial diagnosis of MTNKN, and censoring occurred for patients without CNS relapse at death or last follow-up (FU). In the descriptive analysis of the larger MTNKN cohort (n = 135) as well as in the training (n = 226) and validation sets (n = 745), we report progression-free survival (PFS) and OS from initial diagnosis. In the descriptive analysis of the larger MTNKN cohort (n = 135), we report OS after CNS relapse (CNS-OS). Progression was defined as progression, relapse, or death. For PFS and OS analyses, patients were censored if alive at last FU.
Clinicopathologic risk factors for CNS relapse
Baseline clinicopathologic variables were considered based on clinical relevance, standard data captured in clinical trials, and previous data reported to be associated with CNS relapse (Table 1). We grouped patients with enteropathy–associated T-cell lymphoma (EATL) or monomorphic epitheliotropic intestinal T-cell lymphoma together as “EATL” and patients with SPTCL and PCGD-TCL together as “SPTCL” because patients may have been diagnosed before the separation of these into individual disease entities. Minimum possible International Prognostication Index (IPI) score was calculated if any variables were missing. Except for histology, all variables in our risk model were considered as binary variables.
Baseline characteristics for patients with MTNKN and CNS relapse
| Patient characteristics (n = 135) . | |
|---|---|
| Age, median (range), y | 59.5 (20-81) |
| Age | n (%) |
| ≤60 y | 71 (53) |
| >60 y | 63 (47) |
| Missing | 1 (1) |
| Sex | |
| Male | 56 (41) |
| Female | 79 (59) |
| MTNKN histology | |
| PTCL, NOS | 34 (25) |
| TFH-TCL (including AITL) | 20 (15) |
| ALCL, ALK+ | 1 (1) |
| ALCL, ALK– | 18 (13) |
| ALCL, ALKu | 0 (0) |
| ALCL, BIA | 0 (0) |
| ENKTL | 7 (5) |
| SPTCL (includes SPTCL and PCGD-TCL) | 2 (1) |
| EATL (includes EATL and MEITL) | 7 (5) |
| HSTCL | 2 (1) |
| MF/SS | 18 (13) |
| Other primary cutaneous TCL | 2 (1) |
| ATLL | 14 (10) |
| T-PLL | 3 (2) |
| T-LGL | 2 (1) |
| ANKL | 3 (2) |
| CLD-NK | 1 (1) |
| T-IOL | 1 (1) |
| TCL UNS | 0 (0) |
| ECOG PS | |
| 0 | 48 (36) |
| 1 | 46 (34) |
| 2 | 15 (11) |
| 3 | 4 (3) |
| 4 | 3 (2) |
| Missing | 19 (14) |
| B symptoms | |
| Yes | 69 (51) |
| No | 47 (35) |
| Missing | 19 (14) |
| Elevated LDH | |
| Yes | 55 (41) |
| No | 76 (56) |
| Missing | 4 (3) |
| Hemoglobin | |
| <11 g/dL | 40 (30) |
| ≥11 g/dL | 86 (64) |
| Missing | 9 (7) |
| Platelet count | |
| <150 × 109/L | 30 (22) |
| ≥150 × 109/L | 90 (67) |
| Missing | 15 (11) |
| Ann Arbor stage | |
| I | 18 (13) |
| II | 11 (8) |
| III | 17 (13) |
| IV | 78 (58) |
| Missing/not applicable | 11 (8) |
| Sites of EN involvement (excluding BM/PB) | |
| None | 45 (33) |
| Liver | 13 (10) |
| GI tract | 18 (13) |
| GU tract | 4 (3) |
| Breast | 3 (2) |
| Testicles | 1 (1) |
| Skin | 34 (25) |
| Bone | 16 (12) |
| Other | 14 (10) |
| Lung | 19 (14) |
| Soft tissue | 14 (10) |
| Sinus | 6 (4) |
| Pleura | 2 (1) |
| Peritoneum | 3 (2) |
| Missing | 3 (2) |
| BM/PB involvement | |
| Yes | 61 (45) |
| No | 56 (41) |
| Missing | 18 (13) |
| ≥2 sites of EN involvement (including BM/PB) | |
| Yes | 59 (44) |
| No | 68 (50) |
| Missing | 8 (6) |
| IPI score | |
| 0 | 19 (14) |
| 1 | 21 (16) |
| 2 | 33 (24) |
| 3 | 45 (33) |
| 4 | 13 (10) |
| 5 | 4 (3) |
| Patient characteristics (n = 135) . | |
|---|---|
| Age, median (range), y | 59.5 (20-81) |
| Age | n (%) |
| ≤60 y | 71 (53) |
| >60 y | 63 (47) |
| Missing | 1 (1) |
| Sex | |
| Male | 56 (41) |
| Female | 79 (59) |
| MTNKN histology | |
| PTCL, NOS | 34 (25) |
| TFH-TCL (including AITL) | 20 (15) |
| ALCL, ALK+ | 1 (1) |
| ALCL, ALK– | 18 (13) |
| ALCL, ALKu | 0 (0) |
| ALCL, BIA | 0 (0) |
| ENKTL | 7 (5) |
| SPTCL (includes SPTCL and PCGD-TCL) | 2 (1) |
| EATL (includes EATL and MEITL) | 7 (5) |
| HSTCL | 2 (1) |
| MF/SS | 18 (13) |
| Other primary cutaneous TCL | 2 (1) |
| ATLL | 14 (10) |
| T-PLL | 3 (2) |
| T-LGL | 2 (1) |
| ANKL | 3 (2) |
| CLD-NK | 1 (1) |
| T-IOL | 1 (1) |
| TCL UNS | 0 (0) |
| ECOG PS | |
| 0 | 48 (36) |
| 1 | 46 (34) |
| 2 | 15 (11) |
| 3 | 4 (3) |
| 4 | 3 (2) |
| Missing | 19 (14) |
| B symptoms | |
| Yes | 69 (51) |
| No | 47 (35) |
| Missing | 19 (14) |
| Elevated LDH | |
| Yes | 55 (41) |
| No | 76 (56) |
| Missing | 4 (3) |
| Hemoglobin | |
| <11 g/dL | 40 (30) |
| ≥11 g/dL | 86 (64) |
| Missing | 9 (7) |
| Platelet count | |
| <150 × 109/L | 30 (22) |
| ≥150 × 109/L | 90 (67) |
| Missing | 15 (11) |
| Ann Arbor stage | |
| I | 18 (13) |
| II | 11 (8) |
| III | 17 (13) |
| IV | 78 (58) |
| Missing/not applicable | 11 (8) |
| Sites of EN involvement (excluding BM/PB) | |
| None | 45 (33) |
| Liver | 13 (10) |
| GI tract | 18 (13) |
| GU tract | 4 (3) |
| Breast | 3 (2) |
| Testicles | 1 (1) |
| Skin | 34 (25) |
| Bone | 16 (12) |
| Other | 14 (10) |
| Lung | 19 (14) |
| Soft tissue | 14 (10) |
| Sinus | 6 (4) |
| Pleura | 2 (1) |
| Peritoneum | 3 (2) |
| Missing | 3 (2) |
| BM/PB involvement | |
| Yes | 61 (45) |
| No | 56 (41) |
| Missing | 18 (13) |
| ≥2 sites of EN involvement (including BM/PB) | |
| Yes | 59 (44) |
| No | 68 (50) |
| Missing | 8 (6) |
| IPI score | |
| 0 | 19 (14) |
| 1 | 21 (16) |
| 2 | 33 (24) |
| 3 | 45 (33) |
| 4 | 13 (10) |
| 5 | 4 (3) |
AITL, angioimmunoblastic T-cell lymphoma; ALKu, ALK status unknown; ANKL, aggressive NK-cell lymphoma; ATLL, adult T-cell leukemia/lymphoma; BIA, breast implant associated; CLD-NK, chronic lymphoproliferative disorder of NK-cells; ECOG PS, Eastern Cooperative Oncology Group performance status; EN, extranodal; GI, gastrointestinal; GU, genitourinary; HSTCL, hepatosplenic T-cell lymphoma; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma; T-IOL, T-cell intraocular lymphoma; T-LGL, T-cell large granular lymphocytic leukemia; T-PLL, T-cell prolymphocytic leukemia; TFH-TCL, T-follicular helper T-cell lymphoma; UNS, unspecified.
Statistical analysis
All statistical analyses were performed in R version 4.1.2. P values are based on Fisher exact test for categorical variables and log-rank test for FU, PFS, and OS. A P value <.05 was used for statistical significance. The median FU, PFS, OS, and CNS-OS and 95% confidence interval (CI) are estimated by the Kaplan-Meier or reverse Kaplan-Meier method using the “survival” package in R (version 3.2-13).
We fitted a least absolute shrinkage and selection operator (LASSO) Cox regression model20 to the training set using clinicopathologic characteristics at initial diagnosis: sex, age >60 years, MTNKN histology, Eastern Cooperative Oncology Group (ECOG) performance status ≥2, elevated lactate dehydrogenase (LDH), Ann Arbor stage ≥III, presence of B symptoms, and ≥2 sites of extranodal involvement including bone marrow/peripheral blood (BM/PB) to identify risk factors for CNS relapse using the “glmnet” package in R (version 4.1-3). We excluded hemoglobin, platelet count, and IPI score as variables to match available validation set data, as well as anatomic sites of extranodal involvement because no specific site was significantly different in the training set except for BM/PB. The tuning parameter lambda for the LASSO model was selected using a 10-fold cross validation technique. As previously discussed, the CITI risk score was derived using a complete case analysis (n = 182). Coefficient weights for risk factors were calculated by the natural log of hazard ratios (HRs), so variables with a HR of 1 were not selected by the model. Low-, intermediate-, and high-risk groups in the training set were stratified by CITI risk score terciles. These cutoffs were applied to a complete case analysis of the external validation set (n = 566), in which we calculated the cumulative incidence and risk of CNS relapse and HR for CNS relapse.
For exploratory analysis of frontline treatment modifications in the training set (n = 182) and high-risk subgroup (n = 55), we performed a multivariate analysis of variables selected by the LASSO model with the following frontline treatment modifications: frontline etoposide use, frontline anthracycline use, hematopoietic cell transplant (HCT) in first complete remission (CR1), and CNS prophylaxis use defined as intrathecal (IT) therapy (methotrexate [MTX] and/or cytarabine) or high-dose (at least 3 g/m2) MTX (HD-MTX). We fitted a Cox proportional hazards model using the “survival” package in R (version 3.2-13).
The study met the eligibility criteria for Institutional Review Board (IRB) review exemption at the University of Pennsylvania.
Results
Characterization of patients and outcomes with CNS relapse
Baseline characteristics of patients with CNS relapse
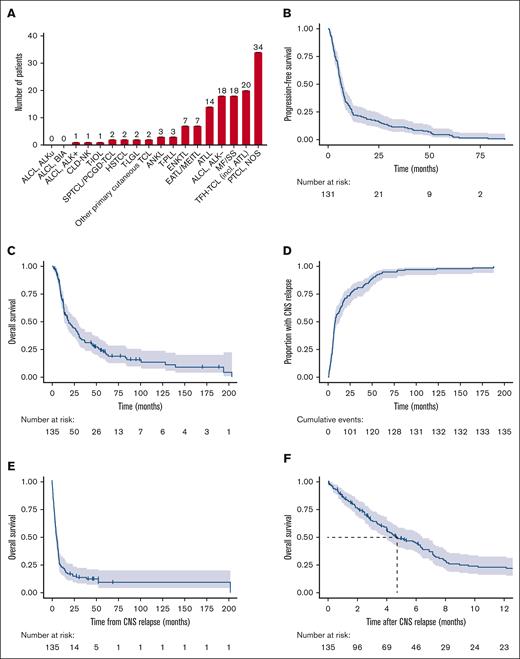
Baseline characteristics for patients with CNS relapse are shown in Table 1. Most patients had stage IV disease at diagnosis (n = 78 [58%]), and n = 59 (44%) had the involvement of ≥2 extranodal sites including BM/PB. The most frequent histologic subtypes observed were peripheral T-cell lymphoma (PTCL), not otherwise specified (NOS) (n = 34 [25%]), nodal T-follicular helper cell lymphoma (TFH-TCL) (n = 20 [15%]), anaplastic large cell lymphoma (ALCL), anaplastic lymphoma kinase (ALK)– (n = 18 [13%]), and mycosis fungoides/Sézary syndrome (MF/SS; n = 18 [13%]; Figure 2A). Nine patients (50%) with MF/SS had biopsy or cytology obtained at the time of CNS relapse, of whom 6 (66%) had evidence of large cell transformation; 2 patients were known to have transformed MF/SS at a prior relapse, whereas 4 patients had de novo large cell transformation upon CNS involvement.
MTNKN subtypes with CNS relapse and outcomes. (A) Frequency of MTNKN subtypes is depicted with number of patients displayed above each bar. Kaplan-Meier estimates for PFS and OS after initial diagnosis are shown in panels B and C, respectively. Four patients were excluded from PFS calculation due to absence of data on time to initial progression. Cumulative incidence of CNS relapse and OS after CNS relapse are shown by reverse and standard Kaplan-Meier estimate in panels D and E-F, respectively. Median OS after CNS relapse is indicated by dashed line (F). For all Kaplan-Meier curves, hashmarks denote censored patients, and shading indicates 95% CI. AITL, angioimmunoblastic T-cell lymphoma; ALKu, ALK status unknown; ANKL, aggressive NK-cell lymphoma; ATLL, adult T-cell leukemia/lymphoma; CLD-NK, chronic lymphoproliferative disorder of NK-cells; HSTCL, hepatosplenic T-cell lymphoma; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma; T-IOL, T-cell intraocular lymphoma; T-LGL, T-cell large granular lymphocytic leukemia; T-PLL, T-cell prolymphocytic leukemia; TCL, T-cell lymphoma; TFH-TCL, T-follicular helper T-cell lymphoma.
MTNKN subtypes with CNS relapse and outcomes. (A) Frequency of MTNKN subtypes is depicted with number of patients displayed above each bar. Kaplan-Meier estimates for PFS and OS after initial diagnosis are shown in panels B and C, respectively. Four patients were excluded from PFS calculation due to absence of data on time to initial progression. Cumulative incidence of CNS relapse and OS after CNS relapse are shown by reverse and standard Kaplan-Meier estimate in panels D and E-F, respectively. Median OS after CNS relapse is indicated by dashed line (F). For all Kaplan-Meier curves, hashmarks denote censored patients, and shading indicates 95% CI. AITL, angioimmunoblastic T-cell lymphoma; ALKu, ALK status unknown; ANKL, aggressive NK-cell lymphoma; ATLL, adult T-cell leukemia/lymphoma; CLD-NK, chronic lymphoproliferative disorder of NK-cells; HSTCL, hepatosplenic T-cell lymphoma; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma; T-IOL, T-cell intraocular lymphoma; T-LGL, T-cell large granular lymphocytic leukemia; T-PLL, T-cell prolymphocytic leukemia; TCL, T-cell lymphoma; TFH-TCL, T-follicular helper T-cell lymphoma.
Outcomes of patients with CNS relapse
The median FU for patients with CNS relapse was 77.1 months (95% CI, 55.7 to not reached), with a PFS and OS from initial diagnosis of 6.4 months (95% CI, 5.5-7.7) and 18.4 months (95% CI, 13.7-29.0), respectively (Figure 2B-C). The median time to CNS involvement was 8.0 months (95% CI, 6.7-12.4; Figure 2D). The median CNS-OS was 4.7 months (95% CI, 4.0-6.4; Figure 2E-F). By 12 months after CNS relapse, 93 patients (69%) had died.
Patterns of CNS relapse and cause of death are shown in supplemental Table 2. CNS relapse was leptomeningeal in 70 patients (52%). Sixty patients (44%) experienced at least 1 occurrence of systemic relapse before CNS involvement, and 92 patients (68%) had concurrent systemic disease upon CNS relapse; 77% of deaths (n = 78) were attributable to lymphoma (26% systemic disease; 16% CNS disease; 36% both).
Treatment characteristics of patients with CNS relapse
Treatment characteristics are shown in supplemental Table 3. Patients received a median of one line of therapy (range, 1-7) before CNS relapse. Frontline regimens included etoposide in 51 (38%) and anthracycline in 94 patients (70%). The overall response rate (ORR) to frontline therapy was 60% (n = 81); 49 patients (36%) had a complete response (CR). Of patients in CR1, 19 (39%) underwent consolidative HCT (autologous HCT, n = 12; allogeneic HCT, n = 7); 6 patients received salvage HCT for systemic relapse before CNS involvement. Frontline CNS prophylaxis was used in 21 patients (16%).
Treatment of CNS relapse/progression included IT therapy (n = 45 [33%]), HD-MTX (n = 34 [25%]), or a combination of IT chemotherapy and HD-MTX (n = 32 [24%]; supplemental Table 4). The ORR to CNS-directed therapy after CNS relapse was 36% (n = 49), with 21% CR (n = 29). Responding and nonresponding patients did not significantly differ by age at the time of CNS relapse (>60 years or ≤60 years; P > .999), pattern of CNS relapse (leptomeningeal or parenchymal; P = .569), or common histologies (PTCL, NOS, P = .807; TFH-TCL, P = .497; ALCL, ALK–, P > .999). The ORRs were 31% (CR, 20%), 47% (CR, 29%), and 38% (CR, 31%) for patients receiving IT therapy, HD-MTX, or combined IT therapy and HD-MTX, respectively (IT vs HD-MTX, P = .407; IT vs combined, P = .160; HD-MTX vs combined, P = .456).
Risk factors for CNS relapse or progression
Baseline characteristics of patients in training and validation sets
Baseline characteristics of these patients are shown in Table 2 (complete case analysis) and supplemental Table 5 (including patients with missing variables). Patients in the validation set more frequently were aged >60 years (P < .001) and had B symptoms at presentation (P < .001) but less frequently had ≥2 sites of extranodal involvement (P < .001). Tumor histology varied between the training and validation sets. The median FU for patients in the training and validation sets were 37.6 months (range, 2.6-150.9) and 95.8 months (range 40.2-160.8), respectively. Patients in the training set had a median PFS and OS of 9.8 months (95% CI, 7.9-13.4) and 33.2 months (95% CI, 25.8-52.7), respectively, and patients in the validation set had a median PFS and OS of 8.5 months (95% CI, 7.3-9.6) and 17.9 months (95% CI, 14.3-21.5), respectively.
Baseline characteristics for patients in training and validation sets with complete case analysis
| Baseline characteristics . | Training set . | P value . | Training set (n = 182) . | Validation set (n = 566) . | P value . | |
|---|---|---|---|---|---|---|
| CNS relapse cohort (n = 63) . | University of Pennsylvania control cohort (n = 119) . | |||||
| Median (range) . | Median (range) . | |||||
| Age, y | 62 (24-81) | 58 (20-85) | .161 | 60 (20-85) | 65 (18-94) | <.001 |
| Age | n (%) | n (%) | ||||
| >60 y | 35 (56) | 55 (46) | .276 | 90 (49) | 366 (65) | <.001 |
| ≤60 y | 28 (44) | 64 (54) | 92 (51) | 200 (35) | ||
| Sex | ||||||
| Male | 38 (60) | 67 (56) | .639 | 105 (58) | 345 (61) | .435 |
| Female | 25 (40) | 52 (44) | 77 (42) | 221 (39) | ||
| MTNKN histology | ||||||
| PTCL, NOS | 25 (40) | 17 (14) | <.001 | 42 (23) | 195 (35) | .004 |
| TFH-TCL (includes AITL) | 14 (22) | 31 (26) | .594 | 45 (25) | 84 (15) | .003 |
| ALCL, ALK+ | 1 (2) | 17 (14) | .007 | 18 (10) | 50 (9) | .658 |
| ALCL, ALK– | 9 (14) | 17 (14) | 1 | 26 (14) | 93 (16) | .561 |
| ALCL, ALKu | 0 (0) | 0 (0) | 1 | 0 (0) | 27 (5) | .001 |
| ENKTL | 5 (8) | 14 (12) | .611 | 19 (10) | 24 (4) | .003 |
| SPTCL (includes SPTCL and PCGD-TCL) | 2 (3) | 13 (11) | .091 | 15 (8) | 9 (2) | <.001 |
| EATL (includes EATL and MEITL) | 6 (10) | 2 (2) | .021 | 8 (4) | 48 (9) | .075 |
| HSTCL | 1 (2) | 5 (4) | .666 | 6 (3) | 8 (1) | .118 |
| TCL UNS | 0 (0) | 0 (0) | 1 | 0 (0) | 28 (5) | <.001 |
| ALCL, BIA | 0 (0) | 3 (3) | .552 | 3 (2) | 0 (0) | .014 |
| ECOG PS | .851 | .12 | ||||
| 2-4 | 14 (22) | 25 (21) | 39 (21) | 156 (28) | ||
| 0-1 | 49 (78) | 94 (79) | 143 (79) | 410 (72) | ||
| Elevated LDH | .271 | .728 | ||||
| Yes | 41 (65) | 67 (56) | 108 (59) | 344 (61) | ||
| No | 22 (35) | 52 (44) | 74 (41) | 222 (39) | ||
| Ann Arbor stage | .043 | .415 | ||||
| III or IV | 50 (79) | 77 (65) | 127 (70) | 375 (66) | ||
| I or II | 13 (21) | 42 (35) | 55 (30) | 191 (34) | ||
| B symptoms | .435 | <.001 | ||||
| Yes | 30 (48) | 49 (41) | 79 (43) | 340 (60) | ||
| No | 33 (52) | 70 (59) | 103 (57) | 226 (40) | ||
| Site of EN involvement (excluding BM/PB) | ||||||
| None | 19 (30) | 54 (45) | .057 | 73 (40) | 248 (44) | .391 |
| Liver | 6 (10) | 5 (4) | .193 | 11 (6) | 28 (5) | .567 |
| GI tract | 13 (21) | 8 (7) | .007 | 21 (12) | 80 (14) | .454 |
| GU tract | 2 (3) | 2 (2) | .61 | 4 (2) | 1 (0.5) | .014 |
| Breast | 1 (2) | 3 (3) | 1 | 4 (2) | 2 (0.5) | .034 |
| Testicles | 1 (2) | 2 (2) | 1 | 3 (2) | 4 (1) | .37 |
| Skin | 14 (22) | 20 (17) | .425 | 34 (19) | 37 (7) | <.001 |
| Bone | 7 (11) | 10 (8) | .597 | 17 (9) | 24 (4) | .014 |
| Other | 5 (8) | 1 (1) | .019 | 6 (3) | 28 (5) | .419 |
| Lung | 12 (19) | 14 (12) | .189 | 26 (14) | 29 (5) | <.001 |
| Soft tissue | 7 (11) | 6 (5) | .142 | 13 (7) | 19 (3) | .035 |
| Sinus | 4 (6) | 7 (6) | 1 | 11 (6) | 9 (2) | .003 |
| Pleura | 1 (2) | 2 (2) | 1 | 3 (2) | 27 (5) | .08 |
| Peritoneum | 3 (5) | 0 (0) | .04 | 3 (2) | 8 (1) | .734 |
| BM/PB involvement | .001 | .037 | ||||
| Yes | 26 (41) | 23 (19) | 49 (27) | 115 (20) | ||
| No | 31 (49) | 94 (79) | 125 (69) | 451 (80) | ||
| Missing | 6 (10) | 2 (2) | 8 (4) | 0 (0) | ||
| ≥2 sites of EN involvement (including BM/PB) | <.001 | <.001 | ||||
| Yes | 30 (48) | 20 (17) | 50 (27) | 79 (14) | ||
| No | 33 (52) | 99 (83) | 132 (73) | 487 (86) | ||
| IPI score | ||||||
| 0-1 | 9 (14) | 43 (36) | .002 | 52 (29) | 146 (26) | .499 |
| 2-3 | 42 (67) | 57 (48) | .019 | 99 (54) | 318 (56) | .732 |
| 4-5 | 12 (19) | 19 (16) | .679 | 31 (17) | 96 (17) | 1 |
| Baseline characteristics . | Training set . | P value . | Training set (n = 182) . | Validation set (n = 566) . | P value . | |
|---|---|---|---|---|---|---|
| CNS relapse cohort (n = 63) . | University of Pennsylvania control cohort (n = 119) . | |||||
| Median (range) . | Median (range) . | |||||
| Age, y | 62 (24-81) | 58 (20-85) | .161 | 60 (20-85) | 65 (18-94) | <.001 |
| Age | n (%) | n (%) | ||||
| >60 y | 35 (56) | 55 (46) | .276 | 90 (49) | 366 (65) | <.001 |
| ≤60 y | 28 (44) | 64 (54) | 92 (51) | 200 (35) | ||
| Sex | ||||||
| Male | 38 (60) | 67 (56) | .639 | 105 (58) | 345 (61) | .435 |
| Female | 25 (40) | 52 (44) | 77 (42) | 221 (39) | ||
| MTNKN histology | ||||||
| PTCL, NOS | 25 (40) | 17 (14) | <.001 | 42 (23) | 195 (35) | .004 |
| TFH-TCL (includes AITL) | 14 (22) | 31 (26) | .594 | 45 (25) | 84 (15) | .003 |
| ALCL, ALK+ | 1 (2) | 17 (14) | .007 | 18 (10) | 50 (9) | .658 |
| ALCL, ALK– | 9 (14) | 17 (14) | 1 | 26 (14) | 93 (16) | .561 |
| ALCL, ALKu | 0 (0) | 0 (0) | 1 | 0 (0) | 27 (5) | .001 |
| ENKTL | 5 (8) | 14 (12) | .611 | 19 (10) | 24 (4) | .003 |
| SPTCL (includes SPTCL and PCGD-TCL) | 2 (3) | 13 (11) | .091 | 15 (8) | 9 (2) | <.001 |
| EATL (includes EATL and MEITL) | 6 (10) | 2 (2) | .021 | 8 (4) | 48 (9) | .075 |
| HSTCL | 1 (2) | 5 (4) | .666 | 6 (3) | 8 (1) | .118 |
| TCL UNS | 0 (0) | 0 (0) | 1 | 0 (0) | 28 (5) | <.001 |
| ALCL, BIA | 0 (0) | 3 (3) | .552 | 3 (2) | 0 (0) | .014 |
| ECOG PS | .851 | .12 | ||||
| 2-4 | 14 (22) | 25 (21) | 39 (21) | 156 (28) | ||
| 0-1 | 49 (78) | 94 (79) | 143 (79) | 410 (72) | ||
| Elevated LDH | .271 | .728 | ||||
| Yes | 41 (65) | 67 (56) | 108 (59) | 344 (61) | ||
| No | 22 (35) | 52 (44) | 74 (41) | 222 (39) | ||
| Ann Arbor stage | .043 | .415 | ||||
| III or IV | 50 (79) | 77 (65) | 127 (70) | 375 (66) | ||
| I or II | 13 (21) | 42 (35) | 55 (30) | 191 (34) | ||
| B symptoms | .435 | <.001 | ||||
| Yes | 30 (48) | 49 (41) | 79 (43) | 340 (60) | ||
| No | 33 (52) | 70 (59) | 103 (57) | 226 (40) | ||
| Site of EN involvement (excluding BM/PB) | ||||||
| None | 19 (30) | 54 (45) | .057 | 73 (40) | 248 (44) | .391 |
| Liver | 6 (10) | 5 (4) | .193 | 11 (6) | 28 (5) | .567 |
| GI tract | 13 (21) | 8 (7) | .007 | 21 (12) | 80 (14) | .454 |
| GU tract | 2 (3) | 2 (2) | .61 | 4 (2) | 1 (0.5) | .014 |
| Breast | 1 (2) | 3 (3) | 1 | 4 (2) | 2 (0.5) | .034 |
| Testicles | 1 (2) | 2 (2) | 1 | 3 (2) | 4 (1) | .37 |
| Skin | 14 (22) | 20 (17) | .425 | 34 (19) | 37 (7) | <.001 |
| Bone | 7 (11) | 10 (8) | .597 | 17 (9) | 24 (4) | .014 |
| Other | 5 (8) | 1 (1) | .019 | 6 (3) | 28 (5) | .419 |
| Lung | 12 (19) | 14 (12) | .189 | 26 (14) | 29 (5) | <.001 |
| Soft tissue | 7 (11) | 6 (5) | .142 | 13 (7) | 19 (3) | .035 |
| Sinus | 4 (6) | 7 (6) | 1 | 11 (6) | 9 (2) | .003 |
| Pleura | 1 (2) | 2 (2) | 1 | 3 (2) | 27 (5) | .08 |
| Peritoneum | 3 (5) | 0 (0) | .04 | 3 (2) | 8 (1) | .734 |
| BM/PB involvement | .001 | .037 | ||||
| Yes | 26 (41) | 23 (19) | 49 (27) | 115 (20) | ||
| No | 31 (49) | 94 (79) | 125 (69) | 451 (80) | ||
| Missing | 6 (10) | 2 (2) | 8 (4) | 0 (0) | ||
| ≥2 sites of EN involvement (including BM/PB) | <.001 | <.001 | ||||
| Yes | 30 (48) | 20 (17) | 50 (27) | 79 (14) | ||
| No | 33 (52) | 99 (83) | 132 (73) | 487 (86) | ||
| IPI score | ||||||
| 0-1 | 9 (14) | 43 (36) | .002 | 52 (29) | 146 (26) | .499 |
| 2-3 | 42 (67) | 57 (48) | .019 | 99 (54) | 318 (56) | .732 |
| 4-5 | 12 (19) | 19 (16) | .679 | 31 (17) | 96 (17) | 1 |
Abbreviations are explained in Table 1.
Development of CITI
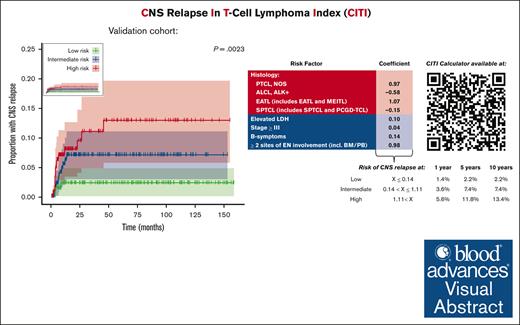
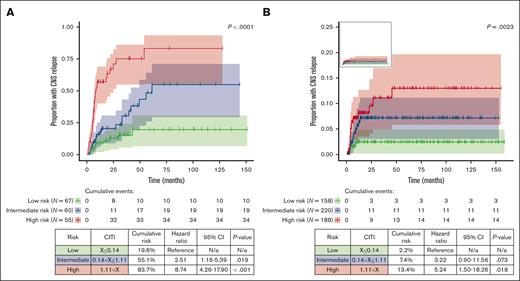
We used a LASSO Cox regression model for risk factor analysis, which uses variable selection and regularization to reduce overfitting and improve predictive accuracy.20 Histology (PTCL, NOS; EATL; ALCL, ALK+; SPTCL), LDH, stage, B symptoms, and ≥2 sites of extranodal involvement were selected by the LASSO model (Table 3). We next applied the CITI score to patients in the training set and stratified them into low- (n = 67; CITI ≤0.14), intermediate- (n = 60; 0.14 < CITI ≤1.11), and high-risk terciles (n = 55; CITI >1.11) (P < .0001). The cumulative risk of CNS relapse in each respective risk group was 19.6%, 55.1%, and 83.7% (Figure 3A).
CITI risk factor coefficients
| Risk factor . | HR . | Weighted coefficient . |
|---|---|---|
| MTNKN histology | ||
| PTCL, NOS | 2.64 | 0.97 |
| ALCL, ALK+ | 0.56 | −0.58 |
| EATL (includes EATL and MEITL) | 2.92 | 1.07 |
| SPTCL (includes SPTCL and PCGD-TCL) | 0.86 | −0.15 |
| Elevated LDH | 1.10 | 0.10 |
| Stage ≥III | 1.04 | 0.04 |
| B symptoms | 1.15 | 0.14 |
| ≥2 sites of EN involvement (including BM/PB) | 2.66 | 0.98 |
| Risk factor . | HR . | Weighted coefficient . |
|---|---|---|
| MTNKN histology | ||
| PTCL, NOS | 2.64 | 0.97 |
| ALCL, ALK+ | 0.56 | −0.58 |
| EATL (includes EATL and MEITL) | 2.92 | 1.07 |
| SPTCL (includes SPTCL and PCGD-TCL) | 0.86 | −0.15 |
| Elevated LDH | 1.10 | 0.10 |
| Stage ≥III | 1.04 | 0.04 |
| B symptoms | 1.15 | 0.14 |
| ≥2 sites of EN involvement (including BM/PB) | 2.66 | 0.98 |
Abbreviations are explained in Table 1.
Cumulative incidence of CNS relapse stratified by CITI risk group. Cumulative incidence of CNS relapse in training set (A) and validation set (B) is depicted by reverse Kaplan-Meier curve, stratified by CITI risk group. Reverse Kaplan-Meier curve for validation set without scaled y-axis is shown in top-left panel inset. For all Kaplan-Meier curves, hashmarks denote censored patients, and shading indicates 95% CI. Log-rank P values for trend are shown above the Kaplan-Meier curves. Cumulative risk, HRs with 95% CI, and Holm-Bonferroni adjusted P values for pairwise risk group comparisons are depicted in tables beneath Kaplan-Meier curves. Low-risk groups were used as reference values for training and validation sets.
Cumulative incidence of CNS relapse stratified by CITI risk group. Cumulative incidence of CNS relapse in training set (A) and validation set (B) is depicted by reverse Kaplan-Meier curve, stratified by CITI risk group. Reverse Kaplan-Meier curve for validation set without scaled y-axis is shown in top-left panel inset. For all Kaplan-Meier curves, hashmarks denote censored patients, and shading indicates 95% CI. Log-rank P values for trend are shown above the Kaplan-Meier curves. Cumulative risk, HRs with 95% CI, and Holm-Bonferroni adjusted P values for pairwise risk group comparisons are depicted in tables beneath Kaplan-Meier curves. Low-risk groups were used as reference values for training and validation sets.
Validation of CITI
To validate the generalizability of the CITI score, we applied it to patients from the Swedish Lymphoma Registry with complete case information (n = 566). The cumulative risk of CNS relapse in low- (n = 158), intermediate- (n = 220), and high-risk patients (n = 188) was 2.2%, 7.4%, and 13.4%, respectively (Figure 3B); as expected, the risk of CNS relapse in the validation set was considerably lower than in the training set because there was no enrichment for patients with CNS involvement, but risk stratification remained significant (P = .0023). The risk of CNS relapse in the high-risk group was significantly higher than that in the low-risk group (HR, 5.24; 95% CI, 1.50-18.26; P = .018); the risk of CNS relapse in intermediate-risk patients was numerically higher than that in low-risk patients, although it did not meet the threshold for statistical significance (HR, 3.22; 95% CI, 0.90-11.56; P = .073). To provide a simple tool for clinical use, we developed an open-access web-based CITI calculator (https://redcap.link/citicalc), which calculates the CITI risk score in real time and provides 1-, 5-, and 10-year estimates of CNS relapse risk based on the validation set data.
Effect of frontline treatment modifications
We performed a multivariate analysis of frontline treatment modifications in the CNS relapse–enriched training set and found that HCT in CR1 was associated with a lower risk of CNS relapse (HR, 0.38; 95% CI, 0.17-0.85; P = .018) when controlling for clinicopathologic factors selected by the CITI model (supplemental Tables 6 and 7). The increased risk of CNS relapse in patients receiving frontline anthracycline therapy can be attributed to anthracycline use in most patients (76%) in this heavily CNS relapse–enriched cohort.
In high-risk patients (n = 55), HCT in CR1 was associated with a lower incidence of CNS relapse but did not meet the threshold for statistical significance (HR, 0.36; 95% CI, 0.10-1.28; P = .113; supplemental Table 8). Similarly, high-risk validation set patients who underwent consolidative HCT (n = 39) did not have a statistically significant reduction in CNS relapse compared with those who did not (n = 148; HR, 0.62; 95% CI, 0.20-1.93; P = .454).
To account for biases from immortal time and response to frontline treatment, we evaluated the impact of frontline treatment modifications for training set patients who both completed frontline therapy and had CR1 (n = 110); completion of frontline therapy was set as a landmark time point to adjust for immortal time bias. HCT in CR1 in this group was associated with a lower incidence of CNS relapse, but it no longer met the threshold for statistical significance (HR, 0.37; 95% CI, 0.14-1.01; P = .051), including for CITI-stratified high-risk patients with CR1 (HR, 0.25; 95% CI, 0.02-2.82; P = .262; supplemental Tables 9 and 10).
Discussion
Here, we report, to our knowledge, the largest descriptive case series to date of CNS relapse in MTNKN. Despite enrichment for CNS relapse, patients in the training set had a considerably longer median OS than those in the validation set (33.2 vs 17.9 months, respectively); this may be in part explained by worse performance status and differences in tumor histology. Nevertheless, the CNS-OS for both groups was still under 6 months, reflecting the poor outcomes for these patients. Using these data, we developed the CITI prognostic model, which has been externally validated and can discriminate patients with MTNKN at high risk of CNS relapse based on clinicopathologic features at initial diagnosis. The 5- and 10-year risks of CNS relapse in high-risk patients exceed 10%, meeting the standard set by the CNS-IPI in B-cell NHL.21
In a study of 625 patients from the Swedish Lymphoma Registry,13 the investigators observed an increased risk of CNS relapse with ≥2 sites of extranodal involvement (HR, 2.60; 95% CI, 1.07-6.29; P = .035), particularly in patients with skin (HR, 3.51; 95% CI, 1.26-9.74; P = .016) and gastrointestinal involvement (HR, 3.06; 95% CI, 1.30-7.18; P = .010), consistent with patterns of extranodal involvement in our cohort (Tables 1-2; supplemental Table 5). Several other retrospective studies corroborated the increased CNS relapse risk with the involvement of ≥2 extranodal sites.14-16,19,22,23 Other factors associated with tumor bulk (LDH,12 IPI score,14,19 and B symptoms16) and anatomic sites of extranodal involvement (paranasal sinus12 and testicles16) have also been reported as risk factors for CNS involvement in single retrospective studies. The CITI model expands upon these data by creating a comprehensive and dynamic score to personalize CNS relapse risk assessment for patients with MTNKN.
The most common histology associated with CNS relapse in our study was PTCL, NOS, which is consistent with several other retrospective studies.18 The risk of CNS relapse was strongly associated with the presence of ≥2 involved extranodal sites and the PTCL, NOS subtype. In general, PTCL, NOS is the most commonly encountered MTNKN subtype in Northern America and Europe, which may partially explain the higher prevalence in our CNS relapse cohort; however, other common subtypes, which were similarly represented, such as TFH-TCL or ALCL, ALK– were not more likely to have CNS relapse. Moreover, similar proportions of patients with PTCL, NOS in the CNS relapse and non-CNS relapse groups in the training set had advanced stage disease (91 vs 89%, respectively) or ≥2 sites of extranodal involvement (35% vs 28%, respectively). When we repeated the LASSO Cox regression in the PTCL, NOS subgroup of our training set (n = 42), we noted similar clinical risk factors as our multihistology model, particularly ≥2 sites of extranodal involvement (data not shown). Together, these findings suggest a plausible biological risk of CNS relapse associated with PTCL, NOS. However, most patients with PTCL, NOS in the training and validation cohorts did not have ≥2 sites of extranodal involvement at diagnosis, and only approximately one-third of MTNKN cases in North American, European, and Asian populations are attributable to PTCL, NOS; thus, the incorporation of multiple clinical and histologic risk factors enables the CITI score to be prognostic for most patients with MTNKNs.
No prospective studies have evaluated CNS relapse in MTNKNs to date, and the National Comprehensive Cancer Network24 does not currently recommend screening asymptomatic patients for CNS involvement. Furthermore, the utility of CNS-directed prophylaxis or frontline treatment modifications remains equivocal in MTNKNs. Although consideration should be given to CNS-directed prophylaxis for nodal or leukemic (acute) forms of ATLL in which the incidence of CNS involvement may be up to 30%,14,25,26 its benefit remains uncertain and solely based on comparisons with historical controls.27,28 Consistent with retrospective studies from the Swedish Lymphoma Registry13 and Memorial Sloan Kettering Cancer Center,14 we did not observe a lower risk of CNS relapse with frontline treatment modifications, with the exception of HCT in CR1. We were unable to demonstrate statistical significance of this benefit at a threshold of P value <.05, specifically in patients in CR1 and/or the high-risk cohorts, although our analysis of treatment modifications is purely hypothesis generating, and we acknowledge that it is limited in part by the small number of patients who underwent consolidative HCT in these subgroups.
Nevertheless, the high concordance of concurrent systemic disease in patients with CNS relapse (68%) suggests that the poor outcomes associated with CNS involvement reflect an overall aggressive nature of the lymphoma because 62% of deaths were ultimately attributed in part to systemic lymphoma. Additionally, we found that patients with MF/SS are among the most frequent with CNS relapse, likely in part due to its prevalence compared with other MTNKN. Although the longer latency period from diagnosis to CNS relapse reflects the more indolent nature of MF/SS, most patients with MF/SS and biopsy-proven CNS relapse in our study had large cell transformation, underscoring an association with more aggressive disease phenotypes. Thus, therapies offering better systemic disease control, such as HCT, may lead to improved outcomes irrespective of CNS relapse. An analogous example of how better initial systemic control inherently leads to a reduced risk of CNS relapse may be the addition of rituximab to chemotherapy in B-cell NHL; although rituximab is not typically considered to have CNS activity, its near universal incorporation into upfront treatment of B-cell NHL has been associated with a reduction in CNS relapse.21,29-32
Our study does have limitations, including its retrospective nature, differences in patient populations and practice patterns across multiple institutions, biological heterogeneity of MTNKN, and selection bias for patients seen at academic institutions. We addressed this through validation of our selected variables in an independent population-based cohort, underscoring that a combination of histologic and clinical features is most reliably able to predict the risk of CNS relapse. Moreover, we excluded leukemic and cutaneous (except SPTCL and PCGD-TCL) forms of MTNKNs from the CITI model to improve generalizability and allow for validation with external data sets, although future models would benefit from incorporation of these patients. We also attempted to collect genetic and cytogenetic data from patients’ diagnostic specimens, although the lack of central pathology review and inclusion of cases dating back to 2009 prevented us from collecting sufficient data for analysis.
Lastly, MTNKN display significant geographic and ethnic heterogeneity, and the CITI model has only been studied in North American and European cohorts, thus extrapolation to other populations will require broader validation. For example, the CNS-prognostic index of NK (CNS-PINK) model was developed and validated in South Korean and Japanese patients with ENKTL, respectively.23,33 Although we did not observe an increased risk of CNS relapse with ENKTL, patients with ≥2 sites of extranodal involvement and stage III to IV disease would be at intermediate risk of CNS relapse regardless of histology using the CITI model, with a cumulative risk of CNS relapse of 7.4% vs 13.9% in the CNS-PINK study.23 Although specific factors such as nonnasal type ENKTL in the CNS-PINK score may improve CNS relapse prediction, developing individual prognostic tools for each histology is unlikely feasible given the rarity of many subtypes and CNS events, supporting the utility of the CITI score across most MTNKN histologies.
In conclusion, using a collaborative multi-institutional network, we analyzed, to our knowledge, the largest cohort to date of patients with MTNKN and CNS relapse. Our study identified slightly improved outcomes of patients with CNS relapse compared with historical controls and found a novel association between MF/SS and CNS involvement. Using these data, we created a predictive risk model, CITI, that was validated in an independent population-based cohort. Although our data about the use of frontline treatment modifications are exploratory, the CITI risk score may provide a more nuanced approach to selecting frontline treatment modifications for preventing and managing CNS disease in MTNKNs, as current practices are extrapolated from B-cell NHL, in which there is no convincing evidence supporting the efficacy of currently available CNS-directed prophylaxis.34-36 However, because most patients with CNS relapse eventually succumb to systemic relapse, more effective therapies in the upfront and relapsed setting may promise the largest impact on outcomes in patients with and without CNS relapse.
Acknowledgments
The authors thank the patients who were included in the study, as well as their family members and the staff from participating institutions.
This work received support, in part, from the Hematopoiesis Training Program Grant at the University of Pennsylvania, National Institutes of Health (NIH; T32DK07780 [R.S.B.]); the Abramson Cancer Center Support Grant, NIH (P30CA016520 [W.L. and Q.L.]); the National Cancer Institute Cancer Center Support Grant, NIH (P30CA008748 [S.M.H]); and the Leukemia & Lymphoma Society (Clinical Research Scholar Award [A.J.O.]).
Authorship
Contribution: R.S.B., and S.K.B. participated in conception and design; R.S.B., F.E., T.R., N.G., R.S., S.M.H., K.W., S.R.H., N.N.B., J.C., L.S., H.S., F.D., P. Porcu, P. Pullarkat, N.M.-S., J.M.Z., M.R., J.E.B., R.P., S.P.I., A.J.O., A.M., P.A.R., S.M.S., C.G., B. Haverkos, B. Hu, T.Z.Z., P.B.A., W.T., M. Janakiram, T.R.B., D.J., N.H., A.M.G., P.G., F.F., J.M.R., E.A.C., J.N.G., D.J.L., S.D.N., S.J.S., J.S., M. Jerkeman, and S.K.B. were responsible for provision of study materials or patients; R.S.B., F.E., T.R., M.C., N.G., R.S., S.M.H., K.W., S.R.H., N.N.B., J.C., L.S., H.S., F.D., P. Porcu, P. Pullarkat, N.M.-S., J.M.Z., M.R., J.E.B., R.P., S.P.I., A.J.O., A.M., P.A.R., S.M.S., C.G., B. Haverkos, B. Hu, T.Z.Z., P.B.A., W.T., M. Janakiram, T.R.B., D.J., N.H., A.M.G., P.G., F.F., J.M.R., E.A.C., J.N.G., D.J.L., S.D.N., S.J.S., J.S., M. Jerkeman, and S.K.B. were responsible for collection and assembly of data; R.S.B. and S.K.B. wrote the manuscript; and all authors participated in data analysis and interpretation, gave the final approval of the manuscript, and are accountable for all aspects of work.
Conflict-of-interest disclosure: R.S.B. reports consulting or advisory role for ALVA10. S.K.B. reports consulting fees or honoraria from Acrotech Biopharma, Affimed, Daiichi Sankyo, Janssen, Seagen, and Kyowa Kirin. M. Jerkeman reports honoraria from AbbVie, AstraZeneca, Kite/Gilead, Janssen, Pierre Fabre, Sobi, and Roche. A.M.G. reports consulting or advisory role for Seattle Genetics. J.N.G. reports research funding from Loxo Oncology. H.S. reports consulting or advisory role for Seattle Genetics, Acrotech Biopharma, MorphoSys, Incyte Corporation, and ADC Therapeutics; and research funding from Secura Bio and Bristol Myers Squibb (BMS). P.B.A. reports consulting or advisory role for Kyowa Hakko Kirin, Daiichi Sankyo, Secura Bio, and Seattle Genetics. P.A.R. reports consulting or advisory role for AbbVie, Genmab, ADC Therapeutics, Pharmacyclics, Novartis, BMS, Kite/Gilead, Nurix Therapeutics, Nektar Therapeutics, Takeda, Intellia Therapeutics, Sana Biotechnology, BeiGene, Janssen, and CVS Caremark; honoraria from Novartis; and research funding from BMS, Kite/Gilead, Novartis, MorphoSys, CRISPR Therapeutics, Calibr, Xencor, Fate Therapeutics, Roche, and Tessa Therapeutics. B. Hu reports consulting or advisory role for BeiGene, ADC Therapeutics, Incyte, and Roche/Genentech. N.M.-S. reports consulting or advisory role for AstraZeneca, Secura Bio/Verastem, Genentech, Kyowa Hakko Kirin, and Janssen; and research funding from AstraZeneca, BMS, Celgene, C4 Therapeutics, Corvus Pharmaceuticals, Daiichi Sankyo, Genentech/Roche, Innate Pharmaceuticals, and Secura Bio/Verastem. N.N.B. reports consulting or advisory role for Secura Bio, Affimed GmbH, Astellas Pharma, and Acrotech Biopharma; scientific advisory committee fees from Acrotech Biopharma. L.S. reports consulting or advisory role for Kyowa Hakko Kirin, Secura Bio, Daiichi Sankyo, CRISPR Therapeutics, and Dren Bio; and research funding from Kyowa Hakko Kirin and EUSA. A.J.O. reports consulting or advisory role for Genmab, Blue Cross/Blue Shield of Rhode Island, Schrodinger, ADC Therapeutics, and BeiGene; and research funding from Leukemia & Lymphoma Society, Genentech, Inc/F. Hoffmann-La Roche Ltd, Adaptive Biotechnologies, Precision Biosciences, and Genmab. J.S. reports consulting or advisory role for Seagen, BMS, AstraZeneca, Pharmacyclics, Adaptive Biotechnologies, and Atara; and research funding from Seagen, Celgene, Pharmacyclics, Merck, BMS, Incyte, AstraZeneca, and Adaptive Biotechnologies. S.M.H. reports honoraria from Affimed, Abcuro Inc, Corvus, Daiichi Sankyo, Kyowa Hakko Kirin, March Biosciences, Ono Pharmaceuticals, Pfizer Pharmaceutical, Seagen, SimBio Pharmaceuticals, Secura Bio, and Takeda; and research funding from ADC Therapeutics, Affimed, C4, Celgene, CRISPR Therapeutics, Daiichi Sankyo, Dren Bio, Kyowa Hakko Kirin, Takeda, Seattle Genetics, and Secura Bio. The remaining authors declare no competing financial interests.
Correspondence: Stefan K. Barta, Lymphoma Program, Abramson Cancer Center, University of Pennsylvania, Perelman Center, South Pavilion, 12th Floor, 3400 Civic Center Blvd, Philadelphia, PA 19104; email: stefan.barta@pennmedicine.upenn.edu.
References
Author notes
Deidentified data that underlie the reported results can be made available upon reasonable request at the time of publication with a data use agreement. Proposals for access should be sent to the corresponding author, Stefan K. Barta (stefan.barta@pennmedicine.upenn.edu). Requests for data from the Swedish Lymphoma Registry must be made directly.
The full-text version of this article contains a data supplement.