Microclots are present in increased number and size in critically ill patients compared with healthy controls and are associated with sepsis.
Microclot levels measured on admission to critical care predict risks of disseminated intravascular coagulation development and mortality.
Visual Abstract
Microclots have been associated with various conditions, including postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. They have been postulated to be amyloid-fibrin(ogen) aggregates, but their role as a prognostic biomarker remains unclear. To examine their possible clinical utility, blood samples were collected for the first 96 hours from critically ill patients (n = 104) admitted to the intensive care unit (ICU). Detection was by staining platelet-poor plasma samples with thioflavin T and visualized by fluorescent microscopy. Image J software was trained to identify and quantify microclots, which were detected in 44 patients (42.3%) on ICU admission but not in the remaining 60 (57.7%) or the 20 healthy controls (0.0%). Microclots on admission to ICU were associated with a primary diagnosis of sepsis (microclots present in sepsis, 23/44 [52.3%] vs microclots absent in sepsis, 19/60 [31.7%]; P = .044). Multicolor immunofluorescence demonstrated that microclots consisted of amyloid-fibrinogen aggregates, which was supported by proteomic analysis. Patients with either a high number or larger-sized microclots had a higher likelihood of developing disseminated intravascular coagulation (odds ratio [OR], 51.4; 95% confidence interval [CI], 6.3-6721.1; P < .001) and had an increased probability of 28-day mortality (OR, 5.3; 95% CI, 2.0-15.6; P < .001). This study concludes that microclots, as defined by amyloid-fibrin(ogen) aggregates, are potentially useful in identifying sepsis and predicting adverse coagulopathic and clinical outcomes.
Introduction
Microclots have been reported in the postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection1 and thrombotic conditions, such as pulmonary embolism.2 They have been detected by staining for amyloid protein and contain fibrin(ogen) as well as other coagulation factors and molecules involved in inflammation.3 Because these amyloid-fibrin(ogen)–containing aggregates are relatively resistant to fibrinolysis, they have been postulated to cause widespread microthrombosis with adverse clinical consequences.4 However, their role as a biomarker of disease or in immunothrombotic pathogenesis remains unclear. This study aims to examine the relevance of microclots, as defined by amyloid-fibrin(ogen) aggregates, in critical illness in which patients often develop coagulation and circulatory disturbances that affect their prognosis.5
Methods
Study design
Blood samples were collected for the first 96 hours (study duration) from patients (n = 104) admitted to the intensive care unit (ICU) at the Royal Liverpool University Hospital between June 2009 and June 2013. Patients were enrolled in accordance with the protocol approved by the National Research Ethics Service Committee North West - Greater Manchester West and Liverpool Central (reference numbers: 07/H1009/64 and 13/NW/0089). Written informed consent or assent from next of kin was obtained. Exclusion criteria were transfers from other ICUs, ICU readmissions within 30 days, or insufficient plasma preserved to perform analysis. Measurements included whole-blood cell counts and coagulation parameters. Age, sex, ethnicity, admission Acute Physiology and Chronic Health Evaluation II scores, daily Sequential Organ Failure Assessment (SOFA) scores, and modified SOFA scores (platelet component removed to avoid bias from thrombocytopenia) were recorded together with 28-day mortality (from ICU admission). Sepsis was originally defined using the American College of Chest Physicians/Society of Critical Care Medicine 2001 international sepsis definition6 and revised to meet Sepsis-3 criteria.7 Disseminated intravascular coagulation (DIC) scoring was performed daily according to International Society for Thrombosis and Haemostasis criteria.8 DIC was diagnosed with a score ≥5 from the platelet count, fibrinogen, D-dimer, and prothrombin time (PT).8 Blood samples were collected from 10 healthy controls recruited in September 2023 (reference number: 16/NW/0170) and 10 historical healthy controls (reference number: 13/NW/0089).
Platelet-poor plasma (PPP) was obtained from venous blood that had been collected into 3.2% sodium citrate tubes without stasis (1 part citrate and 9 parts blood) and centrifuged at 2600g at room temperature (RT) for 10 minutes. PPP was aliquoted and stored at –80°C until analysis.
Microclot detection
On the day of analysis, frozen plasma samples were thawed at 37°C for 10 minutes. Microclots were detected by incubating citrated PPP with thioflavin T (ThT) at 5 μM for 30 minutes at RT, protected from light. Then, 3 μL was smeared on a slide and analyzed by fluorescent microscopy (Olympus IX83; excitation: 467-498 nm; and emission: 513-556 nm). Five representative fields per sample were imaged (magnification ×20) and analyzed using Fiji (ImageJ) Labkit plugin. Software classifier was used to analyze all samples and trained to identify and quantify microclots according to number (count per field) and size (pixels per field). Receiver operating characteristic (ROC) analysis for DIC was used to develop cutoff values to define microclot absence (number <1.0 per field; size <200 pixels per field) and microclot presence (number >1.0 per field; area >200 pixels per field; supplemental Figure 1).
Microclot proteomics
Citrated PPP was diluted with phosphate-buffered saline (volume-to-volume) and centrifuged and washed twice at 20 000g for 30 minutes. Pellets were observed in patients with microclots but not from healthy controls or patients without microclot, and therefore, microclot analysis was performed against a control plasma-derived library. The samples were digested with Trypsin/Lys-C Mix (Promega, Madison, WI) at a 25:1 protein-to-protease ratio (weight-to-weight) overnight at 37°C. Digested microclots from 3 individual ICU patients were processed and subjected to sequential window acquisition of all theoretical fragment ion spectra/data-independent acquisition and data-dependent acquisition (for quality control purposes). Mass spectrometry data were acquired on a TripleTOF 6600 (Sciex) using a 120-minute gradient (bioZen 2.6 um Peptide XB-C18 nano Column, 250 × 0.075 mm, Phenomenex). The sequential window acquisition of all theoretical fragment ion spectra method comprising a total of 100 overlapping windows with variable isolation width spanning a mass-to-charge ratio (m/z) range of 400 to 1500 were acquired for 50 milliseconds. The m/z range for product ion scans was 100 to 1650 with an accumulation time of 33 milliseconds. The total cycle time was 3.7 seconds. Retention time alignment and peptide/protein quantification were performed against a previously acquired control plasma-derived spectral library using version 1.8 of Data-Independent Acquisition by Neural Networks, with a false discovery rate of 1% and with both match between runs and unrelated runs selected (Figure 1).
Microclot analysis methodology. (A) Microclot Quantification: venous blood samples from critically ill patients and healthy controls were collected into sodium citrate tubes. PPP was isolated through centrifugation. PPP was incubated with ThT and the presence of microclots assessed using fluorescence microscopy. Software was trained to quantify microclots. (B) Microclot composition: after centrifugation of PPP, those from critically ill patients with microclots contained visible pellets that were not observed in patients without microclots or in healthy controls. The pellet was digested with trypsin and analyzed by mass spectrometry. (C) Amyloid-fibrin(ogen) colocalization: PPP was incubated with fluorescently labeled anti-fibrinogen antibody and ThT. Colocalization was assessed by fluorescence microscopy.
Microclot analysis methodology. (A) Microclot Quantification: venous blood samples from critically ill patients and healthy controls were collected into sodium citrate tubes. PPP was isolated through centrifugation. PPP was incubated with ThT and the presence of microclots assessed using fluorescence microscopy. Software was trained to quantify microclots. (B) Microclot composition: after centrifugation of PPP, those from critically ill patients with microclots contained visible pellets that were not observed in patients without microclots or in healthy controls. The pellet was digested with trypsin and analyzed by mass spectrometry. (C) Amyloid-fibrin(ogen) colocalization: PPP was incubated with fluorescently labeled anti-fibrinogen antibody and ThT. Colocalization was assessed by fluorescence microscopy.
Microclot composition
To determine whether the ThT-positive aggregates contained fibrinogen, plasma was mixed with AlexaFluor647–labeled anti-fibrinogen β antibody (SantaCruz; 1:100 dilution) for 60 minutes at RT, protected from light. After 60 minutes, ThT was added (final concentration, 5 μM) and incubated for a further 30 minutes. To exclude nonspecific interactions, an AlexaFluor647-labeled isotype control was used (Thermo Fisher; 1:100 dilution). Then, 3 μl was smeared on a slide and analyzed by fluorescent microscopy (Olympus IX83) to assess for colocalization.
Assay reliability
To assess the effect of freeze-thaw and long-term storage on the presence of microclots, samples from 10 healthy donors were analyzed fresh and after storage (at –80°C) for 7 days. Historical healthy control samples (stored at –80°C), recruited simultaneously with the ICU cohort, were also used.
Five microscope fields at ×20 magnification were used after assessment of coefficient of variation (CV) from 1 to 10 fields, which demonstrated no statistically significant improvement on increasing the number of fields above 5 (supplemental Figure 2).
To determine intra-assay and interassay variability, PPP from healthy controls and ICU patients with low and high microclot levels were assessed. Each sample was analyzed in triplicate and repeated on subsequent days. The overall intra-assay CV was 3.2% (range, 0.0%-10.8%) for numbers and 4.5% (range, 0.0%-17.9%) for size, and the interassay CV was 2.5% (range, 0.0%-13.1%) for numbers and 6.3% (range, 0.0%-22.0%) for size. Specifically, the intra-assay and interassay CV was 0.0% in samples in which microclots were absent (normal controls and ICU with low microclot levels). In samples with high levels, the intra-assay CV was 9.8% (range, 8.7%-10.8%) for numbers and 12.4% (range, 6.8%-17.9%) for size, and the interassay CV was 9.3% (range, 5.5%-13.1%) for numbers and 17.0% (range, 12.2%-22.0%) for size.
Statistical analysis
The Kruskal-Wallis test was used for comparison of continuous variables, presented as median (interquartile range). The Fisher exact/χ2 test for comparison of categorical variables were presented as counts (percentage). Microclots were analyzed as continuous variables or categorized. Mann-Whitney U test was used to compare categorical microclot levels with continuous clinical variables. Correlation was assessed using Spearman rank. Receiver operating characteristic curve and multivariate regression (adjusted for age and sex) assessed admission aggregate levels in predicting DIC and 28-day mortality. Statistical tests were performed on SPSS software (IBM, version 29). A 2-tailed P value of <.05 was considered significant.
Results
Microclots are present in critically ill patients and are associated with sepsis
Of 104 ICU patients, microclots were present on admission in 44 (42.3%) but absent in the remaining 60 (57.7%) and healthy controls (0.0%; Figure 2A-D). Microclots were detected in a range of diseases requiring ICU admission, and there was a significant association with sepsis (microclots present in sepsis, 23/44 [52.3%] vs microclots absent in sepsis, 19/60 [31.7%]; P = .044; Figure 2E; Table 1). Microclots were also significantly associated with the development of coagulopathy (Table 1). Approximately half of patients with microclots (21/44) had thrombocytopenia (platelets <150 × 109/L) on admission compared with 12 of 60 (20.0%) without. Platelet counts dropped in those with microclots but remained stable in those without. Their presence was also associated with prolonged PT on admission and reduced fibrinogen levels at 72 hours after admission. Elevated D-dimers were observed regardless of whether microclots were present. Collectively, these changes suggested the possibility of DIC, and the application of the International Society for Thrombosis and Haemostasis score showed that patients with DIC had significantly higher microclot levels (Table 1; number, 2.2 [1.4-4.4]; size, 582 [248-822]) than those without (number, 0.4 [0.1-1.0]; size, 59 [0-190]; P < .001; Figure 2F).
Microclots are associated with critical illness. (A) Microclots were characterized according to number of microclots per field (counts per field). Typical images are presented. (B) The number of microclots (counts per field) were compared between patients on admission to the ICU (n = 104) and paired fresh and frozen (n = 10) and historical (n = 10) normal healthy controls. (C) Microclots were characterized according to size (pixels per field). Typical images are presented. (D) The size of microclots (pixels per field) were compared between patients on admission to the ICU (n = 104) and paired fresh and frozen (n = 10) and historical (n = 10) normal healthy controls. (E) Microclot number and size were compered between ICU patients with nonsepsis (n = 62) and sepsis (n = 42). (F) Microclot number and size were compared between patients diagnosed with DIC (n = 19) and those without DIC (n = 85). Continuous data presented in panels B,D,E-F were analyzed using Mann-Whitney U test.
Microclots are associated with critical illness. (A) Microclots were characterized according to number of microclots per field (counts per field). Typical images are presented. (B) The number of microclots (counts per field) were compared between patients on admission to the ICU (n = 104) and paired fresh and frozen (n = 10) and historical (n = 10) normal healthy controls. (C) Microclots were characterized according to size (pixels per field). Typical images are presented. (D) The size of microclots (pixels per field) were compared between patients on admission to the ICU (n = 104) and paired fresh and frozen (n = 10) and historical (n = 10) normal healthy controls. (E) Microclot number and size were compered between ICU patients with nonsepsis (n = 62) and sepsis (n = 42). (F) Microclot number and size were compared between patients diagnosed with DIC (n = 19) and those without DIC (n = 85). Continuous data presented in panels B,D,E-F were analyzed using Mann-Whitney U test.
Clinical and laboratory characteristics of the ICU patients with and without microclots
| . | Total . | Microclots . | ||||
|---|---|---|---|---|---|---|
| Number R value . | Size R value . | Absent . | Present . | P value . | ||
| Total number, n | 104 | 104 | 104 | 60 | 44 | |
| Age, median (IQR), y | 62.0 (49.0-74.0) | –0.423 | –0.410 | 63.0 (49.0-74.8) | 57.5 (47.5-71.8) | .233 |
| Male, n (%) | 52 (50%) | – | – | 32 (53.3%) | 20 (45.5%) | .552 |
| White ethnicity, n (%) | 95 (91.3%) | – | – | 54 (90%) | 41 (93.2%) | .730 |
| APACHE II score, median (IQR) | 19.0 (14.5-24.0) | 0.279 | 0.249 | 19.0 (14.0-23.8) | 19.5 (16.0-25.5.0) | .566 |
| Admission diagnosis, n (%) | ||||||
| Sepsis | 42 (40.4%) | – | – | 19 (31.7%) | 23 (52.3%) | .044 |
| Trauma | 16 (15.4%) | – | – | 10 (16.7%) | 6 (13.6%) | .787 |
| Cardiovascular | 12 (11.5%) | – | – | 9 (15.0%) | 3 (6.8%) | .231 |
| Respiratory | 16 (15.4%) | – | – | 10 (18.3%) | 6 (13.6%) | .415 |
| Gastrointestinal | 12 (11.5%) | – | – | 8 (16.7%) | 4 (9.1%) | .553 |
| Renal | 3 (2.9%) | – | – | 2 (3.3%) | 1 (2.3%) | 1.000 |
| Central nervous system | 3 (2.9%) | – | – | 2 (3.3%) | 1 (2.3%) | 1.000 |
| Coagulation parameters | ||||||
| Platelets, median (IQR), ×109/L | ||||||
| Admission | 194.0 (129.0-294.3) | –0.526 | –0.527 | 202.0 (156.3-302.3) | 156.0 (65.5-256.8) | .005 |
| 24 h after admission | 206.0 (105.0-260.0) | –0.484 | –0.486 | 214.0 (152.0-270.0) | 162.0 (51.8-249.8) | .012 |
| 48 h after admission | 181.0 (102.3-243.3) | –0.517 | –0.444 | 190.0 (128.5-244.0) | 107.0 (44.0-244.0) | .014 |
| 72 h after admission | 183.0 (99.0-234.0) | –0.539 | –0.515 | 191.0 (127.0-277.0) | 115.5 (50.5-199.5) | .006 |
| PT, median (IQR), s | ||||||
| Admission | 14.6 (13.1-17.0) | 0.597 | 0.542 | 13.7 (12.8-15.6) | 16.2 (13.3-20.8) | .002 |
| 24 h after admission | 14.5 (12.8-16.3) | 0.530 | 0.454 | 13.9 (12.7-15.6) | 15.6 (13.3-20.3) | .007 |
| 48 h after admission | 14.0 (12.2-16.4) | 0.560 | 0.508 | 13.1 (11.9-15.0) | 15.7 (13.0-20.3) | .004 |
| 72 h after admission | 13.6 (12.1-15.7) | 0.523 | 0.469 | 13.4 (11.8-14.4) | 15.2 (12.8-19.3) | .006 |
| aPTT, median (IQR), s | ||||||
| Admission | 32.8 (29.1-40.2) | 0.458 | 0.483 | 30.8 (28.4-37.0) | 35.1 (30.1-44.0) | .038 |
| 24 h after admission | 33.5 (29.1-40.6) | 0.338 | 0.366 | 32.3 (28.3-38.3) | 35.7 (30.1-43.4) | .087 |
| 48 h after admission | 32.2 (28.8-37.9) | 0.387 | 0.366 | 30.8 (28.5-36.0) | 34.9 (29.1-45.8) | .068 |
| 72 h after admission | 31.7 (28.5-37.2) | 0.476 | 0.425 | 30.7 (28.2-36.1) | 34.8 (29.8-42.0) | .042 |
| Fibrinogen, median (IQR), g/L | ||||||
| Admission | 3.7 (2.3-4.9) | –0.332 | –0.164 | 3.7 (2.5-4.8) | 3.8 (1.8-5.4) | .903 |
| 24 h after admission | 4.1 (2.8-5.1) | –0.390 | –0.286 | 4.2 (2.8-5.0) | 3.8 (2.2-5.6) | .512 |
| 48 h after admission | 4.4 (3.2-5.2) | –0.518 | –0.456 | 4.5 (3.6-5.2) | 3.9 (2.9-5.2) | .173 |
| 72 h after admission | 4.4 (3.4-5.4) | –0.565 | –0.514 | 4.8 (3.6-5.5) | 3.7 (2.8-5.0) | .033 |
| D-dimer, median (IQR), ng/mL | ||||||
| Admission | 3969 (1497-9328) | 0.348 | 0.329 | 3490 (1393-7798) | 5 341 (2015-13 456) | .800 |
| 24 h after admission | 4587 (1302-6492) | 0.272 | 0.255 | 3492 (1149-5549) | 5 044 (2 455-7395) | .321 |
| 48 h after admission | 3849 (2540-5974) | 0.418 | 0.445 | 3458 (1950-6887) | 4 221 (2732-6567) | .528 |
| 72 h after admission | 3762 (2348-7084) | 0.569 | 0.664 | 3488 (1972-5636) | 6 036 (2 692-13 734) | .084 |
| Total DIC, n (%) | – | – | – | 1 (1.7%) | 18 (40.9%) | <.001 |
| Time to develop DIC, n (%) | ||||||
| Admission | 8 (7.7%) | – | – | 1 (1.7%) | 7 (15.9%) | .010 |
| 24 h after admission | 4 (3.8%) | – | – | 0 (0.0%) | 4 (9.1%) | .030 |
| 48 h after admission | 3 (2.9%) | – | – | 0 (0.0%) | 3 (6.8%) | .073 |
| 72 h after admission | 4 (3.8%) | – | – | 0 (0.0%) | 4 (9.1%) | .030 |
| Developed DIC ≥24 h after admission, n (%) | 11 (10.6%) | – | – | 0 (0.0%) | 11 (29.7%) | <.001 |
| Organ injury | ||||||
| SOFA score, median (IQR) | ||||||
| Admission | 7.0 (4.0-10.0) | 0.293 | 0.330 | 7.0 (4.0-8.0) | 8.0 (5.0-10.0) | .076 |
| 24 h after admission | 8.0 (5.0-10.0) | 0.437 | 0.448 | 7.0 (4.0-9.0) | 8.0 (7.0-12.0) | .003 |
| 48 h after admission | 7.0 (5.0-9.5) | 0.574 | 0.543 | 6.0 (4.0-8.3) | 9.0 (7.0-13.0) | <.001 |
| 72 h after admission | 7.0 (4.0-10.0) | 0.642 | 0.607 | 5.0 (3.0-8.0) | 10.0 (7.0-12.0) | <.001 |
| Modified SOFA score, median (IQR) | ||||||
| Admission | 6.0 (4.0-9.0) | –0.105 | 0.084 | 6.5 (3.8-8.0) | 6.0 (4.0-9.0) | .393 |
| 24 h after admission | 7.0 (5.0-10.0) | 0.324 | 0.338 | 6.0 (4.0-9.0) | 8.0 (6.0-10.0) | .031 |
| 48 h after admission | 6.0 (4.0-8.0) | 0.486 | 0.449 | 6.0 (4.0-8.0) | 7.0 (6.0-10.0) | .002 |
| 72 h after admission | 6.0 (4.0-8.0) | 0.576 | 0.536 | 5.0 (3.0-7.0) | 7.0 (5.5-10.0) | .001 |
| Mortality, n (%) | 23 (22.1%) | – | – | 6 (10.0%) | 17 (38.6%) | <.001 |
| . | Total . | Microclots . | ||||
|---|---|---|---|---|---|---|
| Number R value . | Size R value . | Absent . | Present . | P value . | ||
| Total number, n | 104 | 104 | 104 | 60 | 44 | |
| Age, median (IQR), y | 62.0 (49.0-74.0) | –0.423 | –0.410 | 63.0 (49.0-74.8) | 57.5 (47.5-71.8) | .233 |
| Male, n (%) | 52 (50%) | – | – | 32 (53.3%) | 20 (45.5%) | .552 |
| White ethnicity, n (%) | 95 (91.3%) | – | – | 54 (90%) | 41 (93.2%) | .730 |
| APACHE II score, median (IQR) | 19.0 (14.5-24.0) | 0.279 | 0.249 | 19.0 (14.0-23.8) | 19.5 (16.0-25.5.0) | .566 |
| Admission diagnosis, n (%) | ||||||
| Sepsis | 42 (40.4%) | – | – | 19 (31.7%) | 23 (52.3%) | .044 |
| Trauma | 16 (15.4%) | – | – | 10 (16.7%) | 6 (13.6%) | .787 |
| Cardiovascular | 12 (11.5%) | – | – | 9 (15.0%) | 3 (6.8%) | .231 |
| Respiratory | 16 (15.4%) | – | – | 10 (18.3%) | 6 (13.6%) | .415 |
| Gastrointestinal | 12 (11.5%) | – | – | 8 (16.7%) | 4 (9.1%) | .553 |
| Renal | 3 (2.9%) | – | – | 2 (3.3%) | 1 (2.3%) | 1.000 |
| Central nervous system | 3 (2.9%) | – | – | 2 (3.3%) | 1 (2.3%) | 1.000 |
| Coagulation parameters | ||||||
| Platelets, median (IQR), ×109/L | ||||||
| Admission | 194.0 (129.0-294.3) | –0.526 | –0.527 | 202.0 (156.3-302.3) | 156.0 (65.5-256.8) | .005 |
| 24 h after admission | 206.0 (105.0-260.0) | –0.484 | –0.486 | 214.0 (152.0-270.0) | 162.0 (51.8-249.8) | .012 |
| 48 h after admission | 181.0 (102.3-243.3) | –0.517 | –0.444 | 190.0 (128.5-244.0) | 107.0 (44.0-244.0) | .014 |
| 72 h after admission | 183.0 (99.0-234.0) | –0.539 | –0.515 | 191.0 (127.0-277.0) | 115.5 (50.5-199.5) | .006 |
| PT, median (IQR), s | ||||||
| Admission | 14.6 (13.1-17.0) | 0.597 | 0.542 | 13.7 (12.8-15.6) | 16.2 (13.3-20.8) | .002 |
| 24 h after admission | 14.5 (12.8-16.3) | 0.530 | 0.454 | 13.9 (12.7-15.6) | 15.6 (13.3-20.3) | .007 |
| 48 h after admission | 14.0 (12.2-16.4) | 0.560 | 0.508 | 13.1 (11.9-15.0) | 15.7 (13.0-20.3) | .004 |
| 72 h after admission | 13.6 (12.1-15.7) | 0.523 | 0.469 | 13.4 (11.8-14.4) | 15.2 (12.8-19.3) | .006 |
| aPTT, median (IQR), s | ||||||
| Admission | 32.8 (29.1-40.2) | 0.458 | 0.483 | 30.8 (28.4-37.0) | 35.1 (30.1-44.0) | .038 |
| 24 h after admission | 33.5 (29.1-40.6) | 0.338 | 0.366 | 32.3 (28.3-38.3) | 35.7 (30.1-43.4) | .087 |
| 48 h after admission | 32.2 (28.8-37.9) | 0.387 | 0.366 | 30.8 (28.5-36.0) | 34.9 (29.1-45.8) | .068 |
| 72 h after admission | 31.7 (28.5-37.2) | 0.476 | 0.425 | 30.7 (28.2-36.1) | 34.8 (29.8-42.0) | .042 |
| Fibrinogen, median (IQR), g/L | ||||||
| Admission | 3.7 (2.3-4.9) | –0.332 | –0.164 | 3.7 (2.5-4.8) | 3.8 (1.8-5.4) | .903 |
| 24 h after admission | 4.1 (2.8-5.1) | –0.390 | –0.286 | 4.2 (2.8-5.0) | 3.8 (2.2-5.6) | .512 |
| 48 h after admission | 4.4 (3.2-5.2) | –0.518 | –0.456 | 4.5 (3.6-5.2) | 3.9 (2.9-5.2) | .173 |
| 72 h after admission | 4.4 (3.4-5.4) | –0.565 | –0.514 | 4.8 (3.6-5.5) | 3.7 (2.8-5.0) | .033 |
| D-dimer, median (IQR), ng/mL | ||||||
| Admission | 3969 (1497-9328) | 0.348 | 0.329 | 3490 (1393-7798) | 5 341 (2015-13 456) | .800 |
| 24 h after admission | 4587 (1302-6492) | 0.272 | 0.255 | 3492 (1149-5549) | 5 044 (2 455-7395) | .321 |
| 48 h after admission | 3849 (2540-5974) | 0.418 | 0.445 | 3458 (1950-6887) | 4 221 (2732-6567) | .528 |
| 72 h after admission | 3762 (2348-7084) | 0.569 | 0.664 | 3488 (1972-5636) | 6 036 (2 692-13 734) | .084 |
| Total DIC, n (%) | – | – | – | 1 (1.7%) | 18 (40.9%) | <.001 |
| Time to develop DIC, n (%) | ||||||
| Admission | 8 (7.7%) | – | – | 1 (1.7%) | 7 (15.9%) | .010 |
| 24 h after admission | 4 (3.8%) | – | – | 0 (0.0%) | 4 (9.1%) | .030 |
| 48 h after admission | 3 (2.9%) | – | – | 0 (0.0%) | 3 (6.8%) | .073 |
| 72 h after admission | 4 (3.8%) | – | – | 0 (0.0%) | 4 (9.1%) | .030 |
| Developed DIC ≥24 h after admission, n (%) | 11 (10.6%) | – | – | 0 (0.0%) | 11 (29.7%) | <.001 |
| Organ injury | ||||||
| SOFA score, median (IQR) | ||||||
| Admission | 7.0 (4.0-10.0) | 0.293 | 0.330 | 7.0 (4.0-8.0) | 8.0 (5.0-10.0) | .076 |
| 24 h after admission | 8.0 (5.0-10.0) | 0.437 | 0.448 | 7.0 (4.0-9.0) | 8.0 (7.0-12.0) | .003 |
| 48 h after admission | 7.0 (5.0-9.5) | 0.574 | 0.543 | 6.0 (4.0-8.3) | 9.0 (7.0-13.0) | <.001 |
| 72 h after admission | 7.0 (4.0-10.0) | 0.642 | 0.607 | 5.0 (3.0-8.0) | 10.0 (7.0-12.0) | <.001 |
| Modified SOFA score, median (IQR) | ||||||
| Admission | 6.0 (4.0-9.0) | –0.105 | 0.084 | 6.5 (3.8-8.0) | 6.0 (4.0-9.0) | .393 |
| 24 h after admission | 7.0 (5.0-10.0) | 0.324 | 0.338 | 6.0 (4.0-9.0) | 8.0 (6.0-10.0) | .031 |
| 48 h after admission | 6.0 (4.0-8.0) | 0.486 | 0.449 | 6.0 (4.0-8.0) | 7.0 (6.0-10.0) | .002 |
| 72 h after admission | 6.0 (4.0-8.0) | 0.576 | 0.536 | 5.0 (3.0-7.0) | 7.0 (5.5-10.0) | .001 |
| Mortality, n (%) | 23 (22.1%) | – | – | 6 (10.0%) | 17 (38.6%) | <.001 |
Microclot absent: number <1.0 per field and size <200 pixels per field; microclot present: number >1.0 per field and/or area >200 pixels per field. R value represents correlation with aggregate presence or absence performed using Spearman rank correlation. P value of comparison of patients with absent vs presence of amyloid-fibrin(ogen) aggregates, calculated using Mann-Whitney U test for continuous variables and χ2 test for categorical variables.
APACHE II, Acute Physiology and Chronic Health Evaluation II; aPTT, activated partial thromboplastin time; IQR, interquartile range.
Characterization of microclots isolated from critically ill patients demonstrate amyloid-fibrin(ogen) aggregates
To assess microclot composition, proteomic analysis was performed. Centrifugation of ICU patient plasma with microclots resulted in a visible pellet, which was not observed in healthy controls or ICU patients without microclots. A similar observation has been made previously between patients with and without detectable microclots.4 The pellet stained strongly when incubated with ThT, confirming the presence of microclots. Mass spectrometry analysis revealed consistent components of the microclots (Figure 3A), including a predominance of fibrinogen-α chain (Figure 3B), fibrinogen-β chain (Figure 3C), fibrinogen-γ chain (Figure 3D), inflammatory molecules, and lipoproteins, when compared with a control plasma-derived library. To further confirm that microclots were amyloid-fibrinogen aggregates, a fluorescently labeled fibrinogen-β chain antibody identifying the same protein sequence obtained from proteomics was used alongside amyloid staining. Multicolor immunofluorescence microscopy confirmed colocalization of amyloid and fibrinogen (Figure 4; supplemental Figure 3), which was not observed in the isotype control (supplemental Figure 4).
Proteomic analysis of microclots from critically ill patients. (A) Trypsin-digested microclots from 3 individual ICU patients were analyzed by sequential window acquisition of all theoretical fragment ion spectra–mass spectrometry (SWATH-MS). The 20 proteins with the highest relative SWATH-MS signal intensities across all 3 patients (total) were identified and represented for each individual critically ill patient and compared against a control plasma-derived library. Representative MS/MS spectra of peptides identified by both data-dependent acquisition and SWATH are shown for fibrinogen-α chain (B), -β chain (C), and -γ chain (D). ALB, albumin; APOA1, apolipoprotein A-I; C5, complement C5; CILP2, cartilage intermediate layer protein 2; FGB, fibrinogen beta chain; FSIP2, Fibrous sheath-interacting protein 2; GSTP1, glutathione S-transferase P; HRG, histidine-rich glycoprotein; KIF4A, chromosome-associated kinesin KIF4A; IGHA1, immunoglobulin heavy constant alpha 1; ILF3, interleukin enhancer-binding factor 3; MSN, moesin; POSTN, periostin; SAA2, serum amyloid A-2 protein; SERPINA1, alpha-1-antitrypsin; TGFBI, transforming growth factor-beta–induced protein ig-h3.
Proteomic analysis of microclots from critically ill patients. (A) Trypsin-digested microclots from 3 individual ICU patients were analyzed by sequential window acquisition of all theoretical fragment ion spectra–mass spectrometry (SWATH-MS). The 20 proteins with the highest relative SWATH-MS signal intensities across all 3 patients (total) were identified and represented for each individual critically ill patient and compared against a control plasma-derived library. Representative MS/MS spectra of peptides identified by both data-dependent acquisition and SWATH are shown for fibrinogen-α chain (B), -β chain (C), and -γ chain (D). ALB, albumin; APOA1, apolipoprotein A-I; C5, complement C5; CILP2, cartilage intermediate layer protein 2; FGB, fibrinogen beta chain; FSIP2, Fibrous sheath-interacting protein 2; GSTP1, glutathione S-transferase P; HRG, histidine-rich glycoprotein; KIF4A, chromosome-associated kinesin KIF4A; IGHA1, immunoglobulin heavy constant alpha 1; ILF3, interleukin enhancer-binding factor 3; MSN, moesin; POSTN, periostin; SAA2, serum amyloid A-2 protein; SERPINA1, alpha-1-antitrypsin; TGFBI, transforming growth factor-beta–induced protein ig-h3.
Amyloid aggregates colocalize with fibrin(ogen). Multicolor immunofluorescence microscopy of PPP from a healthy control and critically patients (n = 3) costained with ThT (left) and anti-fibrinogen–AlexaFluor647 antibody (middle). Colocalization is demonstrated in the merged image (right); magnification 60×.
Amyloid aggregates colocalize with fibrin(ogen). Multicolor immunofluorescence microscopy of PPP from a healthy control and critically patients (n = 3) costained with ThT (left) and anti-fibrinogen–AlexaFluor647 antibody (middle). Colocalization is demonstrated in the merged image (right); magnification 60×.
Microclots predict DIC development and mortality
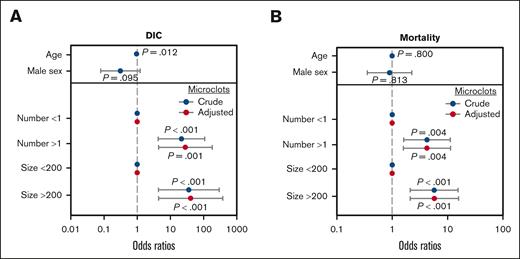
To examine whether microclot detection could predict DIC development, patients with DIC on ICU admission were excluded (8/104 [7.7%]). Patients with microclots on ICU admission had significantly (P < .001) increased risk of developing DIC (11/37 [29.7%]) compared with patients without (0/59 [0.0%]). Univariate analysis demonstrated that patients with microclots had an increased probability of developing DIC (odds ratio, 51.4; 95% confidence interval, 6.3-6721.1; P < .001), and multivariate analysis (adjusted for age and sex) demonstrated that microclots independently predicted DIC development (Figure 5A; Supplemental Table 1).
Presence of microclots is an independent predictor of DIC and mortality in critically ill patients. (A) Multivariate analysis of crude and adjusted odds ratios (with patients adjusted for age and sex). The presence of microclots, as defined by microclot number (>1 per field) or size (>200 pixels per field), were independently associated with DIC development. (B) Multivariate analysis of crude and adjusted odds ratios (with patients adjusted for age and sex). The presence of microclots were independently associated with 28-day mortality.
Presence of microclots is an independent predictor of DIC and mortality in critically ill patients. (A) Multivariate analysis of crude and adjusted odds ratios (with patients adjusted for age and sex). The presence of microclots, as defined by microclot number (>1 per field) or size (>200 pixels per field), were independently associated with DIC development. (B) Multivariate analysis of crude and adjusted odds ratios (with patients adjusted for age and sex). The presence of microclots were independently associated with 28-day mortality.
DIC is often associated with the multiorgan dysfunction syndrome, and assessment using the SOFA and modified SOFA scores showed that microclots were associated with the development of organ dysfunction 24 hours after ICU admission and remained significant throughout the study duration (Table 1). The presence of microclots also significantly (P < .001) increased the risk of mortality (17/44 [38.6%]) compared with those without (6/60 [10%]). Univariate analysis demonstrated that patients with microclots had an increased probability of 28-day mortality (odds ratio, 5.3; 95% confidence interval, 2.0-15.6; P < .001). Multivariate analysis (adjusted for age and sex) demonstrated that microclots independently predicted 28-day mortality (Figure 5B; supplemental Table 1).
Discussion
Microclots, as defined by amyloid-fibrin(ogen) aggregates, have been detected in various conditions, and their targeted removal has been proposed.9 However, their role in disease pathogenesis remains unclear. Our study was not designed to address disease causation but aimed to objectively clarify the clinical implications of their detection. To our knowledge, our findings are the first to demonstrate their potential as a predictive biomarker in an ICU setting.
These findings of clinically relevant associations do not indicate causality between detection of microclots and development of hemostatic and organ dysfunction. Further research is needed to define their pathophysiological relevance in vivo and understand how and why microclots form. There are several potential pathogen- and host-related mechanisms of amyloid-fibrin(ogen) aggregate formation in critically ill patients. For example, lipopolysaccharide10 and the acute phase reactant, serum amyloid A,11 can bind and alter the structure of fibrinogen to potentiate aggregate formation, which could be consistent with our findings in sepsis. The presence of amyloid and other molecules involved in inflammation, innate immune activation, and coagulation could lead to better understanding of immunothrombosis and the host response to critical illness.12
Microclots are poorly understood in terms of their composition, mechanism of formation, relationship to blood coagulation, and clinical relevance.12,13 Pretorius et al4 demonstrated microclots in patients with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection, which appeared similar to the findings of Baker et al,2 in which they demonstrated fibrin aggregates in patients with pulmonary embolism. Our findings through proteomic and immunofluorescent analyses demonstrate that microclots contain fibrin(ogen) in an ICU cohort and may provide a link to these other independent observations.
A limitation of this study is that the number of patients from a single ICU do not enable reliable information on patients without sepsis. Larger, multicentre, cohort studies and in different conditions are needed. Samples have also been frozen for a period, but we ensured that microclot formation was not due to freeze-thaw or prolonged storage. Assessment of paired fresh and frozen controls (n = 10), along with historical controls (n = 10) obtained concurrently with the ICU samples, did not detect microclots (Figure 2A-D), but we cannot entirely exclude a possible effect of prolonged storage of samples from critically ill patients as opposed to healthy controls. A further but more generic limitation is the lack of universally accepted nomenclature for microclots. Other terms used include fibrinaloid microclots14 and amyloid-fibrin(ogen) particles.15 Central to the discussion is the definition of a clot, which by strict definition, requires soluble fibrinogen to have been cleaved by thrombin into insoluble fibrin.16 Although we have demonstrated amyloid-fibrin(ogen) colocalization, there is no conclusive evidence that fibrin is always present in microclots. However, reports of relative fibrinolytic resistance4 offer fresh insight into the biochemical nature and biophysical effects of molecular structures with potential vascular occlusive effects.
Another limitation due to the sample size is the ability to conduct multivariate analysis on the extent to which microclots, as a standalone biomarker, predict DIC and adverse outcomes independent of parameters currently used in DIC scoring, for example, platelet count, PT, fibrinogen, and D-dimer. Currently, there is no single test that can accurately diagnose DIC and no clinically available assay to predict DIC development.17 In this cohort, all patients who developed DIC after ICU admission had detectable microclots on admission. Although a number of patients with microclots did not develop DIC, the assay has a very high negative predictive value of 100%. This could therefore serve as a rule-out test and influence triaging of care in critically ill patients. Future prospective studies in larger patient cohorts are required to extend and validate upon the findings described here and clarify the clinical utility of microclot detection in the care pathway.
In conclusion, we believe that our novel clinical observations advance the field by demonstrating the prognostic relevance of microclots in the ICU setting. There is also technological advancement through integration of computer learning to objectively calculate microclot number and size in a standardizable and reproducible manner. This would lead to better validation of results and meaningful comparisons between studies to improve the robustness of evidence generation. Overall, we believe that this is an important step forward in the potential utility of this assay as a clinically useful and practically measurable biomarker to identify sepsis and predict DIC and mortality in the ICU.
Acknowledgments
The authors thank all the patients, their families, and staff involved in this study. The authors also thank Colin Downey for his advice and constructive discussions, along with Lance Turtle for providing access to plasma from normal healthy controls. The authors acknowledge the use of the Centre for Drug Safety Science Bioanalytical Facility provided by Liverpool Shared Research Facilities, Faculty of Health and Life Sciences, University of Liverpool.
This study is funded by the Liverpool University Hospitals NHS Foundation Trust (LUHFT) and the Department of Health and Social Care (DHSC) and is supported by the National Institute for Health Research (NIHR) (NIHR135073). The views expressed in this study are those of the authors and not necessarily those of LUHFT, NIHR, or the DHSC.
Authorship
Contribution: J.S. performed experiments; S.T.A. and J.S. analyzed the data, assisted by S.L.; R.J. performed mass spectrometry and analyzed raw data; J.S., S.T.A., G.W., and C.-H.T. wrote, edited, and reviewed the manuscript and figures; and S.T.A., G.W., and C.-H.T. designed and supervised the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cheng-Hock Toh, Department of Clinical Infection, Microbiology and Immunology, University of Liverpool, Ronald Ross Building, 8 West Derby St, Liverpool L69 7BE, United Kingdom; email: toh@liverpool.ac.uk.
References
Author notes
The data presented in this study are available on reasonable request from the corresponding author, Cheng-Hock Toh (toh@liverpool.ac.uk).
The full-text version of this article contains a data supplement.