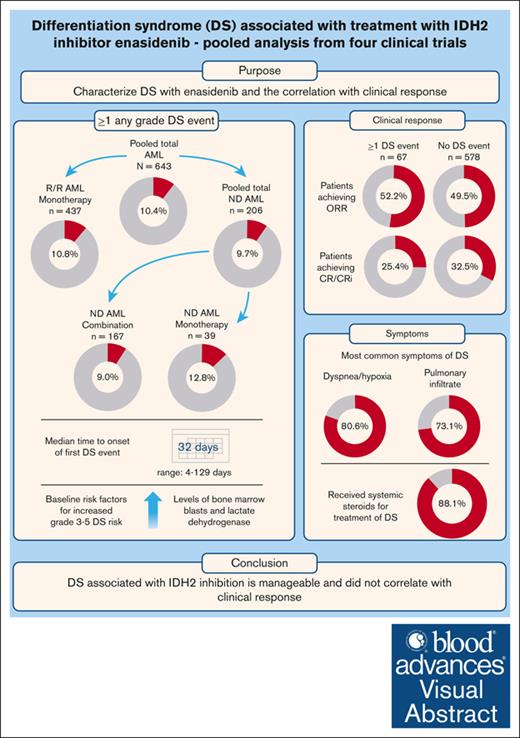
Enasidenib treatment may be associated with DS in patients with AML, yet diagnosis of DS is challenging because of nonspecific symptoms.
IDH inhibition-associated DS is manageable by identifying risk factors, recognizing symptoms, and initiating treatment in a timely manner.
Visual Abstract
Treatment with enasidenib, a selective mutant isocitrate dehydrogenase isoform 2 (IDH2) inhibitor, has been associated with the development of differentiation syndrome (DS) in patients with acute myeloid leukemia (AML). Studies on the incidence and clinical features of DS are limited in this setting, and diagnosis is challenging because of nonspecific symptoms. This study assessed the incidence, diagnostic criteria, risk factors, and correlation with clinical response of DS based on the pooled analysis of 4 clinical trials in patients with IDH2-mutated AML treated with enasidenib as monotherapy, or in combination with azacitidine or with chemotherapy. Across the total AML population, 67 of 643 (10.4%) had ≥1 any-grade DS event, with highest incidence in patients who received enasidenib plus azacitidine and lowest incidence in patients who received enasidenib plus chemotherapy (13/74 [17.6%] and 2/93 [2.2%]). The most common symptoms of DS were dyspnea/hypoxia (80.6%) and pulmonary infiltrate (73.1%). Median time to onset of first DS event across all studies was 32 days (range, 4-129). Most patients (88.1%) received systemic steroids for treatment of DS. Evaluation of baseline risk factors for DS identified higher levels of bone marrow blasts and lactate dehydrogenase as independent factors associated with increased grade 3 to 5 DS risk. Overall, these results suggest that DS associated with IDH inhibition is manageable, given the benefits of enasidenib treatment in IDH2-mutated AML. We further characterized enasidenib-related DS in these patients and identified risk factors, which could be used for DS management in clinical practice. These trials were registered at www.ClinicalTrials.gov as # NCT01915498, NCT02577406, NCT02677922, and NCT02632708.
Introduction
Acute myeloid leukemia (AML) is a heterogenous hematologic malignancy of myeloid precursor cells that is often diagnosed in older populations (with an average age at diagnosis of 68 years).1 Mutations in the isocitrate dehydrogenase (IDH) enzyme isoforms 1 and 2 (IDH1 and IDH2) have been found in ∼10% and 15% of AML cases, respectively.2,3
Enasidenib, a selective inhibitor of mutant IDH2 approved by the US Food and Drug Administration for the treatment of adults with relapsed or refractory (R/R) IDH2-mutated AML, suppresses aberrant levels of the oncometabolite 2-hydroxyglutarate and restores normal myeloid differentiation.4,5 Treatment with enasidenib has been associated with the development of differentiation syndrome (DS), a potentially fatal adverse reaction related to agents that promote myeloid differentiation. DS was initially described in patients with acute promyelocytic leukemia (APL) treated with differentiating agents (ie, all-trans retinoic acid and arsenic trioxide).6,7 DS pathogenesis is not fully understood but believed to arise from therapeutically induced differentiation of blasts, resulting in a “cytokine storm,” and subsequent severe systemic inflammatory response.8-10 Signs and symptoms of IDH inhibitor-induced DS, including fever, dyspnea, acute renal failure, respiratory distress, and pleural/pericardial effusion, are commonly heterogeneous and resemble those observed in AML progression.11-13 Early recognition and management of suspected cases of DS are critical, with symptoms of DS shown to resolve with rapid intervention using corticosteroid treatment.11,13,14
Although the reported mean incidence of DS ranges between 9.6% and 19%,13,15 the true incidence is not well established, given the difficulty in confirming diagnosis. This was evident in the phase 1/2 trial of enasidenib in IDH2-mutated AML (AG221-C-001 [ClinicalTrials.gov identifier: NCT01915498]), which reported a range of DS rates (6%-19%) depending on the definition or algorithm used to identify DS.11,13,16 Because of limited studies and lack of large study populations, the incidence and clinical picture of DS, and its association with enasidenib in patients with IDH2-mutated AML, remains unclear. This study further characterizes enasidenib-related DS in terms of incidence, appropriate diagnostic criteria, risk factors, and correlation with clinical response based on the pooled analysis of 4 clinical trials of enasidenib in adults with AML.
Methods
Study design
This study assessed DS events across 4 clinical trials in patients with newly diagnosed (ND) or R/R IDH2-mutated AML treated with enasidenib as monotherapy (AG221-C-001 and AG221-AML-004 [ClinicalTrials.gov identifier: NCT02577406]) or in a combination setting (with azacitidine in AG221-AML-005 [ClinicalTrials.gov identifier: NCT02677922], or with induction chemotherapy [7 + 3 cytarabine and daunorubicin or idarubicin], with or without consolidation chemotherapy, in AG120-221-C-001 [ClinicalTrials.gov identifier: NCT02632708]; supplemental Table 1).12,17 Each trial was approved by the institutional review board or central ethics committee at each participating institution. All trials were conducted in accordance with the Declaration of Helsinki, and all patients provided written informed consent.
The study aimed to characterize DS (incidence, clinical presentation, and severity) in patients with IDH2-mutated AML treated with enasidenib as monotherapy or in combination therapy; evaluate steroid use for DS treatment; understand DS risk factors; explore the correlation between DS and clinical response; understand the diagnostic criteria for DS based on risk factors and signs and symptoms; and perform time-to-event analyses.
Identification of DS
All serious and nonserious events reported as DS by clinical trial investigators were forwarded for review by the DS review committee (DSRC), which comprised 4 hematologists experienced in the care of patients treated with IDH inhibitors. To identify potential cases of DS for DSRC review, the safety database was searched at least once monthly, and the clinical database was searched periodically for the predefined Medical Dictionary for Regulatory Activities terms listed in the DSRC charter (supplemental Table 2). The DSRC retrospectively reviewed (1) adverse events (AEs) reported as DS; (2) AEs consistent with the signs and symptoms of DS in the absence of infection; and (3) AEs in patients who were not in remission and were not responsive to anti-infectives. DSRC members evaluated potential DS events using modified DS diagnostic criteria initially described by Montesinos et al.9 The diagnostic guideline was adjusted based on data from the enasidenib monotherapy study AG221-C-001 (supplemental Table 2). DS, according to the Montesinos criteria, is based on ≥2 signs and symptoms of APL-DS, in the absence of alternative causes.
To determine the likelihood of an event being DS, the DSRC independently considered the following criteria: (1) signs or symptoms suggestive of DS and not attributed to another cause, or resistant to treatment for the original diagnosis (including fever, dyspnea/hypoxia, pulmonary infiltrates, pleural or pericardial effusions, rapid weight gain or peripheral edema, rash, bone pain/arthralgia, lymphadenopathy, or renal dysfunction); (2) evidence of cellular differentiation or proliferation at the time of, or after, potential DS, as indicated by the bone marrow (BM) aspirate/biopsy and/or peripheral blood (PB) differential in patient reports; and (3) symptoms improved or resolved with use of systemic corticosteroids.
The DSRC identified DS as “probable DS” when signs or symptoms occurred in the absence of other significant secondary causes and in the presence of cellular differentiation or proliferation, and the patient responded to corticosteroid therapy; as “possible DS” when there was response to steroids, no clear evidence of secondary causes, and no signs of cellular differentiation or proliferation; or as “unlikely DS” or “not DS” if the event could have been, or was attributed to, another cause in the absence of evidence of cellular differentiation and/or response to steroids. Probable or possible cases of DS were considered confirmed cases of DS and were included in this analysis. DS was considered confirmed when 3 of 4 DSRC members (or 2 of 3 members if a member was exempted from reviewing their own patient’s records) were in consensus. DSRC assessments and the final adjudication were recorded in a “DSRC Review Form.”
Grading for DS was defined by the sponsor in the study-specific DS management guidelines. The severity of DS events was graded according to the following scale (grade 1 was not used): grade 2, moderate severity not requiring hospitalization; grade 3, severe, requiring hospitalization; grade 4, life threatening, requiring ventilator support or intensive care unit admission; and grade 5, death.
Incidence, severity, signs and symptoms, and time to onset of DS
DS events, including severity and signs and symptoms, were tabulated in count and percentage. Each participant with >1 DS episode was counted once.
Time to onset of a DS event was calculated as time from the start of enasidenib treatment to the onset of a DS event. For patients with >1 event, the onset of the earliest DS event was used. For events not reported as DS by a clinical trial investigator but identified as probable or possible DS by the DSRC, based on signs and symptoms of DS reported as AEs unresponsive to anti-infectives, onset of the earliest event representing DS was used as the DS start date.
Use of systemic steroids and enasidenib interruption for management of DS
Steroid use for treatment of DS (yes/no), time to treatment initiation from DS onset day, and duration of steroid treatment relative to clinical features of DS (duration of event and severity) were recorded for the pooled population. Interruptions to enasidenib treatment during DS management were recorded similarly.
Correlation with clinical response
Disease responses to enasidenib treatment were assessed by investigators per 2003 International Working Group response criteria for AML.18 Rates of overall response (complete remission [CR] + morphologic CR with incomplete neutrophil recovery + morphologic CR with incomplete platelet recovery + partial remission + morphologic leukemia-free state), CR and CR with partial hematologic recovery (CRh; defined as all criteria of CR except absolute neutrophil count >0.5 × 109/L [500/μL] and platelet count >50 × 109/L [50 000/μL]) were summarized in patients with AML treated with enasidenib with or without DS. For patients who experienced DS events, best responses before and after the onset of first such events were also summarized.
Rates of CR or CRh were also summarized for the studies in which CR/CRh assessments were available.
Risk factor modeling
To identify potential prognostic risk factors of DS (any grade and grade 3-5), univariate and multivariate logistic regressions were performed for key baseline and disease characteristics: age, sex, performance status, IDH2 mutation site, history of prior myelodysplastic syndromes, PB and BM blast counts, serum creatinine, albumin, median number of prior anticancer regimens, and lactate dehydrogenase (LDH). The risk factors with univariate P values <.2 were included in a multivariate logistic regression and selected by backward deletion at a significance level of .05, and the main effects with P values of <.05 were retained in the model and identified as potential prognostic factors. Statistical results should be interpreted with caution because multiplicity adjustment was not planned for these exploratory analyses.
Each trial was approved by the institutional review board or central ethics committee at each participating institution.
Results
Incidence and severity of DS
The pooled population comprised 643 patients (437 with R/R AML, and 206 with ND AML) across the 4 individual studies. Patient characteristics and laboratory values at baseline are provided in supplemental Tables 3 and 4. The median age at baseline across all studies was 70 years (range, 19-100). Most patients had IDH2-R140 mutations (73.3%), cytogenetics of intermediate risk or worse (79.6%), and prior anticancer therapies (74.5%).
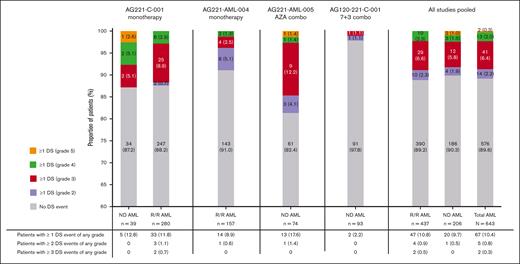
Across all studies, 67 of 643 (10.4%) of the total AML population had ≥1 DS event of any grade. Among the 67 patients with DS, 14 of 67 (20.9%) had ≥1 grade 2 event, 41 of 67 (61.2%) had ≥1 grade 3 event, and 13 of 67 (19.4%) had ≥1 grade 4 event. Two patients with ND AML (2/67 [3.0%]) had a grade 5 event (Figure 1). The proportion of patients who experienced ≥1 DS event was 10.8% (47/437) in patients with R/R AML who received enasidenib monotherapy, 12.8% (5/39) in patients with ND AML who received enasidenib monotherapy, and 9.0% (15/167) in patients with ND AML who received combination therapy. Of patients with ND AML who received combination therapy (n = 167), the proportion who experienced ≥1 DS event was higher in those who received azacitidine combination therapy vs those receiving 7 + 3 combination therapy (17.6% [13/74] vs 2.2% [2/93], 15.42% difference; 95% confidence interval [CI], 6.26-24.57; Figure 1).
Incidence and severity of DS event by study and type of AML (ND or R/R; AML safety population). ND patients received no prior treatment for AML. No patients experienced >3 DS events. There is no grade 1 category per site guidelines for DS. DS events included possible and probable cases of DS. Number and percentage of patients in each group shown as n (%). Patients were treated with enasidenib (100 mg or 200 mg per day) in combination with azacitidine 75 mg/m2 per day. Patients were treated with enasidenib (100 mg once daily) in combination with cytarabine (200 mg/m2 per day for 7 days) and either daunorubicin (60 mg/m2 per day for 3 days) or idarubicin (12 mg/m2 per day for 3 days). AML, acute myeloid leukemia; DS, differentiation syndrome; ND, newly diagnosed; R/R, relapsed or refractory.
Incidence and severity of DS event by study and type of AML (ND or R/R; AML safety population). ND patients received no prior treatment for AML. No patients experienced >3 DS events. There is no grade 1 category per site guidelines for DS. DS events included possible and probable cases of DS. Number and percentage of patients in each group shown as n (%). Patients were treated with enasidenib (100 mg or 200 mg per day) in combination with azacitidine 75 mg/m2 per day. Patients were treated with enasidenib (100 mg once daily) in combination with cytarabine (200 mg/m2 per day for 7 days) and either daunorubicin (60 mg/m2 per day for 3 days) or idarubicin (12 mg/m2 per day for 3 days). AML, acute myeloid leukemia; DS, differentiation syndrome; ND, newly diagnosed; R/R, relapsed or refractory.
Recurrences of DS events were not common and a total of 9 recurrences were reported in 7 patients (7/67 [10.4%]): 5 patients had 2 DS events, and 2 had 3 DS events of any grade. No patient experienced >3 DS events.
Signs and symptoms and time to onset of DS
The most common signs and symptoms of DS among all patients with a DS event (n = 67) were dyspnea/hypoxia (80.6%), pulmonary infiltrate (73.1%), unexplained fever (71.6%), and requirement of oxygen supplementation (55.2%; Table 1).
Signs and symptoms and time of onset of DS by study and type of AML
| Study number/treatment type population . | AG221-C-001 monotherapy . | AG221-AML-004 monotherapy . | AG221-AML-005 AZA combo∗ . | AG120-221-C-001 7 + 3 combo† . | All studies pooled . | |||
|---|---|---|---|---|---|---|---|---|
| ND AML n = 39 n (%) . | R/R AML n = 280 n (%) . | R/R AML n = 157 n (%) . | ND AML n = 74 n (%) . | ND AML n = 93 n (%) . | R/R AML n = 437 n (%) . | ND AML n = 206 n (%) . | Total AML N = 643 n (%) . | |
| Patients with >1 DS event‡ | 5 (12.8) | 33 (11.8) | 14 (8.9) | 13 (17.6) | 2 (2.2) | 47 (10.8) | 20 (9.7) | 67 (10.4) |
| Signs and symptoms for all DS events§ | ||||||||
| Dyspnea/hypoxia | 4 (80.0) | 28 (84.8) | 9 (64.3) | 11 (84.6) | 2 (100.0) | 37 (78.7) | 17 (85.0) | 54 (80.6) |
| Pulmonary infiltrate | 4 (80.0) | 24 (72.7) | 8 (57.1) | 11 (84.6) | 2 (100.0) | 32 (68.1) | 17 (85.0) | 49 (73.1) |
| Unexplained fever | 2 (40.0) | 25 (75.8) | 8 (57.1) | 11 (84.6) | 2 (100.0) | 33 (70.2) | 15 (75.0) | 48 (71.6) |
| Required oxygen supplementation‖ | 3 (60.0) | 18 (54.5) | 7 (50.0) | 9 (69.2) | 0 | 25 (53.2) | 12 (60.0) | 37 (55.2) |
| Pleural effusion | 4 (80.0) | 13 (39.4) | 5 (35.7) | 9 (69.2) | 1 (50.0) | 18 (38.3) | 14 (70.0) | 32 (47.8) |
| Serum creatinine increase | 4 (80.0) | 13 (39.4) | 5 (35.7) | 4 (30.8) | 0 | 18 (38.3) | 8 (40.0) | 26 (38.8) |
| Edema or rapid weight gain | 1 (20.0) | 7 (21.2) | 5 (35.7) | 5 (38.5) | 0 | 12 (25.5) | 6 (30.0) | 18 (26.9) |
| Pericardial effusion | 4 (80.0) | 5 (15.2) | 1 (7.1) | 3 (23.1) | 0 | 6 (12.8) | 7 (35.0) | 13 (19.4) |
| Rash | 0 | 8 (24.2) | 2 (14.3) | 3 (23.1) | 0 | 10 (21.3) | 3 (15.0) | 13 (19.4) |
| Bone pain | 1 (20.0) | 6 (18.2) | 3 (21.4) | 1 (7.7) | 0 | 9 (19.1) | 2 (10.0) | 11 (16.4) |
| Time to onset of first DS, d | ||||||||
| Median | 48.0 | 32.0 | 27.0 | 42.0 | 38.5 | 32.0 | 42.5 | 32.0 |
| Min, max | 10.0, 99.0 | 9.0, 129.0 | 5.0, 122.0 | 4.0, 84.0 | 29.0, 48.0 | 5.0, 129.0 | 4.0, 99.0 | 4.0, 129.0 |
| Study number/treatment type population . | AG221-C-001 monotherapy . | AG221-AML-004 monotherapy . | AG221-AML-005 AZA combo∗ . | AG120-221-C-001 7 + 3 combo† . | All studies pooled . | |||
|---|---|---|---|---|---|---|---|---|
| ND AML n = 39 n (%) . | R/R AML n = 280 n (%) . | R/R AML n = 157 n (%) . | ND AML n = 74 n (%) . | ND AML n = 93 n (%) . | R/R AML n = 437 n (%) . | ND AML n = 206 n (%) . | Total AML N = 643 n (%) . | |
| Patients with >1 DS event‡ | 5 (12.8) | 33 (11.8) | 14 (8.9) | 13 (17.6) | 2 (2.2) | 47 (10.8) | 20 (9.7) | 67 (10.4) |
| Signs and symptoms for all DS events§ | ||||||||
| Dyspnea/hypoxia | 4 (80.0) | 28 (84.8) | 9 (64.3) | 11 (84.6) | 2 (100.0) | 37 (78.7) | 17 (85.0) | 54 (80.6) |
| Pulmonary infiltrate | 4 (80.0) | 24 (72.7) | 8 (57.1) | 11 (84.6) | 2 (100.0) | 32 (68.1) | 17 (85.0) | 49 (73.1) |
| Unexplained fever | 2 (40.0) | 25 (75.8) | 8 (57.1) | 11 (84.6) | 2 (100.0) | 33 (70.2) | 15 (75.0) | 48 (71.6) |
| Required oxygen supplementation‖ | 3 (60.0) | 18 (54.5) | 7 (50.0) | 9 (69.2) | 0 | 25 (53.2) | 12 (60.0) | 37 (55.2) |
| Pleural effusion | 4 (80.0) | 13 (39.4) | 5 (35.7) | 9 (69.2) | 1 (50.0) | 18 (38.3) | 14 (70.0) | 32 (47.8) |
| Serum creatinine increase | 4 (80.0) | 13 (39.4) | 5 (35.7) | 4 (30.8) | 0 | 18 (38.3) | 8 (40.0) | 26 (38.8) |
| Edema or rapid weight gain | 1 (20.0) | 7 (21.2) | 5 (35.7) | 5 (38.5) | 0 | 12 (25.5) | 6 (30.0) | 18 (26.9) |
| Pericardial effusion | 4 (80.0) | 5 (15.2) | 1 (7.1) | 3 (23.1) | 0 | 6 (12.8) | 7 (35.0) | 13 (19.4) |
| Rash | 0 | 8 (24.2) | 2 (14.3) | 3 (23.1) | 0 | 10 (21.3) | 3 (15.0) | 13 (19.4) |
| Bone pain | 1 (20.0) | 6 (18.2) | 3 (21.4) | 1 (7.7) | 0 | 9 (19.1) | 2 (10.0) | 11 (16.4) |
| Time to onset of first DS, d | ||||||||
| Median | 48.0 | 32.0 | 27.0 | 42.0 | 38.5 | 32.0 | 42.5 | 32.0 |
| Min, max | 10.0, 99.0 | 9.0, 129.0 | 5.0, 122.0 | 4.0, 84.0 | 29.0, 48.0 | 5.0, 129.0 | 4.0, 99.0 | 4.0, 129.0 |
DS study and type of AML included ND or R/R AML; and AML safety population.
AML, acute myeloid leukemia; AZA, azacitidine; combo, combination; DS, differentiation syndrome; min, minimum; max, maximum; ND, newly diagnosed; R/R, relapsed or refractory.
Patients were treated with enasidenib (100 mg or 200 mg per day) in combination with azacitidine 75 mg/m2 per day.
Patients were treated with enasidenib (100 mg once daily) in combination with cytarabine (200 mg/m2 per day for 7 days) and either daunorubicin (60 mg/m2 per day for 3 days) or idarubicin (12 mg/m2 per day for 3 days).
Percentage for patients with at >1 DS event was based on number of patients in the safety population.
Percentage for each sign and symptom was calculated based on the number of patients reporting a DS event and not the total number.
This category is separate from “dyspnea/hypoxia” as either event may not require oxygen supplementation.
Across all studies, the median time to onset of the first DS event was 32 days (range, 4-129) from the start of enasidenib treatment, with longer median time to onset for the pooled ND AML population than for the R/R AML population (43 days [range, 4-99] vs 32 days [range, 5-129]; Table 1).
Use of systemic steroids and hydroxyurea for DS management
Across all studies, most patients (59/67 [88.1%]) with DS received treatment with systemic steroids; steroids were used in 66 of 76 (86.8%) DS events (Table 2). The median duration of steroid use was 14 days. Of 60 patients with only 1 DS event, 86.7% (n = 52) received steroids for the event. Of 5 patients with 2 DS events, 4 patients received steroids for both events, and each of the 2 patients with 3 DS events received steroids for third and second events, respectively. For most DS events (49/76 [64.5%]; Table 2), steroid treatment was initiated on the same day or within 1 day of diagnosis. Of 60 patients who experienced 1 DS event, 8 (13.3%) did not receive steroid treatment. Six patients had mild symptoms or symptoms that responded to treatment (eg, nonsteroidal anti-inflammatory drug and hydroxyurea). However, the remaining 2 patients with ND AML were hospitalized because of symptoms and later died (supplemental Table 5).
Duration and severity of DS events by steroid and hydroxyurea use
| . | Duration of DS . | DS severity . | All DS events . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <5 d . | 5-10 d . | 11-20 d . | >20 d . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . | ||
| Number of DS events, n | 4 | 32 | 19 | 21 | 15 | 46 | 13 | 2 | 76 |
| Steroid use, n (%) | |||||||||
| No | 2 (50.0) | 2 (6.3) | 2 (10.5) | 4 (19.0) | 1 (6.7) | 6 (13.0) | 1 (7.7) | 2 (100.0) | 10 (13.2) |
| Yes | 2 (50.0) | 30 (93.8) | 17 (89.5) | 17 (81.0) | 14 (93.3) | 40 (87.0) | 12 (92.3) | 0 | 66 (86.8) |
| Time to initiation of steroid use, n (%) | |||||||||
| 0-1 d | 2 (50.0) | 24 (75.0) | 13 (68.4) | 10 (47.6) | 9 (60.0) | 30 (65.2) | 10 (76.9) | 0 | 49 (64.5) |
| 2-3 d | 0 | 2 (6.3) | 1 (5.3) | 3 (14.3) | 3 (20.0) | 2 (4.3) | 1 (7.7) | 0 | 6 (7.9) |
| ≥4 d | 0 | 4 (12.5) | 3 (15.8) | 4 (19.0) | 2 (13.3) | 8 (17.4) | 1 (7.7) | 0 | 11 (14.5) |
| Duration of steroid use, n (%) | |||||||||
| <5 d | 0 | 5 (15.6) | 4 (21.1) | 0 | 1 (6.7) | 7 (15.2) | 1 (7.7) | 0 | 9 (11.8) |
| 5-10 d | 0 | 12 (37.5) | 3 (15.8) | 1 (4.8) | 3 (20.0) | 8 (17.4) | 5 (38.5) | 0 | 16 (21.1) |
| 11-20 d | 0 | 9 (28.1) | 5 (26.3) | 5 (23.8) | 5 (33.3) | 11 (23.9) | 3 (23.1) | 0 | 19 (25.0) |
| >20 d | 2 (50.0) | 4 (12.5) | 5 (26.3) | 11 (52.4) | 5 (33.3) | 14 (30.4) | 3 (23.1) | 0 | 22 (28.9) |
| Time from end of steroid use for prior DS to recurrence of DS, n/N (%) | |||||||||
| N/A∗ | 0 | 1/5 (20.0) | 1/4 (25.0) | 0 | - | - | - | - | 2/9 (22.2) |
| <5 d | 0 | 0 | 0 | 0 | - | - | - | - | 0 |
| 5-10 d | 0 | 1/5 (20.0) | 0 | 0 | - | - | - | - | 1/9 (11.1) |
| 11-20 d | 0 | 1/5 (20.0) | 2/4 (50.0) | 0 | - | - | - | - | 3/9 (33.3) |
| >20 d | 0 | 2/5 (40.0) | 1/4 (25.0) | 0 | - | - | - | - | 3/9 (33.3) |
| Hydroxyurea use, n (%) | |||||||||
| No | 4 (100.0) | 22 (68.8) | 16 (84.2) | 16 (76.2) | 9 (60.0) | 36 (78.3) | 11 (84.6) | 2 (100.0) | 58 (76.3) |
| Yes | 0 | 10 (31.3) | 3 (15.8) | 5 (23.8) | 6 (40.0) | 10 (21.7) | 2 (15.4) | 0 | 18 (23.7) |
| Time to initiation of hydroxyurea, n (%) | |||||||||
| 0-1 d | 0 | 10 (31.3) | 0 | 2 (9.5) | 3 (20.0) | 7 (15.2) | 2 (15.4) | 0 | 12 (15.8) |
| 2-3 d | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ≥4 d | 0 | 0 | 3 (15.8) | 3 (14.3) | 3 (20.0) | 3 (6.5) | 0 | 0 | 6 (7.9) |
| Duration of hydroxyurea use, n (%) | |||||||||
| <5 d | 0 | 3 (9.4) | 0 | 0 | 0 | 2 (4.3) | 1 (7.7) | 0 | 3 (3.9) |
| 5-10 d | 0 | 5 (15.6) | 2 (10.5) | 0 | 1 (6.7) | 5 (10.9) | 1 (7.7) | 0 | 7 (9.2) |
| 11-20 d | 0 | 2 (6.3) | 1 (5.3) | 2 (9.5) | 3 (20.0) | 2 (4.3) | 0 | 0 | 5 (6.6) |
| >20 d | 0 | 0 | 0 | 3 (14.3) | 2 (13.3) | 1 (2.2) | 0 | 0 | 3 (3.9) |
| . | Duration of DS . | DS severity . | All DS events . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <5 d . | 5-10 d . | 11-20 d . | >20 d . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . | ||
| Number of DS events, n | 4 | 32 | 19 | 21 | 15 | 46 | 13 | 2 | 76 |
| Steroid use, n (%) | |||||||||
| No | 2 (50.0) | 2 (6.3) | 2 (10.5) | 4 (19.0) | 1 (6.7) | 6 (13.0) | 1 (7.7) | 2 (100.0) | 10 (13.2) |
| Yes | 2 (50.0) | 30 (93.8) | 17 (89.5) | 17 (81.0) | 14 (93.3) | 40 (87.0) | 12 (92.3) | 0 | 66 (86.8) |
| Time to initiation of steroid use, n (%) | |||||||||
| 0-1 d | 2 (50.0) | 24 (75.0) | 13 (68.4) | 10 (47.6) | 9 (60.0) | 30 (65.2) | 10 (76.9) | 0 | 49 (64.5) |
| 2-3 d | 0 | 2 (6.3) | 1 (5.3) | 3 (14.3) | 3 (20.0) | 2 (4.3) | 1 (7.7) | 0 | 6 (7.9) |
| ≥4 d | 0 | 4 (12.5) | 3 (15.8) | 4 (19.0) | 2 (13.3) | 8 (17.4) | 1 (7.7) | 0 | 11 (14.5) |
| Duration of steroid use, n (%) | |||||||||
| <5 d | 0 | 5 (15.6) | 4 (21.1) | 0 | 1 (6.7) | 7 (15.2) | 1 (7.7) | 0 | 9 (11.8) |
| 5-10 d | 0 | 12 (37.5) | 3 (15.8) | 1 (4.8) | 3 (20.0) | 8 (17.4) | 5 (38.5) | 0 | 16 (21.1) |
| 11-20 d | 0 | 9 (28.1) | 5 (26.3) | 5 (23.8) | 5 (33.3) | 11 (23.9) | 3 (23.1) | 0 | 19 (25.0) |
| >20 d | 2 (50.0) | 4 (12.5) | 5 (26.3) | 11 (52.4) | 5 (33.3) | 14 (30.4) | 3 (23.1) | 0 | 22 (28.9) |
| Time from end of steroid use for prior DS to recurrence of DS, n/N (%) | |||||||||
| N/A∗ | 0 | 1/5 (20.0) | 1/4 (25.0) | 0 | - | - | - | - | 2/9 (22.2) |
| <5 d | 0 | 0 | 0 | 0 | - | - | - | - | 0 |
| 5-10 d | 0 | 1/5 (20.0) | 0 | 0 | - | - | - | - | 1/9 (11.1) |
| 11-20 d | 0 | 1/5 (20.0) | 2/4 (50.0) | 0 | - | - | - | - | 3/9 (33.3) |
| >20 d | 0 | 2/5 (40.0) | 1/4 (25.0) | 0 | - | - | - | - | 3/9 (33.3) |
| Hydroxyurea use, n (%) | |||||||||
| No | 4 (100.0) | 22 (68.8) | 16 (84.2) | 16 (76.2) | 9 (60.0) | 36 (78.3) | 11 (84.6) | 2 (100.0) | 58 (76.3) |
| Yes | 0 | 10 (31.3) | 3 (15.8) | 5 (23.8) | 6 (40.0) | 10 (21.7) | 2 (15.4) | 0 | 18 (23.7) |
| Time to initiation of hydroxyurea, n (%) | |||||||||
| 0-1 d | 0 | 10 (31.3) | 0 | 2 (9.5) | 3 (20.0) | 7 (15.2) | 2 (15.4) | 0 | 12 (15.8) |
| 2-3 d | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ≥4 d | 0 | 0 | 3 (15.8) | 3 (14.3) | 3 (20.0) | 3 (6.5) | 0 | 0 | 6 (7.9) |
| Duration of hydroxyurea use, n (%) | |||||||||
| <5 d | 0 | 3 (9.4) | 0 | 0 | 0 | 2 (4.3) | 1 (7.7) | 0 | 3 (3.9) |
| 5-10 d | 0 | 5 (15.6) | 2 (10.5) | 0 | 1 (6.7) | 5 (10.9) | 1 (7.7) | 0 | 7 (9.2) |
| 11-20 d | 0 | 2 (6.3) | 1 (5.3) | 2 (9.5) | 3 (20.0) | 2 (4.3) | 0 | 0 | 5 (6.6) |
| >20 d | 0 | 0 | 0 | 3 (14.3) | 2 (13.3) | 1 (2.2) | 0 | 0 | 3 (3.9) |
Data from the pooled AML safety population.
AML, acute myeloid leukemia; d, day(s); DS, differentiation syndrome; N/A, not applicable.
No steroid use for prior event.
Of 9 DS reoccurrences reported in 7 patients (all of which lasted between 5 and 20 days), 7 (77.8%) received steroid treatment for the prior DS event (Table 2). Of these 7 DS reoccurrences, 6 started >10 days after the end of steroid treatment (Table 2).
Hydroxyurea was used in 18 of 76 (23.7%) DS events across all studies, given within a day of DS diagnosis in most cases (12 of 18; 66.7%,), and for duration of ≤20 days in all but 3 DS events (Table 2).
Enasidenib interruption/discontinuation
Of 76 DS events, 46.1% (n = 35) resulted in enasidenib treatment interruption; 4 patients discontinued treatment (Table 3). DS duration was similar regardless of whether interruption of enasidenib treatment was required or not. Of the 38 DS events that did not lead to interruption, the DS duration for 19 events (50.0%) was ≤10 days, and for 19 events (50.0%) was >10 days. Of 35 DS events that led to interruption, the DS duration for 16 events (45.7%) was ≤10 days, and for 19 events (54.3%) was >10 days.
Enasidenib interruption/discontinuation for DS
| . | Duration of DS . | DS severity . | All DS events . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <5 d . | 5-10 d . | 11-20 d . | >20 d . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . | ||
| Number of DS events, n | 4 | 32 | 19 | 21 | 15 | 46 | 13 | 2 | 76 |
| Enasidenib interruption or discontinuation, n (%) | |||||||||
| No | 3 (75.0) | 16 (50.0) | 8 (42.1) | 11 (52.4) | 8 (53.3) | 23 (50.0) | 5 (38.5) | 2 (100.0) | 38 (50.0) |
| Yes | |||||||||
| Interruption | 1 (25.0) | 15 (46.9) | 9 (47.4) | 10 (47.6) | 7 (46.7) | 21 (45.7) | 7 (53.8) | 0 | 35 (46.1) |
| Discontinuation | 0 | 1 (3.1) | 2 (10.5) | 1 (4.8) | 0 | 3 (6.5) | 1 (7.7) | 0 | 4 (5.3) |
| Duration of interruption, n (%) | |||||||||
| <5 d | 1 (25.0) | 4 (12.5) | 1 (5.3) | 0 | 0 | 3 (6.5) | 3 (23.1) | 0 | 6 (7.9) |
| 5-10 d | 0 | 9 (28.1) | 2 (10.5) | 0 | 1 (6.7) | 8 (17.4) | 2 (15.4) | 0 | 11 (14.5) |
| 11-20 d | 0 | 2 (6.3) | 4 (21.1) | 3 (14.3) | 4 (26.7) | 4 (8.7) | 1 (7.7) | 0 | 9 (11.8) |
| >20 d | 0 | 0 | 2 (10.5) | 6 (28.6) | 2 (13.3) | 5 (10.9) | 1 (7.7) | 0 | 8 (10.5) |
| Missing | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (2.2) | 0 | 0 | 1 (1.3) |
| Time from DS start date to dose interruption/discontinuation, n (%) | |||||||||
| Dose stopped before DS event | 0 | 2 (6.3) | 1 (5.3) | 1 (4.8) | 2 (13.3) | 1 (2.2) | 1 (7.7) | 0 | 4 (5.3) |
| 0-1 d | 1 (25.0) | 10 (31.3) | 5 (26.3) | 4 (19.0) | 2 (13.3) | 14 (30.4) | 4 (30.8) | 0 | 20 (26.3) |
| 2-3 d | 0 | 4 (12.5) | 2 (10.5) | 2 (9.5) | 2 (13.3) | 3 (6.5) | 3 (23.1) | 0 | 8 (10.5) |
| ≥4 d | 0 | 0 | 3 (15.8) | 3 (14.3) | 1 (6.7) | 5 (10.9) | 0 | 0 | 6 (7.9) |
| Time from end of prior DS to recurrence of DS, n/N (%) | |||||||||
| No enasidenib interruption∗ | 0 | 0 | 1/3 (33.3) | 3/5 (60.0) | - | - | - | - | 4/9 (44.4) |
| Enasidenib interruption∗ | 0 | 1/1 (100.0) | 2/3 (66.7) | 2/5 (40.0) | - | - | - | - | 5/9 (55.6) |
| . | Duration of DS . | DS severity . | All DS events . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <5 d . | 5-10 d . | 11-20 d . | >20 d . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . | ||
| Number of DS events, n | 4 | 32 | 19 | 21 | 15 | 46 | 13 | 2 | 76 |
| Enasidenib interruption or discontinuation, n (%) | |||||||||
| No | 3 (75.0) | 16 (50.0) | 8 (42.1) | 11 (52.4) | 8 (53.3) | 23 (50.0) | 5 (38.5) | 2 (100.0) | 38 (50.0) |
| Yes | |||||||||
| Interruption | 1 (25.0) | 15 (46.9) | 9 (47.4) | 10 (47.6) | 7 (46.7) | 21 (45.7) | 7 (53.8) | 0 | 35 (46.1) |
| Discontinuation | 0 | 1 (3.1) | 2 (10.5) | 1 (4.8) | 0 | 3 (6.5) | 1 (7.7) | 0 | 4 (5.3) |
| Duration of interruption, n (%) | |||||||||
| <5 d | 1 (25.0) | 4 (12.5) | 1 (5.3) | 0 | 0 | 3 (6.5) | 3 (23.1) | 0 | 6 (7.9) |
| 5-10 d | 0 | 9 (28.1) | 2 (10.5) | 0 | 1 (6.7) | 8 (17.4) | 2 (15.4) | 0 | 11 (14.5) |
| 11-20 d | 0 | 2 (6.3) | 4 (21.1) | 3 (14.3) | 4 (26.7) | 4 (8.7) | 1 (7.7) | 0 | 9 (11.8) |
| >20 d | 0 | 0 | 2 (10.5) | 6 (28.6) | 2 (13.3) | 5 (10.9) | 1 (7.7) | 0 | 8 (10.5) |
| Missing | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (2.2) | 0 | 0 | 1 (1.3) |
| Time from DS start date to dose interruption/discontinuation, n (%) | |||||||||
| Dose stopped before DS event | 0 | 2 (6.3) | 1 (5.3) | 1 (4.8) | 2 (13.3) | 1 (2.2) | 1 (7.7) | 0 | 4 (5.3) |
| 0-1 d | 1 (25.0) | 10 (31.3) | 5 (26.3) | 4 (19.0) | 2 (13.3) | 14 (30.4) | 4 (30.8) | 0 | 20 (26.3) |
| 2-3 d | 0 | 4 (12.5) | 2 (10.5) | 2 (9.5) | 2 (13.3) | 3 (6.5) | 3 (23.1) | 0 | 8 (10.5) |
| ≥4 d | 0 | 0 | 3 (15.8) | 3 (14.3) | 1 (6.7) | 5 (10.9) | 0 | 0 | 6 (7.9) |
| Time from end of prior DS to recurrence of DS, n/N (%) | |||||||||
| No enasidenib interruption∗ | 0 | 0 | 1/3 (33.3) | 3/5 (60.0) | - | - | - | - | 4/9 (44.4) |
| Enasidenib interruption∗ | 0 | 1/1 (100.0) | 2/3 (66.7) | 2/5 (40.0) | - | - | - | - | 5/9 (55.6) |
Data from the pooled AML safety population.
AML, acute myeloid leukemia; d, day(s); DS, differentiation syndrome.
Enasidenib interruption for prior event.
Risk factors for the development of DS
Of the potential baseline risk factors evaluated for DS (any grade), higher levels of white blood cells, PB blasts, BM blasts, and LDH were associated with increased DS risk vs lower levels (12.2%-17.8% vs 4.2%-10.0%; supplemental Table 6). In the final multivariate model, high baseline BM blast counts (baseline BM blast count ≤50% vs >50%; odds ratio [OR], 0.45; 95% CI, 0.23-0.87; P = .0182) was associated with a significantly increased risk of DS across all studies (supplemental Table 7). Multivariate analysis for only grade 3 to 5 DS events showed baseline BM blast count of ≤20% vs >20% (OR, 0.13; 95% CI, 0.02-0.99; P = .0491) and baseline LDH of >1× upper limit of normal (OR, 0.41; 95% CI, 0.19-0.91; P = .0283) were associated with significantly increased risk across all studies (supplemental Table 8).
Correlation with AML clinical response
In the pooled population, the overall response rates (ORRs) in patients with ≥1 DS event vs those with no DS event were 52.2% (n = 35) vs 49.5% (n = 285); CR: 22.4% (n = 15) vs 29.9% (n = 172); CR/CRh: 31.3% (n = 21) vs 31.9% (n = 184; Table 4). Median time to response to CR and ORR between patients with ≥1 DS event and those with no DS event was similar (CR: 3.8 vs 3.7 months; ORR: 2.2 vs 1.9 months; supplemental Table 9). In all patients with ≥1 DS event (n = 67) who achieved CR, CR occurred after onset of the first DS event regardless of what type of treatment they had received.
Correlation of DS events with clinical response
| Population . | AG221-C-001 monotherapy . | AG221-AML-004 monotherapy . | AG221-AML-005 AZA combo∗ . | AG120-221-C-001 7 + 3 combo† . | All studies pooled . | |||
|---|---|---|---|---|---|---|---|---|
| ND AML n = 39 n (%) . | R/R AML n = 280 n (%) . | R/R AML n = 157 n (%) . | ND AML n = 74 n (%) . | ND AML n = 93 n (%) . | R/R AML n = 437 n (%) . | ND AML n = 206 n (%) . | Total AML N = 643 n (%) . | |
| Patients with ≥1 DS event | 5 (12.8) | 33 (11.8) | 14 (8.9) | 13 (17.6) | 2 (2.2) | 47 (10.8) | 20 (9.7) | 67 (10.4) |
| Achieved ORR‡,§ | 0 | 16 (48.5) | 8 (57.1) | 9 (69.2) | 2 (100.0) | 24 (51.1) | 11 (55.0) | 35 (52.2) |
| Before onset of first DS event‡ | 0 | 3 (9.1) | 0 | 1 (7.7) | 0 | 3 (6.4) | 1 (5.0) | 4 (6.0) |
| After onset of first DS event‡ | 0 | 15 (45.5) | 8 (57.1) | 8 (61.5) | 2 (100.0) | 23 (48.9) | 10 (50.0) | 33 (49.3) |
| Achieved CR/CRi‡ | 0 | 8 (24.2) | 2 (14.3) | 6 (46.2) | 1 (50.0) | 10 (21.3) | 7 (35.0) | 17 (25.4) |
| Before onset of first DS event‡ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| After onset of first DS event‡ | 0 | 8 (24.2) | 2 (14.3) | 6 (46.2) | 1 (50.0) | 10 (21.3) | 7 (35.0) | 17 (25.4) |
| Achieved CR/CRp‡ | 0 | 11 (33.3) | 2 (14.3) | 7 (53.8) | 2 (100.0) | 13 (27.7) | 9 (45.0) | 22 (32.8) |
| Before onset of first DS event‡ | 0 | 1 (3.0) | 0 | 0 | 0 | 1 (2.1) | 0 | 1 (1.5) |
| After onset of first DS event‡ | 0 | 10 (30.3) | 2 (14.3) | 6 (46.2) | 2 (100.0) | 12 (25.5) | 8 (40.0) | 20 (29.9) |
| Achieved CR/CRh‡ | NA | 9 (27.3) | 3 (21.4) | 7 (53.8) | 2 (100.0) | 12 (25.5) | NA | NA |
| Before onset of first DS event‡ | NA | 0 | 0 | 0 | 0 | 0 | NA | NA |
| After onset of first DS event‡ | NA | 9 (27.3) | 3 (21.4) | 7 (53.8) | 2 (100.0) | 12 (25.5) | NA | NA |
| Patients with no DS event | 34 (87.2) | 247 (88.2) | 143 (91.1) | 61 (82.4) | 91 (97.8) | 390 (89.2) | 186 (90.3) | 576 (89.6) |
| Achieved ORR§,‖ | 12 (35.3) | 95 (38.5) | 56 (39.2) | 45 (73.8) | 77 (84.6) | 151 (38.7) | 134 (72.0) | 285 (49.5) |
| Achieved CR/CRi‖ | 7 (20.6) | 49 (19.8) | 35 (24.5) | 38 (62.3) | 58 (63.7) | 84 (21.5) | 103 (55.4) | 187 (32.5) |
| Achieved CR/CRp‖ | 8 (23.5) | 61 (24.7) | 35 (24.5) | 36 (59.0) | 57 (62.6) | 96 (24.6) | 101 (54.3) | 197 (34.2) |
| Achieved CR/CRh‖ | NA | 54 (21.9) | 37 (25.9) | 36 (59.0) | 57 (62.6) | 91 (23.3) | NA | NA |
| Population . | AG221-C-001 monotherapy . | AG221-AML-004 monotherapy . | AG221-AML-005 AZA combo∗ . | AG120-221-C-001 7 + 3 combo† . | All studies pooled . | |||
|---|---|---|---|---|---|---|---|---|
| ND AML n = 39 n (%) . | R/R AML n = 280 n (%) . | R/R AML n = 157 n (%) . | ND AML n = 74 n (%) . | ND AML n = 93 n (%) . | R/R AML n = 437 n (%) . | ND AML n = 206 n (%) . | Total AML N = 643 n (%) . | |
| Patients with ≥1 DS event | 5 (12.8) | 33 (11.8) | 14 (8.9) | 13 (17.6) | 2 (2.2) | 47 (10.8) | 20 (9.7) | 67 (10.4) |
| Achieved ORR‡,§ | 0 | 16 (48.5) | 8 (57.1) | 9 (69.2) | 2 (100.0) | 24 (51.1) | 11 (55.0) | 35 (52.2) |
| Before onset of first DS event‡ | 0 | 3 (9.1) | 0 | 1 (7.7) | 0 | 3 (6.4) | 1 (5.0) | 4 (6.0) |
| After onset of first DS event‡ | 0 | 15 (45.5) | 8 (57.1) | 8 (61.5) | 2 (100.0) | 23 (48.9) | 10 (50.0) | 33 (49.3) |
| Achieved CR/CRi‡ | 0 | 8 (24.2) | 2 (14.3) | 6 (46.2) | 1 (50.0) | 10 (21.3) | 7 (35.0) | 17 (25.4) |
| Before onset of first DS event‡ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| After onset of first DS event‡ | 0 | 8 (24.2) | 2 (14.3) | 6 (46.2) | 1 (50.0) | 10 (21.3) | 7 (35.0) | 17 (25.4) |
| Achieved CR/CRp‡ | 0 | 11 (33.3) | 2 (14.3) | 7 (53.8) | 2 (100.0) | 13 (27.7) | 9 (45.0) | 22 (32.8) |
| Before onset of first DS event‡ | 0 | 1 (3.0) | 0 | 0 | 0 | 1 (2.1) | 0 | 1 (1.5) |
| After onset of first DS event‡ | 0 | 10 (30.3) | 2 (14.3) | 6 (46.2) | 2 (100.0) | 12 (25.5) | 8 (40.0) | 20 (29.9) |
| Achieved CR/CRh‡ | NA | 9 (27.3) | 3 (21.4) | 7 (53.8) | 2 (100.0) | 12 (25.5) | NA | NA |
| Before onset of first DS event‡ | NA | 0 | 0 | 0 | 0 | 0 | NA | NA |
| After onset of first DS event‡ | NA | 9 (27.3) | 3 (21.4) | 7 (53.8) | 2 (100.0) | 12 (25.5) | NA | NA |
| Patients with no DS event | 34 (87.2) | 247 (88.2) | 143 (91.1) | 61 (82.4) | 91 (97.8) | 390 (89.2) | 186 (90.3) | 576 (89.6) |
| Achieved ORR§,‖ | 12 (35.3) | 95 (38.5) | 56 (39.2) | 45 (73.8) | 77 (84.6) | 151 (38.7) | 134 (72.0) | 285 (49.5) |
| Achieved CR/CRi‖ | 7 (20.6) | 49 (19.8) | 35 (24.5) | 38 (62.3) | 58 (63.7) | 84 (21.5) | 103 (55.4) | 187 (32.5) |
| Achieved CR/CRp‖ | 8 (23.5) | 61 (24.7) | 35 (24.5) | 36 (59.0) | 57 (62.6) | 96 (24.6) | 101 (54.3) | 197 (34.2) |
| Achieved CR/CRh‖ | NA | 54 (21.9) | 37 (25.9) | 36 (59.0) | 57 (62.6) | 91 (23.3) | NA | NA |
Data from ND or R/R AML populations, and AML safety population.
AML, acute myeloid leukemia; CRi, morphologic CR with incomplete neutrophil recovery; CRp, morphologic CR with incomplete platelet recovery; d, day(s); DS, differentiation syndrome; MLFS, morphologic leukemia-free state; N/A, not available; ND, newly diagnosed; ORR, overall response ratio; PR, partial remission; R/R, relapsed or refractory.
AML, acute myeloid leukemia; d, day(s); DS, differentiation syndrome; ND, newly diagnosed; R/R, relapsed or refractory; ORR, overall response ratio.
Patients were treated with enasidenib (100 mg or 200 mg per day) in combination with azacitidine 75 mg/m2 per day.
Patients were treated with enasidenib (100 mg once daily) in combination with cytarabine (200 mg/m2 per day for 7 days) and either daunorubicin (60 mg/m2 per day for 3 days) or idarubicin (12 mg/m2 per day for 3 days).
Percentage was based on number of patients in the safety AML population with >1 DS event of any grade.
ORR: CR + CRi + CRp + PR + MLFS.
Percentage was based on number of patients in the safety AML population without a DS event.
Discussion
The present analysis assessed DS events based on pooled data from 4 studies in patients with IDH2-mutated AML treated with enasidenib as monotherapy or in combination with azacitidine, or with chemotherapy. Across the 4 studies, the incidence of ≥1 any-grade DS event was 10.4% in patients with ND or R/R IDH2-mutated AML treated with enasidenib. This is in concordance with previously reported DS incidence of ∼10.0% to 20.0% of patients with AML treated with IDH inhibitors.8,10,11,13,15 The incidence of DS was similar in patients with R/R AML (10.8%) and ND AML (9.7%). The similarity in the proportion of DS events among patients in the pooled analysis to previous studies further strengthens our understanding of the likelihood of DS in treated patients.
Among the studies, the incidence of DS was lowest in the chemotherapy combination study (AG120-221-C-001), likely resulting from chemotherapy-induced BM ablation, which precluded rapid differentiation and decreased the burden of leukemic cells. The incidence of DS was similar in both monotherapy arms in patients with R/R AML. Enasidenib plus azacitidine is known to synergically enhance leukemic cell differentiation and apoptosis in vitro.19 Our pooled analysis showed a higher incidence rate of DS in patients with ND AML receiving enasidenib plus azacitidine vs enasidenib alone (17.6% vs 8.9%). Interestingly, the results from the pooled analysis were similar to those observed in the individual study (AG221-AML-005) when comparing enasidenib plus azacitidine vs azacitidine alone in patients with ND AML who were ineligible for intensive chemotherapy.20 Another study reported an incidence rate of 17% in a similar cohort of patients with ND AML treated with ivosidenib (a selective mutant-IDH1 inhibitor) plus azacitidine.21 Similarly, in the phase 3, double-blind AGILE trial (ClinicalTrials.gov identifier: NCT03173248), DS of any grade occurred in 14% (as per investigator judgment) of patients with ND AML receiving ivosidenib plus azacitidine.22 It should be noted that, in the AGILE trial, the DS incidence was 8% among patients receiving azacitidine alone, highlighting the difficulties in making an adequate DS diagnosis, as well as the potential for inducing DS using azacitidine monotherapy. Taken together, enasidenib in combination with azacitidine could induce a stronger effect on driving leukemic cell differentiation and inflammatory response than enasidenib alone, leading to increased risk of DS. An isolated case of DS with azacitidine has been reported.23 Nonetheless, clinicians should be aware of the shared characteristics of side effects with each therapy alone and in combination. Our results are confounded by the modest population size and, therefore, more studies are required to compare the risk of DS with enasidenib alone and in combination with azacitidine or chemotherapy. It should be noted that in the prognostic risk factor modeling, azacitidine was not an independent risk factor for the development of enasidenib-induced DS, thus other factors may require prioritization in order to establish the risk of DS using enasidenib.
Respiratory distress, represented by dyspnea and/or hypoxia and radiologic findings of pulmonary infiltrates, was the most commonly observed manifestation of DS. These results were consistent with previous findings, and with the most common signs and symptoms (>50% of cases) of DS.8,11,13 Heterogeneous manifestations of DS often share similar clinical features with infectious sequelae, cardiac complications, and APL or AML disease progression, which may obscure identification of DS. Increased awareness of signs and symptoms of DS would aid in earlier diagnosis and improvement in the management of patients with IDH1- and IDH2-mutated AML.11,13
Clinical recommendations advise that administration of steroids should be initiated upon first suspicion of DS (eg, dexamethasone 10 mg twice daily), and then tapered until improvement in symptoms of DS.24-26 Concomitant administration of cytoreductive agents, such as hydroxyurea, is recommended when leukocytosis is observed.10 Interruption or discontinuation of IDH inhibition needs to be considered based on the severity of the DS and its initial response to steroids. The data presented in this study, however, are not sufficient to provide guidance on the timing of enasidenib interruption or discontinuation after steroid treatment initiation for DS. In this pooled analysis, the majority of patients (88.1%) received systemic steroids for the treatment of DS. Among 8 patients who did not receive early steroid treatment, there were 2 fatalities, as well as 2 events of grade ≥3 severity. These poor outcomes underscore the need for early initiation of treatment for DS. The approved product label of enasidenib contains a boxed warning highlighting the risk, potentially fatal, of DS as an adverse drug reaction if left untreated. Because of the life-threatening nature of this disorder, prompt recognition and treatment of DS is critical in the prevention of fatal outcomes.8
Approximately half of all DS events resulted in enasidenib treatment interruption within 1 day of DS diagnosis, including in 4 patients who discontinued treatment after a DS event. The duration of DS was the same regardless of whether treatment with enasidenib was stopped or not. Given the extended half-life of enasidenib (∼137 hours), interruption/discontinuation of enasidenib alone is not sufficient to improve symptoms from DS, and high-dose systemic steroids must be administered.27,28
In patients with AML, onset of IDH inhibitor-induced DS can arise weeks to months from treatment initiation.11,13,15,20 The median time to onset of DS with enasidenib (32 days) was similar to a previous report (30 days).11 In addition, the reported median time to onset was longer in the ND AML population vs the R/R AML population (42.5 days vs 32 days, respectively). It is possible that this difference in DS onset could be because of variations in disease and/or patient characteristics between these subgroups.
Findings from a multivariate analysis suggested that higher baseline BM blast counts (>20%) and LDH (>1 upper limit of normal) could potentially increase the risk of DS. Similar potential risk factors of higher baseline BM blasts, PB blasts, and LDH were observed in previous studies with cohorts of patients with APL and AML receiving treatment with enasidenib or ivosidenib.11,13 Collectively, these results suggest that IDH-DS is associated with higher cellular turnover, possibly arising from the rapid myeloid differentiation induced by enasidenib.29 Because of the lack of overall alpha-level control for multiple testing, statistical results should be interpreted with caution and further examination of potential biomarkers of DS are required.
In all patients with ≥1 DS event who achieved CR, CR occurred after onset of the first DS event regardless of what type of treatment they had received (enasidenib as monotherapy or in a combination setting). In all 4 trials with enasidenib, DS typically preceded development of a treatment response or, in some cases, was observed after loss of the initial response after further dose escalation. However, no significant association between experiencing DS and achieving subsequent CR was detected, as in prior studies.
In summary, ∼10% of patients with IDH2-mutated AML treated with enasidenib had ≥1 DS event of any grade across the 4 studies. Incidence of DS was lowest in the chemotherapy combination study (AG120-221-C-001) and was highest in the azacitidine combination study (AG221-AML-005). Respiratory distress, represented by dyspnea and/or hypoxia, and radiologic findings of pulmonary infiltrates, was the most commonly observed manifestation of DS, and most DS cases in the 4 studies were successfully managed with steroids. Taken together, the results of this study suggest that the risk of DS associated with IDH inhibition is manageable, given the benefits of enasidenib treatment in the setting of R/R AML and the effectiveness of corticosteroids to manage DS. These findings highlight the overall safety of enasidenib. Our analyses provide further characterization of DS in a larger data set, and a better understanding of the risk of IDH inhibitor-induced DS, to allow its timely recognition, diagnosis, and management in patients with IDH2-mutated AML in clinical practice.
Acknowledgments
The authors acknowledge Patricia-Riguera Martin for her support during the clinical trial work.
Writing and editorial assistance were provided by Cynthia Li of Ashfield MedComms, an Inizio Company, funded by the study sponsor. The authors had full control of the publication and provided their final approval of all content.
This study was sponsored by Celgene, a Bristol Myers Squibb Company.
Authorship
Contribution: P.M. and A.T.F. contributed equally to the development of this manuscript; P.M., A.T.F., S.D.B., E.S., A.M.Z., and C.D.D. acquired and interpreted data; Y.Z., T.P., C.E.V., I.B., and X.Y. analyzed and interpreted data; and all authors critically reviewed and approved the contents of the manuscript.
Conflict-of-interest disclosure: P.M. serves on the advisory board and speaker bureaus of and reports research support from Bristol Myers Squibb (BMS)/Celgene. A.T.F. received clinical trial support from AbbVie, Agios/Servier, and Celgene/BMS; reports advisory board participation with AbbVie, Agios/Servier, Amgen, Astellas, Blueprint, Celgene/BMS, Daiichi Sankyo, EnClear, Foghorn, Genentech, Immunogen, Kite, Kura Oncology, Mablytics, Menarini, MorphoSys, Novartis, Orum, Pfizer, PureTech, Remix, Rigel, Seattle Genetics, Takeda, and Trillium; and reports consulting for Daiichi Sankyo, Forma, Gilead, Ipsen, Menarini, Remix, and Rigel. S.D.B. reports honoraria from AbbVie, Astellas, BMS, Jazz Pharmaceuticals, Loxo, and Servier; reports a consultancy position with BMS, GlaxoSmithKline, Remix, Servier, and Syndax; reports speakers bureau participation for AbbVie, Astellas, BMS, Jazz Pharmaceuticals, and Servier; reports research funding from Auron and Forma; and reports travel expenses paid by AbbVie and Servier. E.S. received grants and/or contracts from Agios and BMS; and received consulting fees from Agios, BMS, and Servier. A.M.Z. reports membership in Leukemia and Lymphoma Society Scholar in Clinical Research; received research funding (institutional) from AbbVie, Aprea, Astex, Boehringer Ingelheim, Celgene/BMS, Foran, Geron, Incyte, Kura, MedImmune/AstraZeneca, Novartis, Pfizer, Shattuck Labs, and Takeda; serves on advisory boards, and/or had a consultancy with, and received honoraria from, AbbVie, Agios, ALX Oncology, Amgen, Astellas, BeyondSpring, BioCryst, Boehringer Ingelheim, Celgene/BMS, Chiesi, Daiichi Sankyo, Epizyme, Genentech, Geron, Gilead, Incyte, Ionis, Janssen, Jazz Pharmaceuticals, Kura, Mendus, Novartis, Notable, Orum, Pfizer, Regeneron, Schrodinger, Seattle Genetics, Servier, Syndax, Syros, Taiho, Takeda, Tyme, and Zentalis; and serves on clinical trial committees for AbbVie, ALX Oncology, BioCryst, Celgene/BMS, Geron, Gilead, Kura, Novartis, and Syros. C.D.D. received research funding from AbbVie, Astex, Celgene/BMS, Calithera, Immune-Onc, and Loxo; serves as consultant for AbbVie, BMS, GenMab, GlaxoSmithKline, Rigel, Servier, and Schrodinger; received honoraria from AbbVie, Astellas, Celgene/BMS, Daiichi Sankyo, Genentech, Gilead, Jazz Pharmaceuticals, Kura, Novartis, Remix, and Takeda; serves on advisory boards with stock options for Notable Labs; and serves on the data safety monitoring board for GenMab. Y.Z. and C.E.V. are employees of BMS. T.P., I.B., and X.Y. are employees of BMS; and hold stocks with BMS.
Correspondence: Pau Montesinos, Servicio de Hematología y Hemoterapia, Hospital Universitari i Politècnico La Fe, Avenida de Fernando Abril Martorell 106, 46026 Valencia, Spain; email: montesinos_pau@gva.es.
References
Author notes
P.M. and A.T.F. contributed equally to this study.
Bristol Myers Squibb (BMS) shares data from BMS studies that meet their data sharing criteria with qualified researchers who submit a data sharing proposal at http://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
The full-text version of this article contains a data supplement.