Key Points
Upon engaging DNT-susceptible AML, DNTs secrete TNFα, which sensitizes AML to DNT killing, including DNT-resistant AML.
TNFα noncanonically signals through JAK1 to upregulate ICAM-1 on AML for increased DNT antileukemic function.
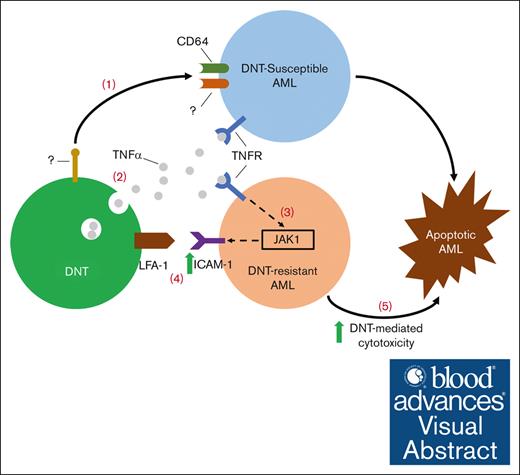
Visual Abstract
Allogeneic double-negative T cells (DNTs) are a rare T-cell subset that effectively target acute myeloid leukemia (AML) without inducing graft-versus-host disease in an allogeneic setting. A phase 1 clinical trial demonstrated the feasibility, safety, and potential efficacy of allogeneic DNT therapy among patients with relapsed AML. However, the molecular mechanisms of DNT-mediated cytotoxicity against AML remain elusive. Thus, we used a flow cytometry–based high throughput screening to compare the surface molecule expression profile on DNTs during their interaction with DNT-susceptible or -resistant AML cells and identified a tumor necrosis factor α (TNFα)-dependent cytotoxic pathway in DNT-AML interaction. TNFα secreted by DNTs, upon encountering susceptible AML targets, sensitized AML cells to DNT-mediated killing, including those otherwise resistant to DNTs. Mechanistically, TNFα upregulated ICAM-1 on AML cells through a noncanonical JAK1-dependent pathway. DNTs then engaged with AML cells more effectively through an ICAM-1 receptor, lymphocyte function–associated antigen 1, leading to enhanced killing. These results reveal a TNFα–JAK1–ICAM-1 axis in DNT-mediated cytotoxicity against AML to improve therapeutic efficacy.
Introduction
Acute myeloid leukemia (AML) is the most common form of adult acute leukemia with poor long-term survival due to high disease relapse rates after induction chemotherapy.1-4 Currently, allogeneic hematopoietic stem cell transplantation provides a potential curative treatment option for patients with high-risk AML by eliciting a graft-versus-leukemia response, in which donor-derived immune cells target residual AML cells.3,5 However, treatment-associated toxicities such as graft-versus-host disease, risks of infection, and significant relapse rates pose a challenge.3 Nevertheless, the potency of the graft-versus-leukemia effect has driven the development of adoptive cellular therapies for AML. With the remarkable success of chimeric antigen receptor (CAR) T-cell therapy in B-cell malignancies,6 a number of studies developed and assessed CAR T-cell therapy for patients with AML.7-9 Although early-phase clinical trials showed some promising results, on-target off-tumor toxicities, logistical obstacles, and AML heterogeneity limit a greater success of CAR T-cell therapy against AML.10-13
CD3+CD4–CD8– double-negative T cells (DNTs) are mature peripheral T cells that account for ∼3% to 5% of T lymphocytes. Ex vivo expanded, nongenetically modified DNTs from healthy donors can target primary and chemotherapy-resistant AML cells without inducing graft-versus-host disease in xenograft models.14,15 Recently, a phase 1 clinical trial (ChiCTR1900022795) demonstrated the feasibility, safety, and potential efficacy of allogeneic DNT therapy to treat patients with AML with relapsed disease after allogeneic hematopoietic stem cell transplantation.16 However, some patients are unresponsive to DNT therapy, and some primary AML samples show high levels of resistance to DNTs in preclinical models.14,16 Further dissecting DNT cytotoxic mechanisms will help identify why DNTs fail to target some AML cells and provide strategies to overcome the resistance to DNT-mediated killing.
In this study, we used a flow cytometry–based high throughput screening (HTS) assay to compare cell surface molecule expression profiles on DNTs after encountering DNT-susceptible or -resistant AML cells. By validating the functions of these molecules in vitro and in vivo, we identified the significance of the tumor necrosis factor α (TNFα)/TNFα-receptor (TNFR) pathway in DNT-AML interactions. Furthermore, our study highlighted the unappreciated contributions of JAK1 in TNFα signaling to induce ICAM-1 expression on AML cells and the subsequent role of lymphocyte function–associated antigen 1 (LFA-1) on DNTs to recognize and target TNFα-sensitized AML cells. These data demonstrate the utility of the flow cytometry–based HTS method to discover important immune–cancer cell pathways and the role of the TNFα–JAK1–ICAM-1 axis in DNT-mediated cytotoxicity against AML.
Methods
Ex vivo DNT expansion
Flow cytometry–based in vitro cytotoxic assays
DNTs were cocultured with primary AML blasts from patients (supplemental Table 1) for 3 hours or AML cell lines for 2 to 24 hours at optimal effector-to-target (E:T) ratios (0.125:1 to 4:1). AML viability was determined by annexin V (2- to 24-hour assays) or DAPI (4′,6-diamidino-2-phenylindole; multiday assays) in CD3–CD33+ or CD3–CD34+ gated populations for AML cell lines and CD45lowCD3–CD33+ or CD34+ populations for primary AML samples. Percentage specific killing was calculated by .
For TNFα pretreatment assays, DNTs or AML cells were treated with recombinant human TNFα (rTNFα; 100 ηg/mL; R&D Systems) for 24 hours (or 16 hours with primary AML), then washed with phosphate-buffered saline prior to conducting the cytotoxicity assay.
For blocking assays, neutralizing antibodies (Biolegend) were added: anti-CD120b (3G7A02), anti-TNFα (MAb1), anti-CD18 (TS1/18), anti-CD54 (HCD54), anti-CD62ε (HAE-1f), or corresponding isotype controls at 5 to 10 μg/mL unless specified. Percentage inhibition of killing was calculated by .
For assays involving small molecule inhibitors, AML cells were incubated with necrostatin 2 racemate (MedChemExpress) or itacitinib (Selleckchem) for 24 hours at specified concentrations in the figure legends, followed by rTNFα treatment prior to cytotoxicity assays.
Flow cytometry–based HTS assay
DNTs from 3 different donors were cocultured with or without OCI-AML3 or KG-1a for 2 hours and stained with anti-CD3, anti-CD33, and anti-CD34 antibodies. Then, the Princess Margaret Genomics Centre conducted highly multiplexed cell surface assessment using 385 commercially available antibodies and flow cytometry, as described by Gedye et al.17 Cell surface protein expressions from 3 groups were analyzed: DNT-OCI-AML3 coculture, DNT-KG-1a coculture, and DNT alone.
Multiday mixed coculture assay
DNTs, DNT-susceptible AML cells (MV4-11 or OCI-AML3WT), and DNT-resistant AML cells (KG-1a or OCI-AML3CD64KO) were cocultured in a 24-well plate at a 2:1:1 ratio (DNT: DNT-susceptible AML: DNT-resistant AML) for 2 days. Anti-TNFα neutralizing antibodies were added on days 0 and 1. Aliquots were taken each day to track cell viability by DAPI and ICAM-1 expression. Cells were distinguished by flow cytometry through surface markers and green fluorescent protein (GFP) expression: KG-1a (CD3−CD34+CD33–), MV4-11 (CD3–CD34–CD33+), OCI-AML3CD64KO (CD3–CD33+GFP+), and OCI-AML3WT (CD3–CD33+GFP–).
Transwell assay
DNTs and AML cells (KG-1a and MV4-11) were cocultured in the Transwell-96 Permeable Support with 0.4 μm PET membrane (Corning). The top compartment contained MV4-11 cells cocultured with or without DNTs (E:T ratio of 1:1). The bottom compartment housed KG-1a cells with or without DNTs (E:T ratio of 2:1). After 2 days, KG-1a viability from the bottom compartment were determined by DAPI and flow cytometry.
Xenograft model
NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice (Jackson Laboratories) were used for xenograft experiments. Female mice aged 8 to 12 weeks were sublethally irradiated (225 cGy) 1 day before an IV injection of 2 × 106 OCI-AML2 cells. OCI-AML2 cells were treated with or without rTNFα (100 ηg/mL) for 24 hours before the injection. A total of 2 × 107 DNTs were IV injected on days 1 and 4 after AML cell injection. A total of 104 IU rIL-2 (recombinant human IL-2, Proleukin) was given IV at the time of DNT infusion and intraperitoneally on day 11. Mice were euthanized on day 14. Cells from mice femurs were harvested to assess the bone marrow engraftments of AML cells by flow cytometry.
Statistical analysis
All graphs and statistical analyses were generated using GraphPad Prism 5. Student t test, analysis of variance, and linear regression tests were used. The following indicate statistical significance between groups: ns, nonsignificant; ∗P < .05; ∗∗P < .01; ∗∗∗P <.001. Error bars represent ± standard error of the mean or ± standard deviation.
Human blood collection and use was in accordance with University Health Network Research Ethics Board (05-0221-T) and National Heart, Lung, and Blood Insitute (NHLBI)-approved protocols. Animal use for experiments was approved by University Health Network Animal Care Committee (Animal Utilization Protocol: 741) and performed according to the Canadian Council on Animal Care Guidelines.
Results
Flow cytometry–based HTS assay identifies the involvement of CD120b/TNFR pathway in DNT-mediated cytotoxicity
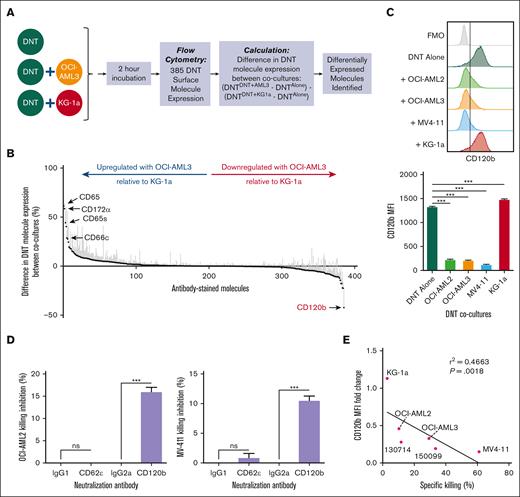
Although the anticancer activity of ex vivo expanded DNTs has been demonstrated,14,15,18,19 the underlying mechanisms by which DNTs mediate their activity is less well characterized. To identify cell surface proteins potentially involved in the DNT-mediated killing of AML cells, we used a flow cytometry–based HTS assay17 to compare the changes in the expression of 385 cell surface proteins on DNTs during their interactions with DNT-susceptible (OCI-AML3) or -resistant (KG-1a) AML cell lines relative to DNT alone control (Figure 1A). KG-1a cells previously exhibited high intrinsic resistance to DNTs in in vitro cytotoxicity assays and in xenograft models compared with other AML cell lines, such as OCI-AML3, OCI-AML2, and MV4-11.20 Using DNTs derived from 3 different donors, we identified molecules that were preferentially downregulated or upregulated on DNTs, when cocultured with OCI-AML3 cells relative to KG-1a cells, with a minimum cutoff of >25% differential expression and relevant immune cytotoxic function (Figure 1B; supplemental Table 2). The screen identified 4 molecules of interest: CD120b/TNFR2, CD65, CD66c, and CD172α.
Flow cytometry–based HTS assay identifies the involvement of CD120b/TNFR pathway in DNT-mediated cytotoxicity. (A) Schematic of the flow cytometry–based HTS assay using DNT-resistant (KG-1a) and -susceptible (OCI-AML3) AML cell lines. (B) DNTs alone or cocultured with OCI-AML3 or KG-1a in 96-well plates were stained with 385 fluorophore-conjugated antibodies. Molecule expression on DNTs (CD3+CD33–CD34–) cocultured with OCI-AML3 (CD3–CD33+CD34–) or KG-1a (CD3–CD33–CD34+) relative to DNT alone was determined. From left to right, the graph shows molecules that are upregulated or downregulated on DNTs during the interaction with OCI-AML3, relative to DNT-KG-1a interactions. The experiment was performed with 3 biological replicates, and the data were pooled together. (C) DNTs stained for CD120b after a 2-hour coculture with DNT-resistant (KG-1a) or -susceptible (OCI-AML2, OCI-AML3, and MV4-11) AML cell lines. A representative histogram (left) and corresponding median fluorescence intensity (MFI) values (right) of CD120b expression are shown. Experiments were done in triplicates. The data shown are representative of 3 independent experiments. (D) DNTs were cocultured with DNT-susceptible AML cell lines, OCI-AML2 (top) or MV4-11 (bottom), for 24 hours in the presence of anti-CD62ε or anti-CD120b neutralizing antibody or corresponding isotype controls. Experiments were done in triplicates. The data shown are representative of 2 independent experiments. (E) Linear regression analysis performed between the MFI fold change in CD120b expression on DNTs cocultured with AML cells relative to DNT alone and the percentage specific killing of AML cell lines and primary AML samples by DNTs. AML cells and DNTs were coincubated for 2 hours. Each symbol represents an AML cell line or primary AML sample. Numbers represent the ID of patients with AML. Experiments were done in triplicates, and the data shown are representative of 2 independent experiments. Student t test, 1-way analysis of variance (ANOVA), and linear regression analysis were used. ∗∗∗P < .001. ns, nonsignificant.
Flow cytometry–based HTS assay identifies the involvement of CD120b/TNFR pathway in DNT-mediated cytotoxicity. (A) Schematic of the flow cytometry–based HTS assay using DNT-resistant (KG-1a) and -susceptible (OCI-AML3) AML cell lines. (B) DNTs alone or cocultured with OCI-AML3 or KG-1a in 96-well plates were stained with 385 fluorophore-conjugated antibodies. Molecule expression on DNTs (CD3+CD33–CD34–) cocultured with OCI-AML3 (CD3–CD33+CD34–) or KG-1a (CD3–CD33–CD34+) relative to DNT alone was determined. From left to right, the graph shows molecules that are upregulated or downregulated on DNTs during the interaction with OCI-AML3, relative to DNT-KG-1a interactions. The experiment was performed with 3 biological replicates, and the data were pooled together. (C) DNTs stained for CD120b after a 2-hour coculture with DNT-resistant (KG-1a) or -susceptible (OCI-AML2, OCI-AML3, and MV4-11) AML cell lines. A representative histogram (left) and corresponding median fluorescence intensity (MFI) values (right) of CD120b expression are shown. Experiments were done in triplicates. The data shown are representative of 3 independent experiments. (D) DNTs were cocultured with DNT-susceptible AML cell lines, OCI-AML2 (top) or MV4-11 (bottom), for 24 hours in the presence of anti-CD62ε or anti-CD120b neutralizing antibody or corresponding isotype controls. Experiments were done in triplicates. The data shown are representative of 2 independent experiments. (E) Linear regression analysis performed between the MFI fold change in CD120b expression on DNTs cocultured with AML cells relative to DNT alone and the percentage specific killing of AML cell lines and primary AML samples by DNTs. AML cells and DNTs were coincubated for 2 hours. Each symbol represents an AML cell line or primary AML sample. Numbers represent the ID of patients with AML. Experiments were done in triplicates, and the data shown are representative of 2 independent experiments. Student t test, 1-way analysis of variance (ANOVA), and linear regression analysis were used. ∗∗∗P < .001. ns, nonsignificant.
Next, we validated the findings using 2 additional DNT-susceptible AML cell lines, OCI-AML2 and MV4-11.20 Among the molecules examined, CD120b and CD65 were consistently downregulated or upregulated after coculture with all 3 DNT-susceptible AML cell lines, respectively (Figure 1C; supplemental Figure 2). However, CD66c was only upregulated in the DNT-OCI-AML3 coculture, and CD172α levels remained elevated regardless of the cocultured AML cell line (supplemental Figure 2). To assess the functional relevance of CD120b and CD65, DNTs were coincubated with AML cells in the presence of neutralizing antibodies against CD120b or CD62ε, a receptor for CD65,21 or their respective isotype antibodies as controls. Notably, anti-CD120b antibodies significantly reduced DNT-mediated cytotoxicity against AML cells, whereas blocking CD62ε had no significant effect (Figure 1D). Furthermore, the magnitude of CD120b downregulation on DNTs correlated with the susceptibility of AML cells to DNT killing (Figure 1E). Collectively, these data demonstrate the involvement of the CD120b/TNFR pathway in DNT-mediated cytotoxicity toward AML cells.
TNFα sensitizes AML cells to the antileukemic activity of DNTs
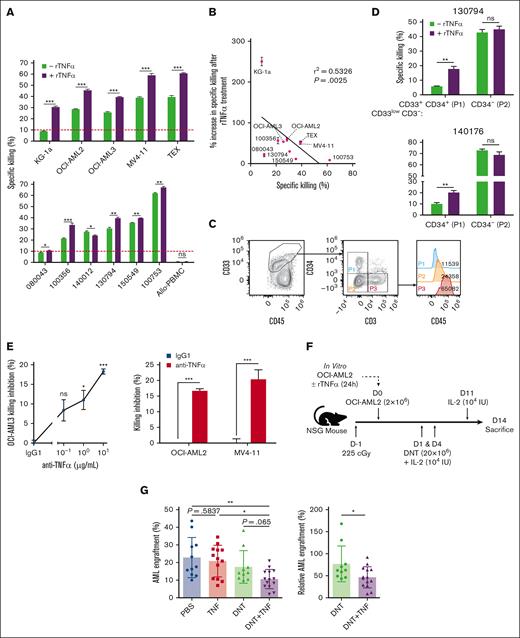
TNFα, the ligand of CD120b, is a well-known inflammatory cytokine involved in various diseases, but whether it has an antitumorigenic or protumorigenic role in AML remains unclear.22-24 Thus, we examined the effects of TNFα in the context of AML and DNT interactions. Unlike ∼28% of cancers directly susceptible to TNFα in vitro, such as breast and colorectal cancers,25-27 a minimal increase in AML apoptosis was observed after a 24-hour exposure to rTNFα in vitro at the highest titrated dose (supplemental Figure 3A). Although direct cytotoxicity was not observed, 10 of 11 rTNFα-pretreated AML cell lines (Figure 2A, top) and primary AML blasts (Figure 2A, bottom) with varying degrees of intrinsic DNT resistance became significantly sensitized to DNT-mediated cytotoxicity (Figure 2A). Similar effects were seen in a highly DNT-resistant B-cell acute lymphoblastic leukemia cell line, NALM-6 (supplemental Figure 3B), in which rTNFα induced minimal direct cytotoxicity (supplemental Figure 3C, left) but sensitized NALM-6 to greater DNT killing (supplemental Figure 3C, right). In contrast to cancer targets, DNTs did not kill healthy allogeneic PBMCs with or without rTNFα pretreatment (Figure 2A). We also observed that AML cells with higher resistance to DNT killing showed a greater relative degree of sensitization by rTNFα (Figure 2B). In line with this trend, DNT-resistant CD34+ populations in primary AML samples (Figure 2C) became susceptible to DNTs after rTNFα treatment, whereas their CD34– counterparts, which were inherently more susceptible to DNTs, were not further sensitized by rTNFα treatment (Figure 2D). To further validate the functional relevance of TNFα in DNT-AML interactions, blocking studies were performed. The addition of anti-TNFα neutralizing antibodies to the DNT-AML cocultures significantly reduced DNT-mediated cytotoxicity against AML cell lines (Figure 2E).
TNFα sensitizes AML cells to the antileukemic activity of DNTs. (A-B) AML cell lines (top), primary AML blasts, and allogeneic PBMCs (bottom) were pretreated with or without rTNFα, washed with phosphate-buffered saline (PBS), then cocultured with DNTs for 2 to 4 hours. Percentage specific killing of AML cells are shown with a red line indicating the 10% specific killing as resistance threshold (A). Linear regression analysis performed between percentage specific killing and the relative percentage increase in specific killing of rTNFα-sensitized AML (B). Each symbol represents an AML cell line or primary AML sample. Experiments were done in triplicates and were performed with at least 2 DNT donors. Numbers represent the ID of patients with AML. (C-D) Primary AML samples (130794 and 140176) were treated with or without rTNFα, washed with PBS, then cocultured with DNTs. Gating strategy (C) and percentage specific killing of rTNFα-treated and nontreated groups of the primary AML samples gated on specified leukemic blast populations in triplicates are shown (D). (E) DNTs were cocultured with AML cell lines for 24 hours, in the presence of increasing (for OCI-AML3) or fixed (for OCI-AML2 and MV4-11, 10 μg/mL) concentration of anti-TNFα blocking antibody or isotype control antibody. The graphs shown are representative of 2 independent experiments. (F-G) Sublethally irradiated (225 cGy) NSG mice were IV injected with OCI-AML2 untreated or treated with rTNFα, followed by 2 infusions of DNTs or PBS. Schematic of the in vivo xenograft mouse model with treatment schedule (F). Bar graphs represent the mean AML bone marrow engraftment levels (left) and engraftment levels normalized to the PBS group (right) (G). Each symbol represents an individual mouse. Data represent the mean ± standard deviation (SD) and pooled from 3 independent experiments (n = 3-5 per group). Student t test, 1-way/2-way ANOVA, and linear regression analysis were used. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ns, nonsignificant.
TNFα sensitizes AML cells to the antileukemic activity of DNTs. (A-B) AML cell lines (top), primary AML blasts, and allogeneic PBMCs (bottom) were pretreated with or without rTNFα, washed with phosphate-buffered saline (PBS), then cocultured with DNTs for 2 to 4 hours. Percentage specific killing of AML cells are shown with a red line indicating the 10% specific killing as resistance threshold (A). Linear regression analysis performed between percentage specific killing and the relative percentage increase in specific killing of rTNFα-sensitized AML (B). Each symbol represents an AML cell line or primary AML sample. Experiments were done in triplicates and were performed with at least 2 DNT donors. Numbers represent the ID of patients with AML. (C-D) Primary AML samples (130794 and 140176) were treated with or without rTNFα, washed with PBS, then cocultured with DNTs. Gating strategy (C) and percentage specific killing of rTNFα-treated and nontreated groups of the primary AML samples gated on specified leukemic blast populations in triplicates are shown (D). (E) DNTs were cocultured with AML cell lines for 24 hours, in the presence of increasing (for OCI-AML3) or fixed (for OCI-AML2 and MV4-11, 10 μg/mL) concentration of anti-TNFα blocking antibody or isotype control antibody. The graphs shown are representative of 2 independent experiments. (F-G) Sublethally irradiated (225 cGy) NSG mice were IV injected with OCI-AML2 untreated or treated with rTNFα, followed by 2 infusions of DNTs or PBS. Schematic of the in vivo xenograft mouse model with treatment schedule (F). Bar graphs represent the mean AML bone marrow engraftment levels (left) and engraftment levels normalized to the PBS group (right) (G). Each symbol represents an individual mouse. Data represent the mean ± standard deviation (SD) and pooled from 3 independent experiments (n = 3-5 per group). Student t test, 1-way/2-way ANOVA, and linear regression analysis were used. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ns, nonsignificant.
To examine TNFα sensitization effects in vivo, NSG mice were engrafted with OCI-AML2 cells, pretreated with or without rTNFα, followed by DNT infusions (Figure 2F; supplemental Figure 3D). Consistent with in vitro results, rTNFα did not significantly alter the AML bone marrow engraftment because rTNFα-treated AML had similar leukemic engraftment levels as phosphate-buffered saline–treated ones (Figure 2G, left). However, we observed significantly reduced engraftment in mice that were infused with rTNFα-treated AML followed by DNT treatment relative to mice that received untreated AML cells and DNT infusions (Figure 2G, right). We also investigated whether rTNFα potentiates DNTs to mediate superior antileukemic activity. Although DNTs pretreated with increasing concentrations of rTNFα for 24 hours showed a dose-dependent downregulation of CD120b (supplemental Figure 3E), the magnitude of DNT-mediated cytotoxicity against AML cells remained unchanged (supplemental Figure 3F). Overall, these findings indicate that TNFα does not directly kill AML or potentiate DNT cytotoxic potency. Instead, TNFα sensitized AML cells to enhanced DNT killing for AML targets that have different levels of resistance to DNT-mediated cytotoxicity.
CD64 expression on susceptible AML cells trigger DNTs to produce TNFα
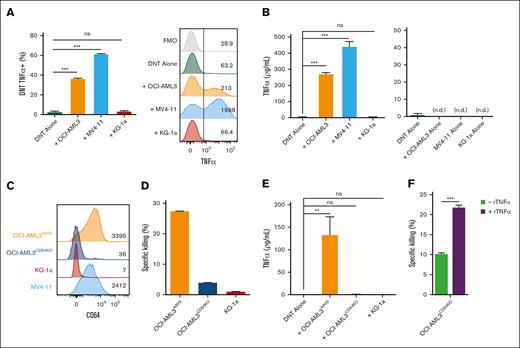
With the observation that rTNFα can sensitize AML targets, we examined whether DNTs produced TNFα when engaging AML cells. We confirmed that DNTs express and secrete TNFα when coincubated with DNT-susceptible AML cell lines but not with the DNT-resistant AML cell line, KG-1a (Figure 3A; Figure 3B, left). TNFα was not detected in the supernatant of AML cell alone or DNT alone cultures (Figure 3B, right).
CD64 expression on susceptible AML cells trigger DNTs to produce TNFα. (A-B) DNTs and AML cells were cultured alone or together for 2 hours. The intracellular expression of TNFα was measured on DNTs (CD3+CD33–) by flow cytometry. The bar graph shows the percentage expression (left), and the flow plot shows a representative histogram with MFI values (right) (A). The level of TNFα in the supernatants of cocultures (left) and AML or DNT cell alone groups (right) were determined by enzyme-linked immunosorbent assay (ELISA) (B). The experiments were performed in triplicates. The data shown are representative of 2 independent experiments. (C) CD64 expression by OCI-AML3AAVS control (orange), OCI-AML3CD64KO (dark blue), KG-1a (red), and MV4-11 (blue) cells. Representative histogram shows expression measured by flow cytometry with MFI values. (D-E) OCI-AML3AAVS control, OCI-AML3CD64KO, and KG-1a cells were cocultured with DNTs for 2 hours. Specific killing of AML cells was measured using flow cytometry (D). The level of TNFα from the coculture supernatants was determined by ELISA (E). The experiment was performed in triplicates, and the data are representative of 2 independent experiments. (F) OCI-AML3CD64KO cells were untreated or pretreated with rTNFα (100 ηg/mL), washed with PBS, then cocultured with DNTs for 24 hours. Specific killing of AML cells by DNT was determined by flow cytometry. Data shown are representative of 2 independent experiments done in triplicates. Student t test and 1-way/2-way ANOVA were used. ∗∗P < .01; ∗∗∗P < .001. n.d., not detected; ns, nonsignificant.
CD64 expression on susceptible AML cells trigger DNTs to produce TNFα. (A-B) DNTs and AML cells were cultured alone or together for 2 hours. The intracellular expression of TNFα was measured on DNTs (CD3+CD33–) by flow cytometry. The bar graph shows the percentage expression (left), and the flow plot shows a representative histogram with MFI values (right) (A). The level of TNFα in the supernatants of cocultures (left) and AML or DNT cell alone groups (right) were determined by enzyme-linked immunosorbent assay (ELISA) (B). The experiments were performed in triplicates. The data shown are representative of 2 independent experiments. (C) CD64 expression by OCI-AML3AAVS control (orange), OCI-AML3CD64KO (dark blue), KG-1a (red), and MV4-11 (blue) cells. Representative histogram shows expression measured by flow cytometry with MFI values. (D-E) OCI-AML3AAVS control, OCI-AML3CD64KO, and KG-1a cells were cocultured with DNTs for 2 hours. Specific killing of AML cells was measured using flow cytometry (D). The level of TNFα from the coculture supernatants was determined by ELISA (E). The experiment was performed in triplicates, and the data are representative of 2 independent experiments. (F) OCI-AML3CD64KO cells were untreated or pretreated with rTNFα (100 ηg/mL), washed with PBS, then cocultured with DNTs for 24 hours. Specific killing of AML cells by DNT was determined by flow cytometry. Data shown are representative of 2 independent experiments done in triplicates. Student t test and 1-way/2-way ANOVA were used. ∗∗P < .01; ∗∗∗P < .001. n.d., not detected; ns, nonsignificant.
We found that susceptible AML cells typically express high levels of CD64 (Figure 3C), and CD64 expression on primary AML cells correlates with their susceptibility to DNT antileukemic activity.28 To determine whether the expression of CD64 was critical for inducing TNFα secretion in DNTs, we first knocked out CD64 on DNT-susceptible OCI-AML3 (OCI-AML3CD64KO) cells (Figure 3C) and assessed their susceptibility to DNT-mediated cytotoxicity. We found that OCI-AML3CD64KO cells were rendered resistant to DNT killing to a similar degree as KG-1a (Figure 3D). When examining a possible CD64-TNFα relationship, we detected a significant drop in TNFα in the supernatant of DNTs cocultured with OCI-AML3CD64KO cells, which was comparable with that of KG-1a, in contrast to OCI-AML3AAVS control (Figure 3E). Furthermore, despite the increased DNT resistance due to CD64 knockout (KO), pretreatment of OCI-AML3CD64KO cells with rTNFα significantly sensitized them to DNT-mediated killing (Figure 3F). These data suggest that DNTs may require CD64 expression on AML cells to release TNFα and initiate cytotoxic activities against susceptible AML cells, but this mechanism can be bypassed by providing exogenous TNFα.
TNFα produced by DNTs upon encountering sensitive AML renders DNT-resistant AML susceptible to DNT killing
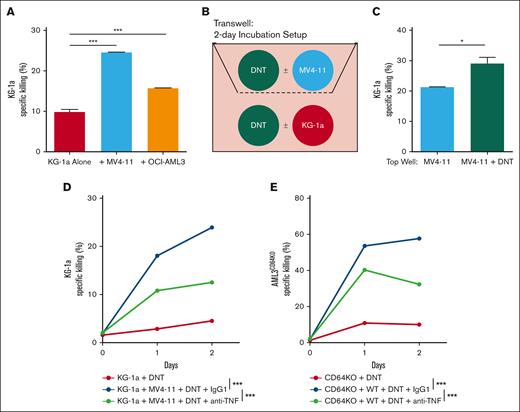
AML is a highly heterogenous disease comprised of subpopulations with varying degrees of susceptibility, contributing to resistance to different forms of therapies.29-31 Now, given that DNTs are potent producers of TNFα, which is crucial for DNT-mediated cytotoxicity against AML, we tested whether DNTs can sensitize nearby DNT-resistant AML cells to DNT killing while interacting with susceptible AML targets. As a surrogate to heterogenous AML cells composed of susceptible and resistant populations, we mixed DNT-susceptible AML cells (MV4-11 or OCI-AML3) and KG-1a cells, respectively, and assessed the cytotoxicity of DNTs against KG-1a. DNTs almost eliminated susceptible AML cells from the mixed coculture regardless of the presence of KG-1a (supplemental Figure 4A), suggesting that KG-1a does not actively suppress DNT antileukemic function. In contrast, DNTs effectively killed KG-1a only in the presence of MV4-11 or OCI-AML3 (Figure 4A). To avoid confounding effects between different AML cells in mixed coculture assays, a transwell assay was conducted in which MV4-11 cells were cultured in the top compartment with or without DNTs, whereas KG-1a cells with or without DNTs were seeded in the bottom wells (Figure 4B). Although having DNTs with MV4-11 in the top compartment had minimal effect on KG-1a cell growth and viability (supplemental Figure 4B), it sensitized KG-1a to DNT-mediated cytotoxicity (Figure 4C).
TNFα produced by DNTs, upon encountering sensitive AML, renders DNT-resistant AML susceptible to DNT killing. (A) DNTs were cocultured with DNT-resistant KG-1a in the presence or absence of DNT-susceptible AML (MV4-11 or OCI-AML3) at a 2:1:1 (DNT: KG-1a: DNT-susceptible AML) ratio for 24 hours. Specific killing of KG-1a was measured by flow cytometry. The experiments were done in triplicates, and the data shown are representative of 2 independent experiments. (B-C) MV4-11 were cultured with or without DNTs in the top compartment of a transwell. KG-1a alone or cocultured with DNTs were placed in the bottom compartment of the transwell. Cells were then incubated for 2 days as shown in the schematic (B). Specific killing of KG-1a cells in the bottom compartment was measured and compared between MV4-11 and MV4-11 + DNT conditions (C). The experiments were done in triplicates. The data shown are representative of 2 independent experiments. (D-E) DNT-resistant AML, KG-1a (D) or OCI-AML3CD64KO (E), were incubated alone or with DNT-susceptible AML, MV4-11 (D) or OCI-AML3WT (E), and DNTs in the presence of anti-TNFα blocking antibody (10 μg/mL) or isotype control for 2 days. Antibodies were added on day 0 and day 1 of the cocultures. Specific killing of DNT-resistant AML cells was measured by flow cytometry. The experiments were done in triplicates. The data shown are representative of 2 independent experiments. Student t test and 1-way/2-way ANOVA were used. ∗P < .05; ∗∗∗P < .001. ns, nonsignificant.
TNFα produced by DNTs, upon encountering sensitive AML, renders DNT-resistant AML susceptible to DNT killing. (A) DNTs were cocultured with DNT-resistant KG-1a in the presence or absence of DNT-susceptible AML (MV4-11 or OCI-AML3) at a 2:1:1 (DNT: KG-1a: DNT-susceptible AML) ratio for 24 hours. Specific killing of KG-1a was measured by flow cytometry. The experiments were done in triplicates, and the data shown are representative of 2 independent experiments. (B-C) MV4-11 were cultured with or without DNTs in the top compartment of a transwell. KG-1a alone or cocultured with DNTs were placed in the bottom compartment of the transwell. Cells were then incubated for 2 days as shown in the schematic (B). Specific killing of KG-1a cells in the bottom compartment was measured and compared between MV4-11 and MV4-11 + DNT conditions (C). The experiments were done in triplicates. The data shown are representative of 2 independent experiments. (D-E) DNT-resistant AML, KG-1a (D) or OCI-AML3CD64KO (E), were incubated alone or with DNT-susceptible AML, MV4-11 (D) or OCI-AML3WT (E), and DNTs in the presence of anti-TNFα blocking antibody (10 μg/mL) or isotype control for 2 days. Antibodies were added on day 0 and day 1 of the cocultures. Specific killing of DNT-resistant AML cells was measured by flow cytometry. The experiments were done in triplicates. The data shown are representative of 2 independent experiments. Student t test and 1-way/2-way ANOVA were used. ∗P < .05; ∗∗∗P < .001. ns, nonsignificant.
Because DNTs may secrete other soluble factors in addition to TNFα, to investigate the role of DNT-derived TNFα in the above assays, the mixed coculture experiment was conducted in the presence of anti-TNFα neutralizing antibodies. These neutralizing antibodies reversed the effect of having MV4-11 cells in the coculture on the cytotoxicity of DNTs against KG-1a cells (Figure 4D). To validate these observations with another DNT-resistant AML cell line and further examine the contribution of CD64, a mixed coculture assay with wild-type OCI-AML3 (OCI-AML3WT) and OCI-AML3CD64KO cells to simulate susceptible and resistant populations, respectively, was used. Similar to the MV4-11–KG-1a mixed assay, the presence of OCI-AML3WT rendered OCI-AML3CD64KO cells susceptible to DNT antileukemic activity, which was partially abrogated by anti-TNFα neutralizing antibodies (Figure 4E). Overall, these data demonstrate that DNTs produce TNFα, upon interacting with DNT-susceptible AML cells, which can then sensitize nearby DNT-resistant AML targets to DNT killing.
TNFα signals through JAK1 to upregulate ICAM-1
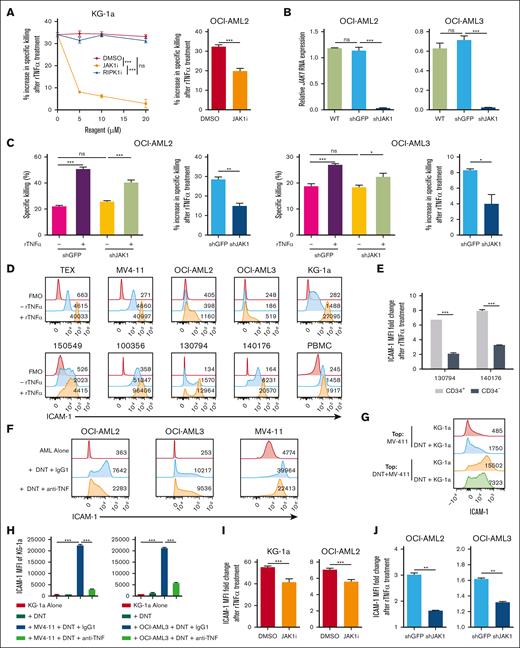
To dissect the molecular mechanisms by which TNFα alters AML susceptibility to DNTs, AML cells were pretreated with inhibitors against potential downstream pathways of TNFα receptor, such as receptor-interacting protein kinase-1 (RIPK1) and JAK1.32-34 Pretreating AML cells with necrostatin 2 racemate (RIPK1 inhibitor) to block canonical TNFα receptor signaling32,33 did not abrogate the effect of rTNFα-mediated sensitization of KG-1a cells to DNTs (Figure 5A). In contrast, itacitinib, a selective JAK1 inhibitor, significantly reduced the ability of rTNFα to increase AML susceptibility to DNTs for both KG-1a and OCI-AML2 (Figure 5A). To further confirm the contribution of JAK1 in TNFα-induced sensitization of AML cells, JAK1 was knocked down in AML cells (AMLshJAK1; Figure 5B), and the effects of rTNFα on AMLshJAK1 cells were compared with that of control cells (AMLshGFP). Consistently, rTNFα was significantly less effective at increasing AML susceptibility to DNTs for AMLshJAK1 cells compared with AMLshGFP cells (Figure 5C). Together, these data support that JAK1 is involved in TNFα-induced sensitization of AML cells.
TNFα signals through JAK1 to upregulate ICAM-1. (A) KG-1a and OCI-AML2 cells were exposed to increasing or fixed (40 μM) concentrations of RIPK1 inhibitor, JAK1 inhibitor, or dimethyl sulfoxide (DMSO) for 24 hours, followed by rTNFα treatment. AML were then cocultured with DNTs for 24 hours (for KG-1a) or 2 hours (for OCI-AML2). Percentage increase in specific killing after rTNFα pretreatment was determined. The data shown are representative of 2 independent experiments. (B-C) WT OCI-AML2 and OCI-AML3 cells were untreated or transduced with shRNAs against JAK1 (shJAK1) or GFP (shGFP). RNA expression of JAK1 was normalized to HPRT housekeeping gene (B). Transduced AML cells were pretreated with or without rTNFα, washed with PBS, then cocultured with DNTs for 2 hours. Specific killing (left) and percentage increase in specific killing (right) after rTNFα pretreatment are shown (C). (D) AML cell lines (top), primary AML samples with patient IDs, and healthy PBMCs (bottom) were untreated (blue) or treated (orange) with rTNFα. Representative histograms of ICAM-1 expression with MFI values including fluorescence-minus-one (FMO) control (red) are shown. (E) CD33+CD45lowCD3−CD34+ (CD34+) and CD33+CD45lowCD3−CD34− (CD34−) primary AML blasts from 2 patients were treated with rTNFα. The ICAM-1 MFI fold change is shown. (F) ICAM-1 expression with MFI values of AML cell lines cocultured with or without DNTs for 24 hours in the presence of anti-TNFα or isotype control. The data shown are representative of 2 independent experiments. (G) MV4-11 and KG-1a were cocultured with or without DNTs in separate compartments of the transwell from Figure 4B. Representative histograms of ICAM-1 expression on KG-1a with MFI values after 2 days is shown. (H) KG-1a cells were alone or were incubated with MV4-11 or OCI-AML3 and DNTs in the presence of anti-TNFα blocking antibody or isotype control for 24 hours. ICAM-1 expression on live KG-1a cells was determined. The data are representative of 2 independent experiments. (I) KG-1a and OCI-AML2 were treated with JAK1 inhibitor or DMSO for 24 hours, followed by stimulation with or without rTNFα and stained for ICAM-1. The MFI fold change of ICAM-1 expression from rTNFα-treated AML cells relative to untreated AML cells is shown and compared between vehicle and JAK1 inhibitor conditions. The data displayed are 2 pooled independent experiments. (J) JAK1 knockdown (shJAK1) or control (shGFP) AML cells were untreated or treated with rTNFα (100 ηg/mL), then stained with anti–ICAM-1 antibody. The MFI fold change of ICAM-1 expression from rTNFα-treated AML cells relative to untreated AML cells is shown and compared between control and JAK1 knockdown conditions. The data shown are representative of 2 independent experiments. Student t test and 1-way/2-way ANOVA were used. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, nonsignificant; shRNA, short hairpin RNA.
TNFα signals through JAK1 to upregulate ICAM-1. (A) KG-1a and OCI-AML2 cells were exposed to increasing or fixed (40 μM) concentrations of RIPK1 inhibitor, JAK1 inhibitor, or dimethyl sulfoxide (DMSO) for 24 hours, followed by rTNFα treatment. AML were then cocultured with DNTs for 24 hours (for KG-1a) or 2 hours (for OCI-AML2). Percentage increase in specific killing after rTNFα pretreatment was determined. The data shown are representative of 2 independent experiments. (B-C) WT OCI-AML2 and OCI-AML3 cells were untreated or transduced with shRNAs against JAK1 (shJAK1) or GFP (shGFP). RNA expression of JAK1 was normalized to HPRT housekeeping gene (B). Transduced AML cells were pretreated with or without rTNFα, washed with PBS, then cocultured with DNTs for 2 hours. Specific killing (left) and percentage increase in specific killing (right) after rTNFα pretreatment are shown (C). (D) AML cell lines (top), primary AML samples with patient IDs, and healthy PBMCs (bottom) were untreated (blue) or treated (orange) with rTNFα. Representative histograms of ICAM-1 expression with MFI values including fluorescence-minus-one (FMO) control (red) are shown. (E) CD33+CD45lowCD3−CD34+ (CD34+) and CD33+CD45lowCD3−CD34− (CD34−) primary AML blasts from 2 patients were treated with rTNFα. The ICAM-1 MFI fold change is shown. (F) ICAM-1 expression with MFI values of AML cell lines cocultured with or without DNTs for 24 hours in the presence of anti-TNFα or isotype control. The data shown are representative of 2 independent experiments. (G) MV4-11 and KG-1a were cocultured with or without DNTs in separate compartments of the transwell from Figure 4B. Representative histograms of ICAM-1 expression on KG-1a with MFI values after 2 days is shown. (H) KG-1a cells were alone or were incubated with MV4-11 or OCI-AML3 and DNTs in the presence of anti-TNFα blocking antibody or isotype control for 24 hours. ICAM-1 expression on live KG-1a cells was determined. The data are representative of 2 independent experiments. (I) KG-1a and OCI-AML2 were treated with JAK1 inhibitor or DMSO for 24 hours, followed by stimulation with or without rTNFα and stained for ICAM-1. The MFI fold change of ICAM-1 expression from rTNFα-treated AML cells relative to untreated AML cells is shown and compared between vehicle and JAK1 inhibitor conditions. The data displayed are 2 pooled independent experiments. (J) JAK1 knockdown (shJAK1) or control (shGFP) AML cells were untreated or treated with rTNFα (100 ηg/mL), then stained with anti–ICAM-1 antibody. The MFI fold change of ICAM-1 expression from rTNFα-treated AML cells relative to untreated AML cells is shown and compared between control and JAK1 knockdown conditions. The data shown are representative of 2 independent experiments. Student t test and 1-way/2-way ANOVA were used. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, nonsignificant; shRNA, short hairpin RNA.
To further investigate how the TNFα-JAK1 axis sensitizes AML cells to DNTs, the expression of inhibitory, cytotoxic, and adhesion molecules on AML cells with or without rTNFα treatment was compared. rTNFα did not affect the expression of inhibitory ligands (transforming growth factor-β (TGF-β) and programmed cell death-ligand 1 (PD-L1); supplemental Figure 5A) or DNAX accessory molecule 1 (DNAM-1) ligands (CD112 and CD155; supplemental Figure 5B) on AML, which were previously reported to influence conventional T-cell and DNT function.14,35,36 Instead, rTNFα treatment drastically upregulated ICAM-1, intercellular adhesion molecule-1 or CD54, on AML cells after overnight stimulation but not on normal PBMCs (Figure 5D; supplemental Figure 5C). Furthermore, rTNFα induced a greater ICAM-1 upregulation on DNT-resistant CD34+ primary AML populations relative to their DNT-susceptible CD34– counterparts (Figure 5E), suggesting that ICAM-1 may contribute to the rTNFα-mediated sensitization observed in the CD34+ population. In addition, DNTs induced ICAM-1 expression on DNT-susceptible AML cells after coculture, which was partially reversed by anti-TNFα neutralizing antibodies (Figure 5F), demonstrating ICAM-1 upregulation by DNT-derived TNFα. Furthermore, soluble factors released from the coculture of DNTs with a DNT-susceptible AML target significantly promoted ICAM-1 upregulation on KG-1a cells (Figure 5G), which was reduced in the presence of anti-TNFα neutralizing antibodies (Figure 5H).
To evaluate the contribution of JAK1 in TNFα-mediated ICAM-1 upregulation, AML cells were treated with itacitinib to selectively inhibit JAK1 followed by rTNFα stimulation. The inhibition of JAK1 effectively disrupted TNFα-mediated ICAM-1 upregulation on KG-1a and OCI-AML2 cells compared with vehicle control (Figure 5I). Similarly, rTNFα was significantly less effective at increasing the expression of ICAM-1 on JAK1 knockdown AML cells relative to control AML cells (Figure 5J). Therefore, these results indicate that the TNFα-JAK1 signal upregulates ICAM-1 on AML cells.
ICAM-1–LFA-1 interaction is critical for TNFα to sensitize AML to DNT-mediated cytotoxicity
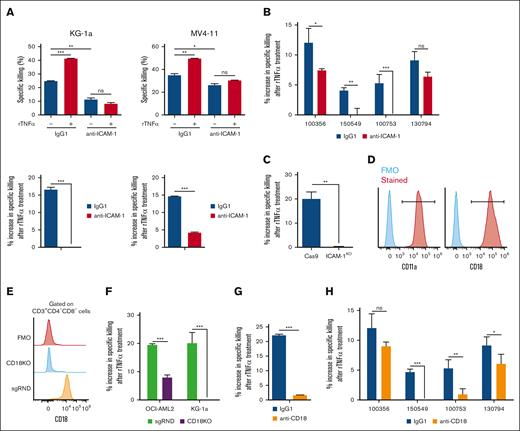
To determine the functional relevance of TNFα-mediated ICAM-1 upregulation in DNT-mediated killing of AML cells, blocking assays were conducted with rTNFα-treated AML. ICAM-1 blocking antibody significantly reduced the intrinsic ability of DNTs to kill AML cell lines and lessened rTNFα-mediated sensitization effects (Figure 6A). A similar effect was also observed in 3 of 4 primary AML samples (Figure 6B). This finding was further confirmed by genetic KO of ICAM-1 in AML cells (supplemental Figure 6), because ICAM-1 KO in OCI-AML2 significantly disrupted the ability of rTNFα to enhance DNT killing compared with control (Figure 6C). These data indicate the importance of ICAM-1 expression on AML cells for the sensitization effect of TNFα.
ICAM-1–LFA-1 interaction is critical for TNFα to sensitize AML to DNT-mediated cytotoxicity. (A-B) AML cell lines (A) and primary AML samples (B) were pretreated with or without rTNFα, washed with PBS, then cocultured with DNTs in the presence of isotype control or anti–ICAM-1 blocking antibody. Percentage specific killing of AML cells (A, left) and percentage increase in specific killing between rTNFα-treated AML and nontreated AML (A, right) were determined. The experiments were performed in triplicates with at least 2 DNT donors. Numbers represent the ID of patients with AML. (C) Cas9 and ICAM-1KO OCI-AML2 cells were untreated or treated with rTNFα, washed with PBS, then cocultured with DNTs for 2 hours. The data shown are the percentage increase in specific killing of rTNFα-treated AML cells by DNTs relative to untreated AML cells. The graph shown is representative of 2 independent experiments. (D) DNTs were stained for LFA-1 subunits (red), CD11a and CD18, and compared with FMO control (blue). (E) Representative histogram of random sgRNA control DNTs (sgRND, orange) and CD18KO DNTs (CD18KO, blue) to show CD18 expression relative to FMO control (red). (F) AML cells were pretreated with or without rTNFα, washed with PBS, then cocultured with CD18KO DNTs (CD18KO) or control DNTs (sgRND) for 24 hours (for KG-1a) and 2 hours (for OCI-AML2). The percentage increase in specific killing due to rTNFα pretreatment was determined. The data shown are representative of 2 independent experiments. (G-H) KG1a (G) and 4 primary AML samples (H) were pretreated with or without rTNFα, washed with PBS, then cocultured with DNTs, in the presence of isotype control or anti-CD18 blocking antibody for 24 hours (for KG-1a) and 3 hours (for primary AML). Percentage change in specific killing between rTNFα-treated AML and nontreated AML is shown. The experiments were performed in triplicates with at least 2 DNT donors. Numbers represent the ID of patients with AML. Student t test and 1-way/2-way ANOVA were used. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, nonsignificant; sgRNA, single guide RNA; sgRND, random single guide RNA.
ICAM-1–LFA-1 interaction is critical for TNFα to sensitize AML to DNT-mediated cytotoxicity. (A-B) AML cell lines (A) and primary AML samples (B) were pretreated with or without rTNFα, washed with PBS, then cocultured with DNTs in the presence of isotype control or anti–ICAM-1 blocking antibody. Percentage specific killing of AML cells (A, left) and percentage increase in specific killing between rTNFα-treated AML and nontreated AML (A, right) were determined. The experiments were performed in triplicates with at least 2 DNT donors. Numbers represent the ID of patients with AML. (C) Cas9 and ICAM-1KO OCI-AML2 cells were untreated or treated with rTNFα, washed with PBS, then cocultured with DNTs for 2 hours. The data shown are the percentage increase in specific killing of rTNFα-treated AML cells by DNTs relative to untreated AML cells. The graph shown is representative of 2 independent experiments. (D) DNTs were stained for LFA-1 subunits (red), CD11a and CD18, and compared with FMO control (blue). (E) Representative histogram of random sgRNA control DNTs (sgRND, orange) and CD18KO DNTs (CD18KO, blue) to show CD18 expression relative to FMO control (red). (F) AML cells were pretreated with or without rTNFα, washed with PBS, then cocultured with CD18KO DNTs (CD18KO) or control DNTs (sgRND) for 24 hours (for KG-1a) and 2 hours (for OCI-AML2). The percentage increase in specific killing due to rTNFα pretreatment was determined. The data shown are representative of 2 independent experiments. (G-H) KG1a (G) and 4 primary AML samples (H) were pretreated with or without rTNFα, washed with PBS, then cocultured with DNTs, in the presence of isotype control or anti-CD18 blocking antibody for 24 hours (for KG-1a) and 3 hours (for primary AML). Percentage change in specific killing between rTNFα-treated AML and nontreated AML is shown. The experiments were performed in triplicates with at least 2 DNT donors. Numbers represent the ID of patients with AML. Student t test and 1-way/2-way ANOVA were used. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, nonsignificant; sgRNA, single guide RNA; sgRND, random single guide RNA.
ICAM-1 is a high-affinity ligand for LFA-1 receptor, an adhesion molecule comprising 2 subunits, CD18 and CD11a,37 highly expressed on DNTs (Figure 6D). To determine the importance of LFA-1 on DNTs to interact with ICAM-1 on AML cells, CD18KO DNTs or control DNTs (sgRND) (Figure 6E) were cocultured with rTNFα-pretreated AML cells. We observed a significant decrease in the ability of rTNFα to sensitize AML cell lines to CD18KO DNTs compared with control DNTs (Figure 6F). Consistent with this, the presence of anti-CD18 neutralizing antibodies to block LFA-138 significantly reduced rTNFα-mediated sensitization of KG-1a (Figure 6G) and 3 of 4 primary AML samples (Figure 6H) to DNTs, compared with isotype controls. Thus, ICAM-1–LFA-1 ligand-receptor interaction is crucial for TNFα-mediated sensitization of AML to DNTs.
Discussion
In this study, we investigated possible mechanisms for DNT-mediated cytotoxicity against AML cells by using a flow cytometry–based HTS assay to compare cell surface protein expression after coculture with DNT-susceptible vs -resistant AML cells. We identified a TNFα–JAK1–ICAM-1 cytotoxic axis, whereby DNTs secreted TNFα upon interacting with CD64+ AML targets, which upregulated ICAM-1 on AML cells. This allowed for DNTs to better target AML cells, including those otherwise resistant to DNTs, through LFA-1. These findings uncovered a novel mechanism involved in the DNT-mediated antileukemic response through a noncanonical TNFα-JAK1 pathway in AML. It also highlighted the potential combination of TNFα-based therapies with DNTs to improve the treatment of heterogenous malignancies such as AML.
Previously, we described the role of interferon gamma, NKG2D, DNAM-1, and CD64 in DNT antileukemic activities. However, interference of these molecules did not completely abrogate DNT killing,14,28 suggesting the presence of additional pathways. Hence, the flow cytometry–based HTS assay explored other potential mechanisms involved in DNT-mediated cytotoxicity against AML. The technique assessed immune–cancer cell interactions by incorporating target cells with differential susceptibilities and cell surface molecule expression data. Given the use of monoclonal antibodies to augment contact-dependent mechanisms and CAR T-cells in cancer immunotherapy,6,39 this approach can be potentially applied to other cancer models to identify targetable cell surface proteins by CARs or antibodies to improve adoptive cellular therapies.
TNFα is a prominent inflammatory cytokine able to elicit protumorigenic and antitumorigenic effects.22,24,40 In AML, the discrepancy remains because high serum TNFα is reported to be an adverse prognostic factor for newly diagnosed patients with AML,23 whereas several clinical case reports describe the development of AML after the use of anti-TNFα inhibitors.41,42 Consistent with the case reports, we demonstrated that anti-TNFα neutralizing antibodies reduced the antitumour activity of DNTs against AML cells in vitro. Furthermore, rTNFα pretreatment sensitized AML cells to DNT-mediated killing in vitro and in vivo in an ICAM-1–LFA-1–dependent manner. Notably, the TNFα effect was more pronounced in AML cells with a higher degree of resistance to DNTs and was not observed in healthy allogeneic PBMCs. These findings support the potential combination therapy of DNT therapy with other approaches that augment the TNFα–ICAM-1 axis43,44 to enhance treatment outcomes.
The release of TNFα appears to be induced by the expression of CD64, a high-affinity Fcγ receptor, typically found on DNT-susceptible AML cells compared with DNT-resistant ones.28 TNFα stimulation can bypass the initial CD64-dependent mechanism and increase DNT-mediated cytotoxicity against AML cells. However, the exact mechanisms between DNTs and CD64+ AML remain unclear. CD64 expression is associated with more mature AML phenotypes, such as AML-M5,45 and AML-M5 blasts are more susceptible to DNTs than other AML subtypes.14 It is possible that DNTs directly bind to CD64 or another molecule regulated by CD64 expression on AML. Nevertheless, these observations uncover a novel function of CD64 on AML to initiate DNT cytotoxic activity and warrant additional investigations to clarify DNT activation mechanisms for improved therapeutic purposes.
Cancer heterogeneity refers to genotypic and phenotypic differences between a tumor across different patients, cancer cells within a single tumor, or the same tumor throughout disease progression.31 The cellular nonuniformity is a major driver for therapeutic resistance and disease relapse, predisposing patients to poorer clinical outcomes, especially in patients with AML.3,29,30 DNT therapy is not an exception in which ∼30% of primary AML blasts show resistance to DNT-mediated cytotoxicity, partially explained by CD64 expression profiles on AML.14,28 Interestingly, TNFα increased the susceptibility of AML to DNTs, expanding the therapeutic window against various AML cells. Furthermore, TNFα produced by DNTs, after encountering susceptible targets, sensitized nearby DNT-resistant AML cell lines. Ultimately, these observations favorably support the use of DNT therapy for AML or other highly heterogeneous cancers,46 possibly composed of a mixture of DNT-susceptible and -resistant populations.
TNFα signals through TNFRs to elicit NF-κB activation and inflammatory responses.32 TNFα produced from DNT-AML cocultures contribute to the downregulation of CD120b on DNTs in an autocrine manner. Although TNFα stimulation of DNTs did not alter cytotoxic function, CD120b signaling has been investigated in regulatory T cells and may be involved in the ability of DNTs to resist host-versus-graft rejection.15,47 In contrast, TNFα sensitized AML to greater DNT-mediated cytotoxicity, independent of a canonical mediator of TNFR/NF-κB, RIPK1. Instead, we identified a TNFR pathway involving JAK1 that consistently mediated ICAM-1 upregulation and AML sensitization. Although the TNFR pathway has been characterized, the molecular relationship and immunotherapeutic significance between TNFRs and JAK1 remain unclear, except some studies demonstrating that TNFRs could signal through JAK1 in B cells, adipocytes, and epithelial cells.34,48,49 JAK1 is 1 of 4 mammalian JAK proteins commonly found at the cytoplasmic domain of interferon and interleukin receptors. Upon ligand-receptor binding, receptor-associated JAKs are activated and lead to phosphorylation of signal transducer and activator of transcription (STAT) proteins. Phosphorylated STAT molecules translocate into the nucleus to upregulate inflammatory factors and cytotoxic ligands.50 Given that DNTs secrete a wide range of inflammatory cytokines,14,51 the significance of JAK-STAT signaling in DNT-AML interactions is currently being explored.
In summary, our findings highlight the utility of the flow cytometry–based HTS platform to identify underlying cytotoxic mechanisms between immune–cancer cell interactions and the significance of the novel TNFα–JAK1–ICAM-1 axis in DNT-mediated cytotoxicity against AML. These data support the potential combinatorial use of drugs that influence this axis to enhance the antileukemic activity of DNT therapy.
Acknowledgments
The authors thank all donors and patients who participated in this study. The authors also thank the Leukemia Tissue Bank (Princess Margaret Cancer Centre) for providing primary acute myeloid leukemia patient samples.
This study was supported by the Canadian Cancer Society Impact Grant (grant 704121) and Canadian Institutes of Health Research (grant 419699).
Authorship
Contribution: E.T., J.B.L., and L.Z. conceived and designed the study experiments; E.T., J.B.L., I.K., and Y.N. conducted experiments; M.D.M. provided primary patient samples; E.T. and J.B.L. prepared the manuscript; and J.B.L., I.K., M.D.M., and L.Z. provided feedback and edited the manuscript.
Conflict-of-interest disclosure: M.D.M. is a consultant for Astellas, Abbvie, and Celgene. L.Z. has financial interests (eg, holdings/shares) in WYZE Biotech Co Ltd and previously received research funding and consulting fee/honorarium from the company. L.Z. and J.B.L. are coinventors of several double-negative T cell technology–related patents and intellectual properties for the treatment of acute myeloid leukemia. The remaining authors declare no competing financial interests.
Correspondence: Li Zhang, Toronto General Hospital Research Institute, University Health Network, 101 College St, Room 2-207, Toronto, ON M5G 1L7, Canada; email: li.zhang@uhnresearch.ca.
References
Author notes
Original data available upon reasonable request from the corresponding author, Li Zhang (li.zhang@uhnresearch.ca).
The full-text version of this article contains a data supplement.