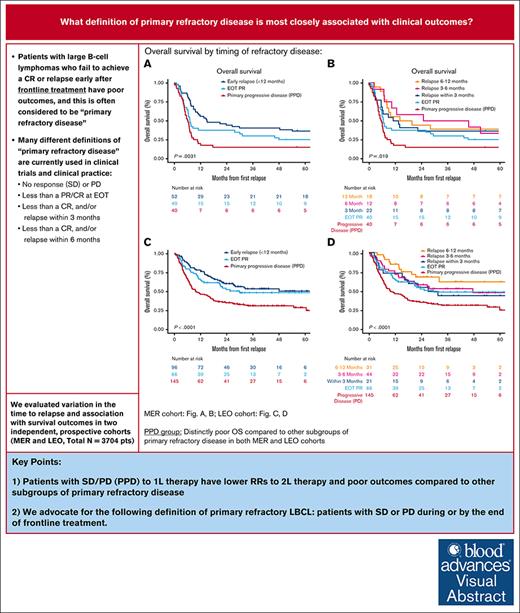
Key Points
Patients with SD/PD to 1L therapy have lower responses to 2L therapy and poor OS compared with other subgroups of primary refractory disease.
We advocate for the following definition of primary refractory LBCL: patients with SD or PD during, or by the end of, 1L treatment.
Visual Abstract
Patients with large B-cell lymphoma (LBCL) that fail to achieve a complete response (CR) or who relapse early after anthracycline-containing immunochemotherapy (IC) have a poor prognosis and are commonly considered to have “primary refractory disease.” However, different definitions of primary refractory disease are used in the literature and clinical practice. In this study, we examined variation in the time to relapse used to define refractory status and association with survival outcomes in patients with primary refractory LBCL in a single-center prospective cohort with validation in an independent multicenter cohort. Patients with newly diagnosed LBCL were enrolled in the Molecular Epidemiological Resource cohort (MER; N = 949) or the Lymphoma Epidemiology of Outcomes cohort (LEO; N = 2755) from September 2002 to May 2021. Primary refractory LBCL was defined as no response (stable disease [SD]) or progressive disease (PD) during, or by the end of, frontline (1L) IC (primary PD; PPD); partial response at end of treatment (EOT PR); or relapse within 3 to 12 months after achieving CR at EOT to 1L IC (early relapse). In the MER cohort, patients with PPD had inferior overall survival (OS; 2-year OS rate: 15% MER, 31% LEO) when compared with other subgroups considered in defining primary refractory disease, EOT PR (2-year OS rate: 38% MER, 50% LEO) and early relapse (2-year OS rate: 44% MER, 58% LEO). Among patients receiving 1L IC with curative intent, we identified that patients with PPD are the key subgroup with poor outcomes. We propose a definition of primary refractory LBCL as SD or PD during, or by the end of, 1L treatment.
Introduction
Patients with diffuse large B-cell lymphoma (DLBCL) or high grade B-cell lymphoma (collectively, large B-cell lymphoma [LBCL]), can be cured with frontline (1L) immunochemotherapy (IC) with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in up to 60% to 70% of the cases.1-3 The clinical course after 1L IC failure is heterogeneous.4,5 LBCL that does not respond adequately to 1L IC or that relapses early after an initial response to IC have poor outcomes and are often considered “primary refractory disease.” However, definitions of primary refractory disease have varied in the literature. The most narrow definition is failure to achieve a partial6,7 or complete response (CR) to 1L treatment.8-10 Another common definition includes LBCL relapsing within 3 months after an initial CR to 1L treatment.4,11 The most broad definition also includes relapses within 3 to 12 months after completing IC.12-15 Historically, patients with these varied definitions of primary refractory disease were treated similarly with salvage chemotherapy and consideration for autologous stem cell transplant (ASCT),6,8 but the outcomes were poor overall (durable remissions in only 20% of patients), because of lack of chemosensitivity that prevents ASCT or results in relapse after ASCT.6,12,13,16 Recently, CD19-directed chimeric antigen receptor (CAR) T-cell therapy (CAR-T) has been approved for eligible patients relapsing within 12 months as an alternative and preferred second line (2L) treatment option for these patients.17,18
Prospective studies of primary refractory disease have been limited, possibly, in part, because of lack of a consensus definition. Even in recent pivotal trials evaluating CAR-T as 2L therapy for patients with primary refractory or early relapsed (within 12 months) aggressive LBCLs, different definitions of primary refractory disease were used.18-20 The International Prognostic Index (IPI) and its modified forms Revised-IPI (R-IPI) and national comprehensive cancer network (NCCN)-IPI capture high risk clinical features but were not developed to identify primary refractory disease.21 Many studies have attempted to identify clinical or pathological predictors of primary refractory disease with conflicting results.4,7,22 In addition to differences in time to treatment failure inclusion criteria, a common limitation in many prior studies is that they do not restrict based on treatment-intensity and therefore the measurements may not be entirely reflective of underlying disease biology; for example, intolerance to therapy because of an adverse event and subsequent progression is likely more of a reflection of underlying comorbidities rather than disease aggressiveness.
In this study, we evaluated clinical and pathological characteristics, 2L treatment responses, and survival outcomes of patients with broadly defined primary refractory LBCL, in 2 large prospective cohorts: (1) the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence Molecular Epidemiological Resource (MER) cohort, and (2) the Lymphoma Epidemiology of Outcomes (LEO) cohort. The aim was to clearly define what time to relapse should be used in defining primary refractory disease to identify patients at the highest risk for poor outcomes to inform clinical practice and future research.
Methods
Patients and methods
MER cohort (cohort 1)
Details on the MER cohort have previously been reported.23 Adult patients with newly diagnosed LBCLs including DLBCL and high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements (HGBCL) were prospectively enrolled in the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence MER cohort from September 2002 to June 2015. Cell-of-origin determination was performed in accordance with the Hans algorithm.24 Immunohistochemical and fluorescence in situ hybridization analyses were performed using available sections from formalin fixed, paraffin embedded tissue blocks that were obtained at initial diagnosis and were documented retrospectively in MER patients with available data.
LEO cohort (cohort 2)
We evaluated whether our definitions of primary refractory disease reflected similar outcomes in the LEO cohort of patients who were treated at 8 academic centers across the United States, reflecting a more diverse patient population than our MER cohort in the Upper Midwest. The LEO prospective cohort enrolled patients between 2015 and 2021 at 8 academic centers. The demographics of this overall cohort has been presented previously.25
Patients in Cohort 1 and Cohort 2 who received curative intent anthracycline-containing IC for LBCL (R-CHOP or similar, including on trial, eg, R-CHOP + lenalidomide or polatuzumab vedotin-R-CHP) or HGBCL (including R-CHOP and more intensive chemotherapy regimens such as rituximab, etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin [R-EPOCH], rituximab, cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosphamide, etoposide, cytarabine [R-CODOX-M/R-IVAC]) were included. Interim response assessment was performed after 2 or 3 cycles in all patients and end of treatment (EOT) response assessment was performed, 4 to 8 weeks after completing chemotherapy, by positron emission tomography -computed tomography (PET-CT) scans in most patients (n = 20 in MER cohort had computed tomography only) by standard Cheson and/or Lugano response criteria.26-28 Patients who did not complete planned IC treatment or who did not initiate anthracycline-based treatment because of intolerance or toxicity were excluded so that our analysis would best capture patients with treatment failure rather than relapses from inadequate treatment or unrelated events. This includes patients who had planned reduced-dose intensity (eg, mini-R-CHOP); were intolerant to, and discontinued treatment, before response assessment; who had toxicity resulting in incomplete treatment (<75% planned dose intensity); and/or who died unrelated to disease progression before EOT response assessment. Patients without complete data of response assessment were also excluded (supplemental Figure 1). Patients with primary central nervous system (CNS) LBCL and transformed LBCL who received prior therapy for the indolent lymphoma and/or received planned consolidative ASCT were also excluded. Patients with secondary CNS involvement at diagnosis were not excluded.
We abstracted baseline data on demographics (age, sex, and race), clinical features (Eastern Cooperative Oncology Group performance status, number of extranodal sites, stage, and IPI), and pathological features (cell of origin, cytogenetic and molecular characteristics such as MYC/BCL2 double expressor, and MYC and BCL2 and/or BCL6 rearrangements). HGBCLs were assessed using World Health Organization WHO 2016 classification criteria, which included BCL6 rearrangements.29 Data on which patients had a MYC/BCL2 vs MYC/BCL6 rearrangement is shown in supplemental Table 1. 2L treatment and response and survival outcomes were abstracted.
Statistical analysis
For the analysis of relapsed patients in Cohort 1 and 2, all available patients meeting inclusion criteria were included in the analysis. We evaluated the functional form of timing of release using restricted cubic splines (supplemental Figure 2). Then, according to different established definitions of primary refractory disease, the following cases were considered serially as components of the definition of primary refractory disease: (1) stable disease or progressive disease (PD) during, or by the end of, 1L IC (including transient interim partial response [PR] or CR, and primary PD [PPD]); (2) PR as the best response at the EOT (EOT PR); or (3) early relapse within 3 months, (4) 3 to 6 months, or (5) 6 to 12 months after achieving CR at EOT to 1L treatment. After individual analysis of subgroups 3 to 5, these were grouped together for further analysis (early relapse).
Overall survival (OS) was defined as time from relapse (or refractoriness) until death from any cause. Different combinations of primary refractory definitions were evaluated based on a priori definitions 1 to 5 as described above. OS was evaluated using Kaplan-Meier curves to visualize the survival probability over time for the different subgroups and statistical significance was quantified using log-rank test P value at an α level of .05. An analysis of baseline features was performed among all patients with PPD in Cohort 2 compared with all remaining newly diagnosed DLBCL with χ2 or t test used to compare the groups and adjusted for multiple comparisons using false discovery rate. For this analysis, OS was defined from time of relapse or the EOT (for patients without relapse) until death from any cause. Analyses were performed using R/RStudio version 4.2.2 and SAS version 9.4M5.
This retrospective study was approved by Mayo Clinic institutional review board.
Results
MER cohort (cohort 1)
Among a total of 949 patients in the MER cohort with newly diagnosed DLBCL (N = 918) or HGBCL (n = 30), 132 (13.9%) met inclusion criteria for primary refractory disease (PPD, n = 40; EOT PR, n = 40; and early relapse, n = 52). Progression or persistent disease was confirmed by biopsy in addition to imaging in 59% of patients, imaging only in 35%, clinically in 2%, or method not documented or missing in 4%. Baseline characteristics are described in Table 1. Median age at diagnosis was 60 years (range, 19-84 years), and 65% were male. Most patients (74%) had advanced stage disease, and 51% had high or high intermediate risk IPI. There were no significant differences in baseline characteristics among the 3 groups (Table 1). The proportions of MYC and BCL2 and/or BCL6 rearrangements (DHL/THL; 12% overall) or MYC/BCL2 double expressor (28% overall) were not different among the 3 groups. In most patients (73%), first-line treatment was R-CHOP or similar treatment on a clinical trial.
Baseline patient characteristics in different subsets of primary refractory disease
| Variable . | MER (N = 949) . | LEO (N = 2755) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PPD (n = 40) . | EOT PR (n = 40) . | Early relapse (<12 mos) (n = 52) . | Total (n = 132) . | P† . | PPD (n = 145) . | EOT PR (n = 66) . | Early relapse (<12 mos) (n = 97) . | Total (n = 308) . | P . | |
| n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | |||
| Age at diagnosis, y | .754 | |||||||||
| Median (range) | 59.5 (19-80) | 62.5 (24-84) | 60.0 (21-83) | 60.0 (19-84) | 63 (19-88) | 62 (28-90) | 64 (25-83) | 63 (19-90) | .829 | |
| Sex | .448 | .996 | ||||||||
| F | 19 (47.5) | 8 (20.0) | 20 (38.5) | 47 (35.6) | 50 (34.7) | 23 (34.8) | 35 (36.5) | 108 (35.3) | ||
| Race | .754 | .485 | ||||||||
| White | 36 (90.0) | 37 (92.5) | 50 (96.2) | 123 (93.2) | 127 (88.2) | 57 (86.4) | 80 (83.3) | 264 (86.3) | ||
| African American | 0 (0.0) | 1 (2.5) | 0 (0.0) | 1 (0.8) | 10 (6.9) | 4 (6.1) | 6 (6.3) | 20 (6.5) | ||
| Unknown/not reported | 4 (10.0) | 2 (5.0) | 2 (3.8) | 8 (6.1) | 4 (2.8) | 1 (1.5) | 4 (4.2) | 9 (2.9) | ||
| Ethnicity | .754 | .889 | ||||||||
| Not Hispanic/Latinx | 33 (82.5) | 37 (92.5) | 47 (90.4) | 117 (88.6) | 126 (87.5) | 60 (90.9) | 84 (87.5) | 270 (88.2) | ||
| Hispanic/Latinx | 0 | 0 | 0 | 0 | 15 (11.0) | 6 (9.1) | 11 (11.5) | 32 (10.5) | ||
| Unknown/not reported | 7 (17.5) | 3 (7.5) | 5 (9.6) | 15 (11.4) | 3 (2.1) | 0 (0) | 1 (1) | 4 (1.3) | ||
| ECOG PS | .853 | .996 | ||||||||
| <2 | 35 (87.5) | 33 (82.5) | 45 (86.5) | 113 (85.6) | 104 (77.0) | 47 (77.0) | 69 (77.5) | 220 (77.2) | ||
| LDH | .782 | .485 | ||||||||
| >Normal | 26 (78.8) | 24 (77.4) | 41 (85.4) | 91 (81.3) | 87 (76.3) | 37 (66.1) | 72 (80.9) | 196 (75.7) | ||
| Extranodal sites | .754 | .829 | ||||||||
| ≤1 | 24 (63.2) | 31 (77.5) | 32 (61.5) | 87 (66.9) | 92 (64.8) | 46 (70.8) | 58 (61.70) | 196 (65.1) | ||
| Ann Arbor stage | .782 | .816 | ||||||||
| III to IV | 32 (80.0) | 29 (72.5) | 37 (71.2) | 98 (74.2) | 107 (80.5) | 46 (76.7) | 80 (85.1) | 233 (81.2) | ||
| IPI group | .754 | .485 | ||||||||
| 0 to 1, low | 9 (22.5) | 11 (27.5) | 10 (19.2) | 30 (22.7) | 37 (25.7) | 19 (28.8) | 14 (14.6) | 70 (22.9) | ||
| 2, low intermediate | 10 (25.0) | 13 (32.5) | 12 (23.1) | 35 (26.5) | 35 (24.3) | 19 (28.8) | 22 (22.9) | 76 (24.8) | ||
| 3, high intermediate | 15 (37.5) | 7 (17.5) | 23 (44.2) | 45 (34.1) | 42 (29.2) | 15 (22.7) | 35 (36.5) | 92 (30.1) | ||
| 4 to 5, high | 6 (15.0) | 9 (22.5) | 7 (13.5) | 22 (16.7) | 30 (20.8) | 13 (19.7) | 25 (26.0) | 68 (22.2) | ||
| Diagnosis to treatment interval | .782 | .889 | ||||||||
| Median (range), days | 12.5 (2-43) | 13 (2-41) | 12.5 (0-40) | 12.5 (0-43) | 15 (1-55) | 16 (3-56) | 16.5 (1-92) | 15 (1-92) | ||
| Cell of origin | .980 | .485 | ||||||||
| Known | ||||||||||
| Non-GCB | 15 (50.0) | 12 (46.2) | 15 (48.4) | 42 (48.3) | 32 (41.5) | 20 (39.2) | 30 (45.4) | 82 (42.2) | ||
| GCB | 15 (50.0) | 14 (53.8) | 16 (51.6) | 45 (51.7) | 45 (58.5) | 31 (60.8) | 36 (54.6) | 112 (57.4) | ||
| Unknown/not done | 1 | 1 | 2 | 4 | 29 | 8 | 11 | 48 | ||
| Double hit/triple hit | .832 | .485 | ||||||||
| Known | ||||||||||
| Yes | 4 (19.0) | 1 (5) | 3 (12) | 8 (12.1) | 23 (27.3) | 7 (14.9) | 11 (16.4) | 41 (20.7) | ||
| No | 17 (81.0) | 19 (95) | 22 (88) | 58 (87.9) | 61 (72.7) | 40 (85.1) | 56 (83.6) | 157 (79.3) | ||
| Unknown/not done | 19 | 20 | 27 | 66 | 61 | 19 | 30 | 110 | ||
| Double expressor | .754 | .889 | ||||||||
| Known | ||||||||||
| Yes | 6 (30) | 3 (20) | 5 (33.3) | 14 (28.0) | 25 (43.1) | 12 (37.5) | 19 (41.3) | 56 (41.2) | ||
| No | 14 (70) | 12 (80) | 10 (66.7) | 36 (72.0) | 33 (56.9) | 20 (62.5) | 27 (58.7) | 80 (58.8) | ||
| Unknown/not done | 20 | 25 | 37 | 82 | 87 | 34 | 50 | 170 | ||
| 1L treatment | .431 | .485 | ||||||||
| R-CHOP | 27 (67.5) | 32 (80.0) | 37 (71.2) | 96 (72.7) | 112 (77.2) | 46 (70.8) | 64 (66.7) | 221 (72.7) | ||
| Other IC∗ | 13 (32.5) | 8 (20.0) | 15 (28.8) | 36 (27.3) | 33 (22.8) | 19 (29.2) | 32 (33.3) | 83 (27.3) | ||
| Variable . | MER (N = 949) . | LEO (N = 2755) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PPD (n = 40) . | EOT PR (n = 40) . | Early relapse (<12 mos) (n = 52) . | Total (n = 132) . | P† . | PPD (n = 145) . | EOT PR (n = 66) . | Early relapse (<12 mos) (n = 97) . | Total (n = 308) . | P . | |
| n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | |||
| Age at diagnosis, y | .754 | |||||||||
| Median (range) | 59.5 (19-80) | 62.5 (24-84) | 60.0 (21-83) | 60.0 (19-84) | 63 (19-88) | 62 (28-90) | 64 (25-83) | 63 (19-90) | .829 | |
| Sex | .448 | .996 | ||||||||
| F | 19 (47.5) | 8 (20.0) | 20 (38.5) | 47 (35.6) | 50 (34.7) | 23 (34.8) | 35 (36.5) | 108 (35.3) | ||
| Race | .754 | .485 | ||||||||
| White | 36 (90.0) | 37 (92.5) | 50 (96.2) | 123 (93.2) | 127 (88.2) | 57 (86.4) | 80 (83.3) | 264 (86.3) | ||
| African American | 0 (0.0) | 1 (2.5) | 0 (0.0) | 1 (0.8) | 10 (6.9) | 4 (6.1) | 6 (6.3) | 20 (6.5) | ||
| Unknown/not reported | 4 (10.0) | 2 (5.0) | 2 (3.8) | 8 (6.1) | 4 (2.8) | 1 (1.5) | 4 (4.2) | 9 (2.9) | ||
| Ethnicity | .754 | .889 | ||||||||
| Not Hispanic/Latinx | 33 (82.5) | 37 (92.5) | 47 (90.4) | 117 (88.6) | 126 (87.5) | 60 (90.9) | 84 (87.5) | 270 (88.2) | ||
| Hispanic/Latinx | 0 | 0 | 0 | 0 | 15 (11.0) | 6 (9.1) | 11 (11.5) | 32 (10.5) | ||
| Unknown/not reported | 7 (17.5) | 3 (7.5) | 5 (9.6) | 15 (11.4) | 3 (2.1) | 0 (0) | 1 (1) | 4 (1.3) | ||
| ECOG PS | .853 | .996 | ||||||||
| <2 | 35 (87.5) | 33 (82.5) | 45 (86.5) | 113 (85.6) | 104 (77.0) | 47 (77.0) | 69 (77.5) | 220 (77.2) | ||
| LDH | .782 | .485 | ||||||||
| >Normal | 26 (78.8) | 24 (77.4) | 41 (85.4) | 91 (81.3) | 87 (76.3) | 37 (66.1) | 72 (80.9) | 196 (75.7) | ||
| Extranodal sites | .754 | .829 | ||||||||
| ≤1 | 24 (63.2) | 31 (77.5) | 32 (61.5) | 87 (66.9) | 92 (64.8) | 46 (70.8) | 58 (61.70) | 196 (65.1) | ||
| Ann Arbor stage | .782 | .816 | ||||||||
| III to IV | 32 (80.0) | 29 (72.5) | 37 (71.2) | 98 (74.2) | 107 (80.5) | 46 (76.7) | 80 (85.1) | 233 (81.2) | ||
| IPI group | .754 | .485 | ||||||||
| 0 to 1, low | 9 (22.5) | 11 (27.5) | 10 (19.2) | 30 (22.7) | 37 (25.7) | 19 (28.8) | 14 (14.6) | 70 (22.9) | ||
| 2, low intermediate | 10 (25.0) | 13 (32.5) | 12 (23.1) | 35 (26.5) | 35 (24.3) | 19 (28.8) | 22 (22.9) | 76 (24.8) | ||
| 3, high intermediate | 15 (37.5) | 7 (17.5) | 23 (44.2) | 45 (34.1) | 42 (29.2) | 15 (22.7) | 35 (36.5) | 92 (30.1) | ||
| 4 to 5, high | 6 (15.0) | 9 (22.5) | 7 (13.5) | 22 (16.7) | 30 (20.8) | 13 (19.7) | 25 (26.0) | 68 (22.2) | ||
| Diagnosis to treatment interval | .782 | .889 | ||||||||
| Median (range), days | 12.5 (2-43) | 13 (2-41) | 12.5 (0-40) | 12.5 (0-43) | 15 (1-55) | 16 (3-56) | 16.5 (1-92) | 15 (1-92) | ||
| Cell of origin | .980 | .485 | ||||||||
| Known | ||||||||||
| Non-GCB | 15 (50.0) | 12 (46.2) | 15 (48.4) | 42 (48.3) | 32 (41.5) | 20 (39.2) | 30 (45.4) | 82 (42.2) | ||
| GCB | 15 (50.0) | 14 (53.8) | 16 (51.6) | 45 (51.7) | 45 (58.5) | 31 (60.8) | 36 (54.6) | 112 (57.4) | ||
| Unknown/not done | 1 | 1 | 2 | 4 | 29 | 8 | 11 | 48 | ||
| Double hit/triple hit | .832 | .485 | ||||||||
| Known | ||||||||||
| Yes | 4 (19.0) | 1 (5) | 3 (12) | 8 (12.1) | 23 (27.3) | 7 (14.9) | 11 (16.4) | 41 (20.7) | ||
| No | 17 (81.0) | 19 (95) | 22 (88) | 58 (87.9) | 61 (72.7) | 40 (85.1) | 56 (83.6) | 157 (79.3) | ||
| Unknown/not done | 19 | 20 | 27 | 66 | 61 | 19 | 30 | 110 | ||
| Double expressor | .754 | .889 | ||||||||
| Known | ||||||||||
| Yes | 6 (30) | 3 (20) | 5 (33.3) | 14 (28.0) | 25 (43.1) | 12 (37.5) | 19 (41.3) | 56 (41.2) | ||
| No | 14 (70) | 12 (80) | 10 (66.7) | 36 (72.0) | 33 (56.9) | 20 (62.5) | 27 (58.7) | 80 (58.8) | ||
| Unknown/not done | 20 | 25 | 37 | 82 | 87 | 34 | 50 | 170 | ||
| 1L treatment | .431 | .485 | ||||||||
| R-CHOP | 27 (67.5) | 32 (80.0) | 37 (71.2) | 96 (72.7) | 112 (77.2) | 46 (70.8) | 64 (66.7) | 221 (72.7) | ||
| Other IC∗ | 13 (32.5) | 8 (20.0) | 15 (28.8) | 36 (27.3) | 33 (22.8) | 19 (29.2) | 32 (33.3) | 83 (27.3) | ||
Early relapse defined as relapse within 12 months after 1L treatment.
Double-hit/triple-hit defined as MYC rearrangement with BCL2 and/or BCL6; and double expressor defined as expression of MYC and BCL2 by immunohistochemistry.
ECOG PS, Eastern Cooperative Oncology Group performance status; F, female; GCB, germinal center B cell; LDH, lactate dehydrogenase; mos, months.
Other IC: includes more intensive 1L chemotherapy including Rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate and cytarabine therapy as part of an investigational clinical trial (eg, R2-CHOP).
False discovery rate correction applied to all analyses.
2L therapies included platinum-based chemotherapy (eg, rituximab, ifosphamide, carboplatin, and etoposide [R-ICE], rituximab, dexamethasone, cytarabine, prednisone [R-DHAP], or similar; n = 89; 69%), primarily CNS-directed therapy (n = 15; 12%), other systemic palliative chemotherapy (n = 17; 13%), or radiotherapy or resection for localized disease (n = 8; 6%; Table 2). The response to salvage chemotherapy was significantly different between the 3 groups. The overall response rate (ORR)/CR/PR rate to 2L was 39.5%/7.9%/31.6%, respectively, for patients with PPD; 62%/10.3%/51.7%, respectively, for patients with EOT PR; and 68.9%/35.6%/33.3%, respectively, for patients with early relapse (Table 2). A higher proportion of patients with PPD had PD as best response to 2L therapy (55.3%) compared than EOT PR (27.5%) or early relapse (24.4%). Among all patients, 83% (n = 105) underwent curative intent 2L therapy (defined as platinum-based chemotherapy or any therapy with intent to proceed to ASCT; Table 3). Patients with PPD had the highest use of curative-intent 2L therapy (90%) but had significantly lower ORR/CR/PR rates than patients with EOT PR or early relapse (Table 3). Among all patients with complete subsequent treatment data (n = 125), 42% (n = 53) of patients underwent ASCT after any 2L therapy. Rates of ASCT were lower in the PPD group (n = 10, 26%) than in the EOT PR (n = 14, 39%) and early relapse (n = 29, 59%) groups.
2L treatment choices and response rate in MER and LEO cohorts
2L therapy . | MER (N = 949) . | LEO (N = 2755) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PPD (n = 40) . | EOT PR (n = 40) . | Early relapse (n = 52) . | Total (n = 132) . | P . | PPD (n = 145) . | EOT PR (n = 66) . | Early relapse (n = 97) . | Total (n = 308) . | P . | |
| n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | |||
| Platinum-based chemotherapy | 33 (82.5) | 29 (76.3) | 27 (52.9) | 89 (69.0) | .002 | 91 (64.5) | 34 (59.6) | 41 (45.6) | 166 (57.6) | .011 |
| Other systemic chemotherapy | 4 (10.0) | 2 (5.3) | 11 (21.6) | 17 (13.2) | 6 (4.2) | 4 (7.0) | 11 (12.2) | 21 (7.3) | ||
| Primarily CNS directed | 3 (7.5) | 1 (2.6) | 11 (21.6) | 15 (11.6) | 7 (4.9) | 9 (15.8) | 17 (18.9) | 33 (11.5) | ||
| Radiation/resection | 0 (0) | 6 (15.8) | 2 (3.9) | 8 (6.2) | 12 (8.5) | 3 (5.3) | 7 (7.8) | 22 (7.7) | ||
| Targeted therapies | N/A | 11 (7.8) | 3 (5.3) | 11 (12.2) | 25 (8.7) | |||||
| CAR-T (no bridging) | N/A | 10 (7.0) | 4 (7.0) | 2 (2.2) | 16 (5.6) | |||||
| No treatment | N/A | 4 (2.8) | 0 (0) | 1 (1.1) | 5 (1.7) | |||||
| Missing/unknown | 0 | 2 | 1 | 3 | 4 | 9 | 7 | 20 | ||
2L therapy . | MER (N = 949) . | LEO (N = 2755) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PPD (n = 40) . | EOT PR (n = 40) . | Early relapse (n = 52) . | Total (n = 132) . | P . | PPD (n = 145) . | EOT PR (n = 66) . | Early relapse (n = 97) . | Total (n = 308) . | P . | |
| n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | |||
| Platinum-based chemotherapy | 33 (82.5) | 29 (76.3) | 27 (52.9) | 89 (69.0) | .002 | 91 (64.5) | 34 (59.6) | 41 (45.6) | 166 (57.6) | .011 |
| Other systemic chemotherapy | 4 (10.0) | 2 (5.3) | 11 (21.6) | 17 (13.2) | 6 (4.2) | 4 (7.0) | 11 (12.2) | 21 (7.3) | ||
| Primarily CNS directed | 3 (7.5) | 1 (2.6) | 11 (21.6) | 15 (11.6) | 7 (4.9) | 9 (15.8) | 17 (18.9) | 33 (11.5) | ||
| Radiation/resection | 0 (0) | 6 (15.8) | 2 (3.9) | 8 (6.2) | 12 (8.5) | 3 (5.3) | 7 (7.8) | 22 (7.7) | ||
| Targeted therapies | N/A | 11 (7.8) | 3 (5.3) | 11 (12.2) | 25 (8.7) | |||||
| CAR-T (no bridging) | N/A | 10 (7.0) | 4 (7.0) | 2 (2.2) | 16 (5.6) | |||||
| No treatment | N/A | 4 (2.8) | 0 (0) | 1 (1.1) | 5 (1.7) | |||||
| Missing/unknown | 0 | 2 | 1 | 3 | 4 | 9 | 7 | 20 | ||
| Response rate to 2L therapy∗ . | n (%) . | n (%) . | n (%) . | n (%) . | P . | n (%) . | n (%) . | n (%) . | n (%) . | P . |
|---|---|---|---|---|---|---|---|---|---|---|
| CR | 3 (7.9) | 3 (10.3) | 16 (35.6) | 21 (20.0) | .009 | 25 (20.8) | 16 (32.7) | 40 (52.6) | 81 (33.1) | <.001 |
| PR | 12 (31.6) | 15 (51.7) | 15 (33.3) | 42 (40.0) | 27 (22.5) | 16 (32.7) | 15 (19.7) | 58 (23.7) | ||
| Stable disease | 2 (5.3) | 3 (10.3) | 3 (6.7) | 8 (7.6) | 9 (7.5) | 4 (8.2) | 4 (5.3) | 17 (6.9) | ||
| PD | 21 (55.3) | 8 (27.5) | 11 (24.4) | 34 (32.4) | 59 (49.2) | 13 (26.4) | 17 (22.4) | 89 (36.3) | ||
| Not applicable/missing† | 2 | 11 | 7 | 27 | 25 | 17 | 21 | 63 |
| Response rate to 2L therapy∗ . | n (%) . | n (%) . | n (%) . | n (%) . | P . | n (%) . | n (%) . | n (%) . | n (%) . | P . |
|---|---|---|---|---|---|---|---|---|---|---|
| CR | 3 (7.9) | 3 (10.3) | 16 (35.6) | 21 (20.0) | .009 | 25 (20.8) | 16 (32.7) | 40 (52.6) | 81 (33.1) | <.001 |
| PR | 12 (31.6) | 15 (51.7) | 15 (33.3) | 42 (40.0) | 27 (22.5) | 16 (32.7) | 15 (19.7) | 58 (23.7) | ||
| Stable disease | 2 (5.3) | 3 (10.3) | 3 (6.7) | 8 (7.6) | 9 (7.5) | 4 (8.2) | 4 (5.3) | 17 (6.9) | ||
| PD | 21 (55.3) | 8 (27.5) | 11 (24.4) | 34 (32.4) | 59 (49.2) | 13 (26.4) | 17 (22.4) | 89 (36.3) | ||
| Not applicable/missing† | 2 | 11 | 7 | 27 | 25 | 17 | 21 | 63 |
Early relapse defined as patients relapsing within 12 months after EOT.
N/A, not applicable; ESHAP-R, Rituximab, etoposide, cisplatin, cytarabine and prednisone; CEPP- cyclophosphamide, etoposide, procarbazine and prednisone; axi-cel, axicabtagene ciloleucel; tisa-cel, tisagenlecleucel.
Treatment groups/Abbreviations: Platinum-based chemotherapy: R-ICE (n = 172), R-DHAP (n = 46), R- guanosine diphosphate (n = 17), R-DHAX (n = 9), HyperCVAD (n = 3), DA-EPOCH-(R)(n = 4), ESHAP-(R) (n = 4); other systemic chemotherapy: CEPP (n = 2), ROAD (n = 5), R-GemOx (n = 16), R-cyclophosphamide(n = 1), R-Bendamustine(n = 1); primarily CNS-directed: single-agent high-dose methotrexate (HD MTX; n = 16), HDMTX, rituximab and temozolomide (MRT; n = 21), cytarabine/HD MTX (n = 7); MATRIX (n = 3), ibrutinib + intrathecal MTX (n = 1). Targeted therapies: ibrutinib (n = 2); polatuzumab vedotin, rituximab with and without bendamustin (n = 2); rituximab-lenalidomide (n = 10); rituximab-lenalidomide-ibrutinib (n = 2); venetoclax (n = 2); loncastuximab tesirine (n = 1); selinexor (n = 1); pembrolizumab (n = 2); and single-agent rituximab with and without prednisone (n = 3). CAR-T: cellular therapy with axi-cel, tisa-cel directly without bridging therapy (n = 15), and mosunetuzumab (n = 1).
Response rate determined by 2014 Lugano response criteria.21
Includes patients who received radiation/resection or no treatment as 2L therapy; in the LEO cohort, also includes patients with no imaging assessment (n = 9).
Response rates to curative-intent 2L treatment in MER and LEO cohorts
| 2L therapy . | MER (N = 949) . | LEO (N = 2755) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PPD (n = 40) . | EOT PR (n = 40) . | Early relapse (n = 52) . | Total (n = 132) . | P . | PPD (n = 145) . | EOT PR (n = 66) . | Early relapse (n = 97) . | Total (n = 308) . | P . | |
| n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | |||
| Curative intent | 35 (90.0) | 32 (86.5) | 38 (74.5) | 105 (82.8) | .174 | 102 (70.8) | 41 (63.1) | 49 (51.0) | 192 (63.0) | .040 |
| Non-curative intent | 4 (10.0) | 5 (13.5) | 13 (25.5) | 22 (17.2) | 42 (29.2) | 24 (36.9) | 47 (49.0) | 113 (37.0) | ||
| Missing/ n/a | 1 | 3 | 1 | 4 | 1 | 1 | 1 | 3 | ||
| 2L therapy . | MER (N = 949) . | LEO (N = 2755) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PPD (n = 40) . | EOT PR (n = 40) . | Early relapse (n = 52) . | Total (n = 132) . | P . | PPD (n = 145) . | EOT PR (n = 66) . | Early relapse (n = 97) . | Total (n = 308) . | P . | |
| n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | |||
| Curative intent | 35 (90.0) | 32 (86.5) | 38 (74.5) | 105 (82.8) | .174 | 102 (70.8) | 41 (63.1) | 49 (51.0) | 192 (63.0) | .040 |
| Non-curative intent | 4 (10.0) | 5 (13.5) | 13 (25.5) | 22 (17.2) | 42 (29.2) | 24 (36.9) | 47 (49.0) | 113 (37.0) | ||
| Missing/ n/a | 1 | 3 | 1 | 4 | 1 | 1 | 1 | 3 | ||
| Response rate to 2L therapya . | n (%) . | n (%) . | n (%) . | n (%) . | P . | n (%) . | n (%) . | n (%) . | n (%) . | P . |
|---|---|---|---|---|---|---|---|---|---|---|
| Curative intent | n = 35 | n = 32 | n = 38 | n = 105 | .002 | n = 102 | n = 41 | n = 49 | n = 192 | ≤.001 |
| CR | 3 (8.6) | 5 (16.6) | 16 (42.1) | 24 (23.5) | 23 (23.9) | 15 (38.5) | 31 (66.0) | 69 (37.9) | ||
| PR | 11 (31.4) | 15 (50.0) | 11 (28.9) | 37 (36.3) | 22 (22.9) | 14 (35.9) | 6 (12.7) | 42 (23.1) | ||
| SD | 1 (2.9) | 2 (6.7) | 3 (7.9) | 6 (5.9) | 6 (6.3) | 1 (2.6) | 2 (4.3) | 9 (4.9) | ||
| PD | 19 (57.1) | 8 (26.7) | 7 (18.9) | 34 (34.3) | 45 (46.9) | 9 (23.0) | 8 (17.0) | 62 (34.1) | ||
| Missing | 1 | 2 | 1 | 4 | 6 | 2 | 2 | 10 | ||
| Non-curative intent | n = 4 | n = 5 | n = 13 | n = 22 | .243 | n = 42 | n = 24 | n = 47 | n = 113 | .045 |
| CR | 0 (0) | 1 (50.0) | 1 (11.1) | 2 (14.3) | 3 (9.6) | 3 (25.0) | 13 (36.1) | 19 (21.6) | ||
| PR | 1 (33.3) | 0 | 4 (44.4) | 5 (35.7) | 5 (16.1) | 2 (16.7) | 11 (30.6) | 18 (20.5) | ||
| SD | 1 (33.3) | 1 (50) | 0 | 2 (14.3) | 3 (9.6) | 3 (25.0) | 2 (5.5) | 8 (9.1) | ||
| PD | 1 (33.3) | 0 | 4 (44.4) | 5 (35.7) | 20 (64.5) | 4 (33.3) | 10 (27.8) | 34 (38.6) | ||
| Not applicableb/ missing | 1 | 3 | 4 | 8 | 11 | 12 | 11 | 34 |
| Response rate to 2L therapya . | n (%) . | n (%) . | n (%) . | n (%) . | P . | n (%) . | n (%) . | n (%) . | n (%) . | P . |
|---|---|---|---|---|---|---|---|---|---|---|
| Curative intent | n = 35 | n = 32 | n = 38 | n = 105 | .002 | n = 102 | n = 41 | n = 49 | n = 192 | ≤.001 |
| CR | 3 (8.6) | 5 (16.6) | 16 (42.1) | 24 (23.5) | 23 (23.9) | 15 (38.5) | 31 (66.0) | 69 (37.9) | ||
| PR | 11 (31.4) | 15 (50.0) | 11 (28.9) | 37 (36.3) | 22 (22.9) | 14 (35.9) | 6 (12.7) | 42 (23.1) | ||
| SD | 1 (2.9) | 2 (6.7) | 3 (7.9) | 6 (5.9) | 6 (6.3) | 1 (2.6) | 2 (4.3) | 9 (4.9) | ||
| PD | 19 (57.1) | 8 (26.7) | 7 (18.9) | 34 (34.3) | 45 (46.9) | 9 (23.0) | 8 (17.0) | 62 (34.1) | ||
| Missing | 1 | 2 | 1 | 4 | 6 | 2 | 2 | 10 | ||
| Non-curative intent | n = 4 | n = 5 | n = 13 | n = 22 | .243 | n = 42 | n = 24 | n = 47 | n = 113 | .045 |
| CR | 0 (0) | 1 (50.0) | 1 (11.1) | 2 (14.3) | 3 (9.6) | 3 (25.0) | 13 (36.1) | 19 (21.6) | ||
| PR | 1 (33.3) | 0 | 4 (44.4) | 5 (35.7) | 5 (16.1) | 2 (16.7) | 11 (30.6) | 18 (20.5) | ||
| SD | 1 (33.3) | 1 (50) | 0 | 2 (14.3) | 3 (9.6) | 3 (25.0) | 2 (5.5) | 8 (9.1) | ||
| PD | 1 (33.3) | 0 | 4 (44.4) | 5 (35.7) | 20 (64.5) | 4 (33.3) | 10 (27.8) | 34 (38.6) | ||
| Not applicableb/ missing | 1 | 3 | 4 | 8 | 11 | 12 | 11 | 34 |
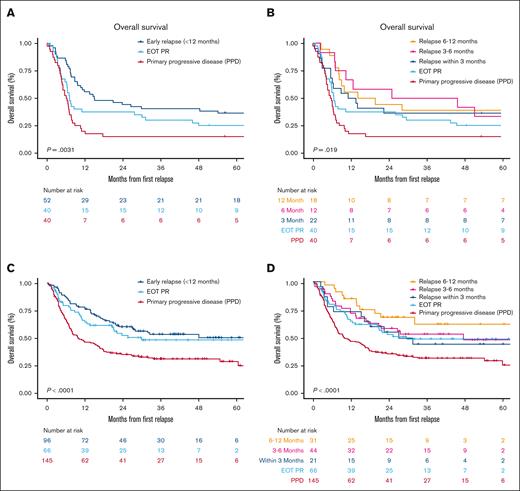
At a median follow-up of 103.2 months, 101 patients (77%) had died. The 2-year OS rate was 15% (95% confidence interval [CI], 7-31) for patients with PPD, which was significantly worse compared with that of patients with EOT PR (2-year OS, 38%; 95% CI, 25-56) or early relapse (3-12 months, 2-year OS, 44%; 95% CI, 33-60; Figure 1A). In patients with early relapse, the 2-year OS rate was not appreciably different among those who relapsed within 3 months (36%; 95% CI, 21-63), between 3 to 6 months (58%; 95% CI, 36-94), or between 6 to 12 months (44%; 95% CI, 27-75; Figure 1B).
OS from time of relapse based on timing of refractory status. (A and B) OS in MER cohort; and (C and D) OS in LEO cohort.
OS from time of relapse based on timing of refractory status. (A and B) OS in MER cohort; and (C and D) OS in LEO cohort.
LEO cohort (validation)
A total of 2755 patients with DLBCL (n = 2522) or HGBCL (n = 233) were enrolled in the LEO cohort from 2015 to 2021. Among 402 patients with relapse before 12 months, 308 (11.3%) met inclusion criteria for primary refractory disease (PPD, n = 145; EOT PR, n = 66; and early relapse, n = 97; supplemental Figure 1). Progression or persistent disease was confirmed by biopsy in addition to imaging in 61% of patients, imaging only in 30%, clinically in 1%, and method not documented or missing in 8%. Baseline characteristics are described in Table 1. Median age was 63 years (range, 19-90 years), and 65% were male. Most patients (81%) had advanced disease, and 52% had high or high intermediate risk IPI. Similar to the MER cohort, there were no significant differences in baseline clinical features between the 3 groups. Among patients with complete data (n = 136), the proportions of DHL/THL (21% overall) or MYC/BCL2 double expressor (41% overall) were not different among the 3 groups. Compared with the MER cohort, the LEO cohort had higher inclusion of non-White races and Hispanic/Latinx ethnicity. Otherwise, baseline clinical, laboratory, and pathology features and treatment were similar between the MER and LEO cohorts.
Similar to the MER cohort, 2L treatment choices and response to 2L treatment were significantly different between the 3 groups. 2L therapies included platinum-based chemotherapy in most patients (n = 166, 58%), primarily CNS-directed therapy (n = 33, 12%), targeted therapies (n = 25; 9%), CAR-T (without bridging, n = 16, 6%; with any bridging therapy, n = 13, 4%), other systemic palliative chemotherapy (n = 21; 7%), radiotherapy or resection (n = 22; 8%), or no treatment (n = 5; 2%). 2L treatment choice was similar among subgroups except a higher use of CNS-directed therapies in those with early relapse (19%) and EOT PR (16%) than PPD (5%). Among all patients, 63% (n = 192) underwent curative intent 2L therapy (defined as platinum-based chemotherapy or any therapy followed by ASCT or CAR-T; Table 3). Patients with PPD had the highest use of curative-intent 2L therapy (71%) but, despite this, had significantly lower ORR/CR/PR rates (46.9%/23.9%/22.9%, respectively) than patients with EOT PR (74.4%/38.5%/35.9%, respectively) or early relapse (78.7%/66.0%/12.7%, respectively; Table 3). Notably, a similar trend was observed when evaluating response rate to 2L treatment groups (Table 2). For patients with PPD, the ORR was similar at ∼40% in both the MER and LEO cohorts, but the CR rate appeared higher in the LEO cohort. Targeted therapies and CAR-T were used more as 2L in the LEO cohort compared with the MER cohort, likely affecting overall and CR rates and outcomes. In the PPD group, 8% of patients received targeted therapies and 7% received CAR-T, with an ORR of 27% (95% CI, 9.8-56.6) and 87% (95% CI, 62.1-96.3), respectively. Among all patients, 18% (n = 54) patients underwent ASCT after any 2L therapy. Rates of ASCT were lower in the PPD group (n = 18, 12%) than EOT PR (n = 12, 18%) and early relapse (n = 24, 25%) groups. The lower ASCT rate than in the MER cohort reflects the availability of CAR-T as an alternative 2L option, although notably all patients who received CAR-T were treated in the context of a clinical trial whereas ASCT was clinical practice.
At a median follow-up of 36.6 months, 187 patients (60%) had died. The 2-year OS rate was 30% (95% CI, 24-39) for patients with PPD, which was significantly worse compared with that of patients with EOT PR (2-year OS, 50%; 95% CI, 38-64) or early relapse (2-year OS, 58%; 95% CI, 49-69; Figure 1C). In patients with early relapse, the 2-year OS rate was not significantly different among those who relapsed within 3 months (52%; 95% CI, 34-79) or between 3 to 6 months (56%; 95% CI, 42-73) but appeared better for those relapsing between 6 to 12 months (66%; 95% CI, 51-86; Figure 1D).
A clear definition of primary refractory LBCL
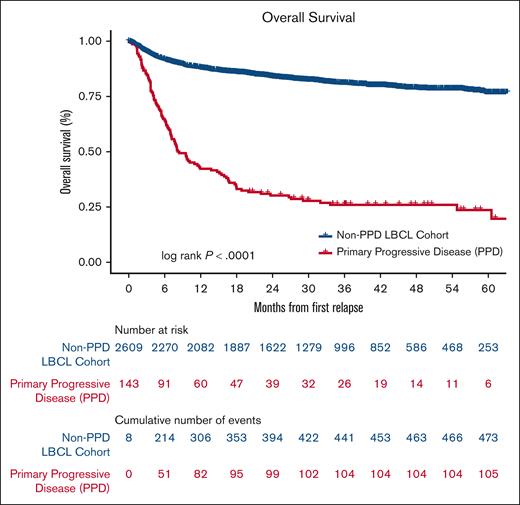
In both cohorts, patients with no response or PD by Lugano response criteria during, or by end of, first line treatment (PPD group) had inferior response rates to 2L therapies and the lowest OS. Clinically, these are the patients with truly refractory disease and will herein be referred to as “primary refractory LBCL.” When evaluating OS among all patients in the LEO cohort from the EOT or relapse, patients with primary refractory LBCL had a 2-year OS of 27% (95% CI, 21-34) and a median OS of 8.4 months compared with patients with nonprimary refractory LBCL who had a 2 year OS of 84% (95% CI, 82-85) and the median OS at 2 years was not reached (Figure 2).
OS for PPD compared with all other patients with newly diagnosed LBCL in the LEO cohort. OS from date of last treatment (nonrelapsed patients) or date of relapse/progression (relapsed patients).
OS for PPD compared with all other patients with newly diagnosed LBCL in the LEO cohort. OS from date of last treatment (nonrelapsed patients) or date of relapse/progression (relapsed patients).
Baseline features associated with primary refractory LBCL
Because patients with primary refractory LBCL have poor outcomes compared with most patients with newly diagnosed LBCLs, we evaluated baseline clinical and pathological features associated with primary refractory LBCL. Primary refractory LBCL was associated with several known adverse clinical and pathological features (Table 4). Most patients had an elevated LDH (76.9%) and advanced stage (80.3%). Patients with primary refractory LBCL had a shorter diagnosis to treatment interval, with a median diagnosis to treatment interval of 12.5 days compared with 16 in non–primary refractory patients. Although there was a shift toward a higher IPI in primary refractory patients, notably 49.5% of patients had a low or low intermediate IPI. The positive predictive value of a high IPI (4-5) was only 8%. Cell of origin was not significantly different between the 2 groups, with a similar distribution of germinal center B-cell and activated B-cell (ABC) subtypes in both groups. Among patients with complete data (n = 1688), DHL/THL was observed in 26% of primary refractory patients compared with 9% in non–primary refractory patients. Presence of Myc/Bcl2 double expressor was observed in 40% and was also significantly higher than that observed in non–primary refractory patients (24%).
Baseline clinical and pathological characteristics of patients with PPD compared with all patients without PPD in the LEO cohort
| Variable . | PPD (N =145) . | All non-PPD (N = 2610) . | Total (N = 2755) . | P value . |
|---|---|---|---|---|
| n (%) . | n (%) . | n (%) . | ||
| Age at diagnosis, y | .610∗ | |||
| Median (range) | 62.5 (19-88) | 63.0 (18-99) | 63.0 (18-99) | |
| Sex | .026† | |||
| F | 50 (34.7%) | 1153 (44.2%) | 1203 (43.7%) | |
| M | 94 (65.3%) | 1456 (55.8%) | 1550 (56.3%) | |
| Race | .892† | |||
| White | 127 (88.2%) | 2217 (85.0%) | 2344 (85.1%) | |
| Black or African American | 10 (6.9%) | 192 (7.4%) | 202 (7.3%) | |
| Unknown/not reported | 4 (2.8%) | 101 (3.9%) | 105 (3.8%) | |
| Asian | 3 (2.1%) | 74 (2.8%) | 77 (2.8%) | |
| >1 race | 0 (0.0%) | 17 (0.7%) | 17 (0.6%) | |
| American Indian/Alaska Native | 0 (0.0%) | 4 (0.2%) | 4 (0.1%) | |
| Native Hawaiian/Pacific Islander | 0 (0.0%) | 4 (0.2%) | 4 (0.1%) | |
| Ethnicity | .764† | |||
| Hispanic/Latinx | 15 (10.4%) | 325 (12.5%) | 340 (12.4%) | |
| Not Hispanic or Latinx | 126 (87.5%) | 2227 (85.4%) | 2353 (85.5%) | |
| Unknown/Not reported | 3 (2.1%) | 57 (2.2%) | 60 (2.2%) | |
| Diagnosis time interval (days) | <.001† | |||
| ≤ 14 | 68 (49.6%) | 755 (30.4%) | 823 (31.4%) | |
| > 14 | 69 (50.4%) | 1731 (69.6%) | 1800 (68.6%) | |
| Missing | 8 | 124 | 132 | |
| ECOG PS | .053† | |||
| <2 | 104 (77.0%) | 2040 (83.4%) | 2144 (83.1%) | |
| ≥2 | 31 (23.0%) | 405 (16.6%) | 436 (16.9%) | |
| Missing | 10 | 165 | 175 | |
| LDH | <.001† | |||
| ≤Normal | 27 (23.7%) | 1038 (45.2%) | 1065 (44.2%) | |
| >Normal | 87 (76.3%) | 1256 (54.8%) | 1343 (55.8%) | |
| Missing | 31 | 316 | 347 | |
| Extranodal sites | .009† | |||
| ≤1 | 92 (64.8%) | 1905 (74.7%) | 1997 (74.2%) | |
| >1 | 50 (35.2%) | 646 (25.3%) | 696 (25.8%) | |
| Missing | 3 | 59 | 62 | |
| Ann Arbor stage | <.001† | |||
| I-II | 26 (19.5%) | 950 (38.5%) | 976 (37.5%) | |
| III-IV | 107 (80.5%) | 1520 (61.5%) | 1627 (62.5%) | |
| Missing | 12 | 140 | 152 | |
| IPI group | .003† | |||
| 0-1, low | 37 (25.7%) | 956 (36.6%) | 993 (36.1%) | |
| 2, low intermediate | 35 (24.3%) | 735 (28.2%) | 770 (28.0%) | |
| 3, high intermediate | 42 (29.2%) | 574 (22.0%) | 616 (22.4%) | |
| 4-5, high | 30 (20.8%) | 344 (13.2%) | 374 (13.6%) | |
| Missing | 1 | 1 | 2 | |
| Cell of origin | .285† | |||
| Known GCB | 45 (58.4%) | 969 (57.3%) | 1014 (57.3%) | |
| Non-GCB | 32 (41.6%) | 721 (42.6%) | 753 (42.6%) | |
| Unknown/not done | 68 | 920 | 988 | |
| Double hit lymphoma | <.001† | |||
| Known DHL | 23 (27.4%) | 165 (10.3%) | 188 (11.1%) | |
| Non-DHL | 61 (72.6%) | 1439 (89.7%) | 1500 (88.9%) | |
| Not done/missing | 61 | 1006 | 1067 | |
| Double Expressor | <.001† | |||
| Known positive | 25 (43.1%) | 337 (25.0%) | 362 (25.8%) | |
| Negative | 33 (56.9%) | 1009 (75.0%) | 1042 (74.2%) | |
| Not done/missing | 87 | 1264 | 1351 |
| Variable . | PPD (N =145) . | All non-PPD (N = 2610) . | Total (N = 2755) . | P value . |
|---|---|---|---|---|
| n (%) . | n (%) . | n (%) . | ||
| Age at diagnosis, y | .610∗ | |||
| Median (range) | 62.5 (19-88) | 63.0 (18-99) | 63.0 (18-99) | |
| Sex | .026† | |||
| F | 50 (34.7%) | 1153 (44.2%) | 1203 (43.7%) | |
| M | 94 (65.3%) | 1456 (55.8%) | 1550 (56.3%) | |
| Race | .892† | |||
| White | 127 (88.2%) | 2217 (85.0%) | 2344 (85.1%) | |
| Black or African American | 10 (6.9%) | 192 (7.4%) | 202 (7.3%) | |
| Unknown/not reported | 4 (2.8%) | 101 (3.9%) | 105 (3.8%) | |
| Asian | 3 (2.1%) | 74 (2.8%) | 77 (2.8%) | |
| >1 race | 0 (0.0%) | 17 (0.7%) | 17 (0.6%) | |
| American Indian/Alaska Native | 0 (0.0%) | 4 (0.2%) | 4 (0.1%) | |
| Native Hawaiian/Pacific Islander | 0 (0.0%) | 4 (0.2%) | 4 (0.1%) | |
| Ethnicity | .764† | |||
| Hispanic/Latinx | 15 (10.4%) | 325 (12.5%) | 340 (12.4%) | |
| Not Hispanic or Latinx | 126 (87.5%) | 2227 (85.4%) | 2353 (85.5%) | |
| Unknown/Not reported | 3 (2.1%) | 57 (2.2%) | 60 (2.2%) | |
| Diagnosis time interval (days) | <.001† | |||
| ≤ 14 | 68 (49.6%) | 755 (30.4%) | 823 (31.4%) | |
| > 14 | 69 (50.4%) | 1731 (69.6%) | 1800 (68.6%) | |
| Missing | 8 | 124 | 132 | |
| ECOG PS | .053† | |||
| <2 | 104 (77.0%) | 2040 (83.4%) | 2144 (83.1%) | |
| ≥2 | 31 (23.0%) | 405 (16.6%) | 436 (16.9%) | |
| Missing | 10 | 165 | 175 | |
| LDH | <.001† | |||
| ≤Normal | 27 (23.7%) | 1038 (45.2%) | 1065 (44.2%) | |
| >Normal | 87 (76.3%) | 1256 (54.8%) | 1343 (55.8%) | |
| Missing | 31 | 316 | 347 | |
| Extranodal sites | .009† | |||
| ≤1 | 92 (64.8%) | 1905 (74.7%) | 1997 (74.2%) | |
| >1 | 50 (35.2%) | 646 (25.3%) | 696 (25.8%) | |
| Missing | 3 | 59 | 62 | |
| Ann Arbor stage | <.001† | |||
| I-II | 26 (19.5%) | 950 (38.5%) | 976 (37.5%) | |
| III-IV | 107 (80.5%) | 1520 (61.5%) | 1627 (62.5%) | |
| Missing | 12 | 140 | 152 | |
| IPI group | .003† | |||
| 0-1, low | 37 (25.7%) | 956 (36.6%) | 993 (36.1%) | |
| 2, low intermediate | 35 (24.3%) | 735 (28.2%) | 770 (28.0%) | |
| 3, high intermediate | 42 (29.2%) | 574 (22.0%) | 616 (22.4%) | |
| 4-5, high | 30 (20.8%) | 344 (13.2%) | 374 (13.6%) | |
| Missing | 1 | 1 | 2 | |
| Cell of origin | .285† | |||
| Known GCB | 45 (58.4%) | 969 (57.3%) | 1014 (57.3%) | |
| Non-GCB | 32 (41.6%) | 721 (42.6%) | 753 (42.6%) | |
| Unknown/not done | 68 | 920 | 988 | |
| Double hit lymphoma | <.001† | |||
| Known DHL | 23 (27.4%) | 165 (10.3%) | 188 (11.1%) | |
| Non-DHL | 61 (72.6%) | 1439 (89.7%) | 1500 (88.9%) | |
| Not done/missing | 61 | 1006 | 1067 | |
| Double Expressor | <.001† | |||
| Known positive | 25 (43.1%) | 337 (25.0%) | 362 (25.8%) | |
| Negative | 33 (56.9%) | 1009 (75.0%) | 1042 (74.2%) | |
| Not done/missing | 87 | 1264 | 1351 |
Double-hit defined as MYC rearrangement with BCL2 and/or BCL6; double expressor defined as expression of MYC and BCL2.
DHL, double hit lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; F, females; GCB, germinal center B cell; LDH, lactate dehydrogenase; M, male; non-GCB, activated B-cell subtype.
Kruskal-Wallis P value.
χ2P value.
Discussion
To the best of our knowledge, this is the largest multicenter, prospective cohort study of outcomes for patients with primary refractory LBCL. Our results showed that broadly defined primary refractory disease occurred at a similar incidence to historical rates (14% in the MER cohort and 11% in the LEO cohort, vs 10%-15% historically).11,14 Our study is consistent with other retrospective studies in demonstrating that patients with refractory disease or early relapse have poor outcomes.13,22,30,31 Importantly, however, the large cohort sizes in our study allowed us to demonstrate that broadly defined primary refractory LBCL has heterogenous survival outcomes. In both MER and LEO cohorts, patients with no response or progression during, or by the end of, 1L treatment (PPD group) have significantly inferior survival compared with other subgroups of primary refractory disease, which is likely driven by poor response to salvage therapies. The particularly poor outcomes in the PPD group, even in the more recent treatment era with more novel therapies available for relapsed/refractory disease, may drive the overall poor outcomes in broadly defined primary refractory LBCL, if examined as a whole in our or other studies. Based on our results, we advocate for the following definition of primary refractory LBCL: patients with stable or PD during or by the EOT (PPD group). This is the group of patients with clear chemoresistance and most in need of better treatment options. Patients with inadequate response or EOT PR (ie, PR as best response by EOT) and early relapse (ie, relapse within 12 months) have similar outcomes and may be better grouped as early relapse.
Evaluating future 1L trials by their ability to decrease patients in the PPD group should be an important clinical end point in future prospective studies. The lack of a consensus definition of primary refractory disease has also limited dedicated clinical trials for this important subgroup of patients and has a major impact on the interpretation of historical studies in relapsed/refractory LBCL. The phase 2 study of tafasitamab and lenalidomide initially excluded patients with primary refractory disease, defined as patients with relapse within 6 months of 1L treatment. The primary analysis observed a high ORR and PFS, which both declined after the inclusion of patients with primary refractory disease.32,33 Both the ZUMA-7 and TRANSFORM trials evaluating CAR-T in the 2L setting included primary refractory and early relapse (<12 months) cases but the definitions for primary refractory were slightly different (lack of CR to 1L therapy in ZUMA-7, and lack of CR to 1L therapy or relapse within 3 months in TRANSFORM).17,18 However, the subgroup analyses by refractory vs relapse status were helpful in interpreting the relative benefits of CAR-T vs standard-of-care therapy in these different subgroups.34
The recognition of PPD may also inform future clinical practice and trial design. Although better therapies are needed for all relapsed patients, selection of 2L therapy appears most crucial for patients with PPD, who had the highest rates of PD to salvage chemotherapy in our study, which led to lower rates of both ASCT and CAR-T than those of patients with EOT PR or early relapse. Our findings are similar to all 3 of the pivotal 2L CAR-T trials that observed high rates of crossover because of inability to achieve a response to salvage chemotherapy and only 33% to 45% of planned patients underwent ASCT.17,18,20 Novel targeted agents may have improved efficacy compared with salvage chemotherapy for these patients and should be explored as bridging strategies for patients with PPD because progression before CAR-T remains a key barrier in both trial and real-world populations.35-37 In contrast, for patients with early relapse, chemotherapy-containing therapies as salvage/bridging might still play a role in select cases, although incorporation of novel therapies is certainly desired as well.
Distinguishing PPD from other subgroups of broadly defined primary refractory disease has important implications in 1L treatment and trial designs. This is the group of patients with clear chemoresistance most in need of better treatment options given treatment failure despite optimal 1L IC. Interim PET and/or circulating tumor DNA assessment–guided treatment strategies may help reduce or salvage such primary progressive cases. One example is the phase 2 ZUMA-12 study, in which high-risk patients (defined as DH/THL or IPI 3-5) who had an incomplete response with positive interim PET after 2 cycles of R-CHOP were treated with axicabtagene ciloleucel. The efficacy was very encouraging, with an ORR of 89%, a CR rate of 78%, and a 12-month PFS of 75%,38 with a randomized 1L trial now enrolling (ClinicalTrials.gov identifier: NCT05605899).39
Ultimately, better 1L therapies are needed to reduce the proportion of patients with PPD. Identifying patients at the highest risk for PPD before treatment is still challenging but becomes evident by the time of the interim PET after 2 or 3 cycles. Our results suggest that using high-intermediate or high IPI alone may miss half of patients who would have PPD, although we acknowledge the small cohort size of refractory patients compared with the total population. Biological features (DHL/THL and double expressor) may be more important than clinical features in predicting primary refractoriness. A better understanding of the molecular alterations in these patients is needed to better understand chemoresistance and risk stratification.40
The strengths of this study include demonstration of our findings in 2 large prospective cohorts with detailed treatment information. We performed a comprehensive analysis of patients who received complete treatment, excluding those with incomplete treatment because of treatment intolerance or toxicity, Thus, our study captures patients whose disease is refractory despite optimal treatment and removes possible confounding factors unrelated to inherent disease biology that may be present in prior event-based analyses.22,31 Detailed 2L treatment and response details were available for >95% of the included patients, and patients in the LEO cohort reflect the diversity representative of the US population.25 The LEO cohort demonstrated improved outcomes compared with the MER cohort across subgroups, but OS for those with PPD still remained significantly inferior to other subgroups. The improvement in outcomes is likely multifactorial with improved supportive care and growth factor support potentially playing an important role, just as outcomes among all patients with newly diagnosed LBCL are improving over time compared with the first studies of R-CHOP.1-3,41-43 There has also been an increase in 2L treatment options in the last 5 to 10 years, including multiple novel targeted therapies and, most recently, CAR-T.17,18,32,44 Limitations include a high number of missing data for double expressor status and MYC rearrangements, mainly in the MER cohort and the inclusion of MYC/BCL6 rearrangements as part of HGBCL, which was removed with the fifth edition of the World Health Organization classification system in 2022. Outcomes are also likely affected by subsequent lines of therapies, and we may be missing the contribution of CAR-T in later lines to improved outcomes in the LEO cohort.
In summary, we evaluated outcomes of patients with primary refractory LBCL who received optimal standard 1L IC in 2 large prospective cohorts. Based on timing of refractoriness or relapse and the associated distinct outcomes, we propose a clear definition of primary refractory disease to 1L therapy: no response (stable disease) or PD during or by the EOT (PPD group). This definition will inform clinical practice, clinical trial design, and aid in interpretation of clinical research and trial results. Better understanding of disease biology is needed to help identify patients at high risk for PPD, to facilitate investigation of novel 1L and salvage therapies.
Acknowledgments
This work was supported by a grant from the National Institutes of Health, National Cancer Institute (to J.R.C; P50 CA97274, U01 CA195568).
Authorship
Contribution: A.M.B., R.M., G.S.N., and L.J.N. conceptualized and designed the study; all authors collected and assembled the data; A.M.B., R.M., M.J.M., G.S.N., and L.J.N. performed data analysis and interpretation; and all authors wrote the manuscript, approved the final version, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: A.M.B. reports consultancy or advisory role with AbbVie; and reports honoraria from Primum. Y.W. reports employment or leadership position with Merck (immediate family member); reports consultant or advisory role with Loxo, Incyte, Innocare, TG Therapeutics, Kite (a Gilead company), Lilly, Janssen, and BeiGene; reports stock ownership in Merck (immediate family member); received honoraria from Kite (a Gilead company); received research funding from InnoCare, Incyte, Novartis, Genentech, Loxo, MorphoSys, and Genmab. M.J.M. reports employment or leadership position with Exact Sciences (immediate family member); reports consultant or advisory role with Genmab and Adpative Biotechnologies; reports stock ownership in Exact Sciences (immediate family member); and received research funding from MorphoSys, Bristol Myers Squibb, Roche/Genentech, and Genmab. B.S.K. declares consultant or advisory role with Celgene, AbbVie, Pharmacyclics, Acerta Pharma, ADC Therapeutics, Genentech, Roche, AstraZeneca, BeiGene, Bayer, MEI Pharma, Kite/Gilead, MorphoSys, Janssen, Celgene, Incyte, and Genmab; and received research funding from Genentech, Acerta Pharma, ADC Therapeutics, and Celgene. P.M. reports consultant or advisory role with Janssen, BeiGene, Karyopharm Therapeutics, Kite/Gilead, Verastem, ADC Therapeutics, Bristol Myers Squibb/Celgene, Epizyme, Merck, MorphoSys, and Takeda; and received research funding from Karyopharm Therapeutics. J.B.C. reports consultant or advisory role with AbbVie, Janssen, Loxo, Kite/Gilead, AstraZeneca, Aptitude Medical, Adicet Bio, and Adaptive Biotechnologies; received research funding from Celgene, Janssen, Novartis, Takeda, AI Therapeutics, Genentech, American Society of Hematology, Lymphoma Research Foundation, Loxo, BioInvent, and AstraZeneca. C.C. reports consultant or advisory role with AbbVie; and received research funding from Gilead, SecuraBio, Genmab, and Genentech. I.S.L. reports consultant or advisory role with Janssen, Seagen, and Verastem; received honoraria from Janssen; and received research funding from the National Cancer Institute. U.F. reports consultant or advisory role with MorphoSys and Caribou; and received honoraria from Kite Pharma and Caribou. T.M.H. reports consultant or advisory role with Celgene, Kite/Gilead; received research funding from Genentech; received other remuneration/uncompensated from Tess Therapeutics, Loxo/Lilly, MorphoSys, Incyte, and BeiGene. C.R.F. and J.R.C. report consultant or advisory roles with Bristol Myers Squibb and Protagonist Therapeutics; received research funding from NanoString Technologies, Celgene, Genentech, and Genmab; and received other remuneration/uncompensated from Regeneron. L.J.N. reports a consultant or advisory role with Interius Biotherapeutics and SIRPant Immunotherapeutics; received honoraria from ADC Therapeutics, Bristol Myers Squibb, Caribou Biosciences, Epizyme, Genentech/Roche, Genmab, Gilead Sciences, Janssen Oncology, MorphoSys, Novartis, Takeda, and TG Therapeutics; and received research funding from Allogene Therapeutics, Bristol Myers Squibb/Celgene, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead Sciences, IgM Biosciences, Janssen Biotech, Novartis, and Takeda. G.S.N. reports a consultant or advisory role for Celgene, MorphoSys, Genentech, Selvita, Debiopharm Group, Kite/Gilead, TG Therapeutics, Kymera, Karyopharm Therapeutics, Ryvu Therapeutics, and Bantham; and received research funding from Celgene, NanoString Technologies, and MorphoSys.
The remaining authors declare no competing financial interests.
Correspondence: Allison Bock, Division of Hematology and Hematologic Malignancies, Huntsman Cancer Institute/University of Utah, 2000 Circle of Hope Dr, Suite 4126, Salt Lake City, UT 84112; email: allison.bock@hci.utah.edu; and Grzegorz S. Nowakowski, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; email: nowakowski.grzegorz@mayo.edu.
References
Author notes
The data sets generated during and/or analyzed during this study are available on request from the corresponding author, Allison Bock (allison.bock@hci.utah.edu); and Grzegorz S. Nowakowski (nowakowski.grzegorz@mayo.edu). The data are not publicly available because of privacy or ethical restrictions.
The full-text version of this article contains a data supplement.