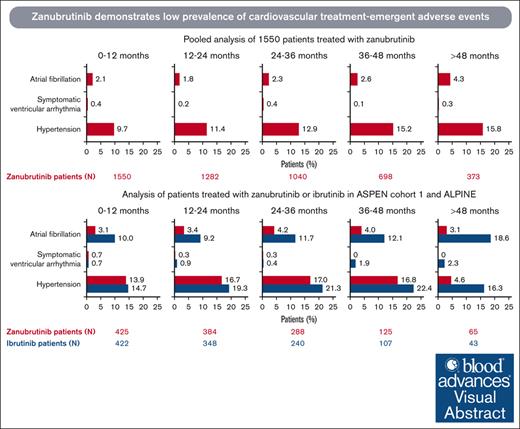
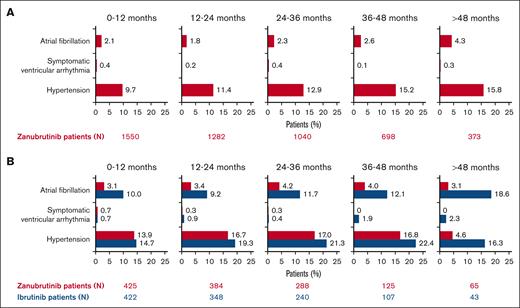
Incidences of atrial fibrillation, symptomatic (grade ≥2) ventricular arrhythmia, and hypertension are generally low with zanubrutinib.
These data support the safer cardiovascular profile of zanubrutinib compared with ibrutinib for patients with B-cell malignancies.
Visual Abstract
First-generation Bruton tyrosine kinase (BTK) inhibitor, ibrutinib, has been associated with an increased risk of cardiovascular toxicities. Zanubrutinib is a more selective, next-generation BTK inhibitor. In this analysis, incidence rates of atrial fibrillation, symptomatic (grade ≥2) ventricular arrhythmia, and hypertension were evaluated in a pooled analysis of 10 clinical studies with zanubrutinib monotherapy in patients (N = 1550) with B-cell malignancies and a pooled analysis of head-to-head studies comparing zanubrutinib with ibrutinib (ASPEN cohort 1; ALPINE). Among the 10 studies, most patients (median age, 67 years) were male (66.3%) and had CLL/SLL (60.5%). Overall incidence and exposure-adjusted incidence rates (EAIR) for atrial fibrillation, symptomatic ventricular arrhythmia, and hypertension were lower with zanubrutinib than ibrutinib. Despite a similar prevalence of preexisting cardiovascular events in ASPEN and ALPINE, atrial fibrillation/flutter incidence rates (6.1% vs 15.6%) and EAIR (0.2 vs 0.64 persons per 100 person-months; P < .0001) were lower with zanubrutinib than with ibrutinib. Symptomatic ventricular arrhythmia incidence was low for both zanubrutinib (0.7%) and ibrutinib (1.7%) with numerically lower EAIR (0.02 vs 0.06 persons per 100 person-months, respectively) for zanubrutinib. The hypertension EAIR was lower with zanubrutinib than ibrutinib in ASPEN but similar between treatment arms in ALPINE. The higher hypertension EAIR in ALPINE was inconsistent with other zanubrutinib studies. However, fewer discontinuations (1 vs 14) and deaths (0 vs 6) due to cardiac disorders occurred with zanubrutinib versus ibrutinib in ALPINE. These data support zanubrutinib as a treatment option with improved cardiovascular tolerability compared with ibrutinib for patients with B-cell malignancies in need of BTK inhibitors. These trials were registered at www.ClinicalTrials.gov as # NCT03053440, NCT03336333, NCT03734016, NCT04170283, NCT03206918, NCT03206970, NCT03332173, NCT03846427, NCT02343120, and NCT03189524.
Introduction
Bruton tyrosine kinase (BTK) is an essential component of signaling pathways regulating B-cell proliferation and survival. In addition to controlling many aspects of cellular function, BTK has been shown to play a crucial role in oncogenic signaling in B-cell malignancies. The advent of ibrutinib, a first-generation covalent small-molecule BTK inhibitor, has transformed the treatment landscape for diseases such as chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), mantle cell lymphoma (MCL), and Waldenström macroglobulinemia (WM).1 Even in patients with previously treated CLL/SLL and molecular markers associated with poor prognoses, ibrutinib demonstrated efficacy.2 However, ibrutinib has been associated with cardiovascular toxicities.3-8 Grade ≥3 atrial fibrillation/flutter has been reported in 4% to 12% of patients treated with ibrutinib (as monotherapy or part of a combination), including patients with treatment-naive (TN) or relapsed/refractory (R/R) CLL/SLL,9-12 MCL,4 or WM.3 Ibrutinib has also been associated with hypertension and life-threatening ventricular arrhythmia.8,13-15 Reports of severe and occasionally fatal ibrutinib-associated cardiovascular complications underscore the need for safer BTK inhibitors.15 Given the need for the long-term use of these agents in patients, maximizing safety is essential.
Although the mechanism underlying BTK inhibitor–associated cardiovascular toxicities is unclear, off-target inhibition of other kinases, such as C-terminal SRC kinase and TEC protein kinase, and disruption of downstream phosphoinositide 3-kinase-Akt signaling may contribute to these effects.16-21 Zanubrutinib is a covalent, irreversible, next-generation BTK inhibitor specifically designed to maximize BTK occupancy and minimize off-target inhibition. In vitro, zanubrutinib inhibits fewer off-target kinases than ibrutinib (>50% inhibition of 7 vs 17 targets, respectively)22 and shows less inhibition of C-terminal SRC–, TEC protein–, and epidermal growth factor receptor–family kinases.23,24 Multiple global clinical studies have demonstrated that zanubrutinib is well tolerated and has favorable efficacy outcomes in patients with B-cell malignancies, which has led to the approval of zanubrutinib for 5 different indications.25,26
Here, in aiming to better understand the cardiovascular safety profile of zanubrutinib, we report an analysis of cardiovascular toxicities, including atrial fibrillation, in 2 phase 3 studies comparing zanubrutinib with ibrutinib in patients with WM (ASPEN cohort 1) or R/R CLL/SLL (ALPINE) and a pooled analysis of 10 studies of zanubrutinib monotherapy in patients with B-cell malignancies.
Methods
Objectives
The primary objective of these analyses was to characterize the atrial fibrillation, symptomatic ventricular arrhythmia, and hypertension profile of zanubrutinib. A secondary objective was to compare the ibrutinib profile, including incidence rates and exposure-adjusted incidence rates (EAIR). Data were assessed from a pooled analysis of 10 clinical studies on zanubrutinib and 2 head-to-head studies comparing zanubrutinib with ibrutinib (ASPEN cohort 1 and ALPINE).
Data source
Two randomized phase 3 studies of zanubrutinib vs ibrutinib were pooled and analyzed for direct comparison of cardiac disorders: ASPEN (cohort 1, patients with MYD88L265P-mutated WM)27 and ALPINE (patients with R/R CLL/SLL),28,29 hereafter referred to as ASPEN/ALPINE. In addition, 10 clinical studies (supplemental Table 1) on patients with B-cell malignancies who received zanubrutinib monotherapy at a dose of 160 mg twice daily or 320 mg once daily, including ASPEN and ALPINE, were pooled and descriptively analyzed. All pooled analyses are post hoc.
Case definitions
In this analysis, treatment-emergent atrial fibrillation/flutter, symptomatic ventricular arrhythmias, and hypertension were assessed. Treatment-emergent adverse event were defined as the occurrence of a new event or worsening of an existing event from baseline, regardless of causality from the date of the first dose of zanubrutinib or ibrutinib to the date of the last dose plus 30 days or the start of new anticancer therapies, whichever occurred earlier. A worsening of a treatment-emergent adverse event to grade 5 more than 30 days after the last dose of zanubrutinib or ibrutinib was considered a treatment-emergent adverse event. Cardiovascular events included atrial fibrillation/flutter, ventricular arrhythmia, and hypertension; cardiac disorders included any event in the Medical Dictionary for Regulatory Activities (MedDRA) system organ class for cardiac disorders. Atrial fibrillation/flutter included the preferred terms for atrial fibrillation and atrial flutter. Ventricular arrhythmia included any event in the Standardized MedDRA Queries of ventricular tachyarrhythmias (narrow) and the MedDRA High-Level term of ventricular arrhythmias and cardiac arrest. Cardiac arrest in the context of COVID-19 was excluded. Symptomatic ventricular arrhythmias were defined as grade ≥2 ventricular arrhythmia events per Common Terminology Criteria for Adverse Events. Hypertension included any event coded as hypertension (Standardized MedDRA Queries narrow).
Medical history and exclusion criteria
Medical history of atrial fibrillation/flutter, ventricular arrhythmia, and hypertension was assessed at the time of enrollment and before treatment with zanubrutinib or ibrutinib using MedDRA v24.0. Patients with active, clinically significant cardiovascular disease, including uncontrolled arrhythmia, class 3 or 4 congestive heart failure as defined by the New York Heart Association functional classification, QT corrected by Fridericia formula (QTcF) prolongation (>480 milliseconds), or a history of myocardial infarction within 6 months of screening, were not eligible to enroll in ASPEN or ALPINE; however, patients with controlled atrial fibrillation were eligible for enrollment. Additional exclusion criteria included active clinically significant electrocardiogram abnormalities in ASPEN, a history of clinically significant arrhythmias, a history of Mobitz II second-degree or third-degree heart block without a permanent pacemaker in place, or uncontrolled hypertension (2 consecutive measurements showing systolic blood pressure >170 mm Hg and diastolic blood pressure >105 mm Hg) in ALPINE.
Statistical analysis
All analyses were conducted with the safety population, defined as all patients who received at least 1 dose of the study treatment. The number and proportion of patients who had an event of interest were summarized and compared using the χ2 test. The prevalence of each event was plotted in every 12-month intervals, and the time to the first onset of each event was analyzed using the Kaplan-Meier method. The EAIR, an average event count per unit of person-time, was calculated as the number of patients having the treatment-emergent adverse event of interest divided by the total exposure time. The total exposure time was the time from the first dose to the first event (or last dose plus 30 days if there was no event), converted to 100 person-months unit of time. The Poisson regression model was used to compare EAIR between the treatment arms, with the number of patients having events as the dependent variable and log(exposure time) as the offset. All statistical tests were 2-sided with P < .05 used to identify significance; there were no adjustments for multiple comparisons.
Correlation analyses were conducted in each treatment arm of the ASPEN/ALPINE populations and the total pooled zanubrutinib population by constructing 2 × 2 tables to represent the frequencies of hypertension and atrial fibrillation and subsequently computing the kappa statistic to measure the correlation between these 2 events.
Furthermore, in the pooled ASPEN/ALPINE treatment populations, logistic regression modeling was used to examine the predictive capabilities of hypertension, treatment, and the potential interaction between hypertension and treatment in determining atrial fibrillation status.
All clinical studies were performed following the Good Clinical Practice per International Conference on Harmonization Guideline E6 requirements under the ethical principles of the Declaration of Helsinki. Study conduct followed the guidance of each site's institutional review board, independent ethics committee, and regulatory authorities. All patients provided written informed consent before study enrollment.
Results
Patient and baseline characteristics
A pooled analysis of 10 clinical trials with zanubrutinib monotherapy involving 1550 patients with various B-cell malignancies was conducted to evaluate cardiovascular safety. The median age was 67 years (range, 20-95), 950 of the 1550 (61.3%) patients were aged ≥65 years, 1027 (66.3%) were male, and 1033 (66.6%) were White. Most patients had CLL/SLL (n = 938; 60.5%); of these, 525 patients had R/R CLL/SLL, and 413 patients had TN CLL/SLL (Table 1). Randomized phase 3 studies, ASPEN cohort 1 and ALPINE, were also analyzed separately to compare cardiac complications in the zanubrutinib and ibrutinib arms. In ASPEN cohort 1, 101 patients with WM and MYD88L265P received zanubrutinib, and 98 patients received ibrutinib; in ALPINE, 324 patients with R/R CLL/SLL received zanubrutinib, and 324 received ibrutinib. Patient characteristics in the pooled ASPEN/ALPINE were overall balanced between the zanubrutinib and ibrutinib arms. The median age was 68 years (range, 35-90) in both arms, 265 of 425 (62.4%) vs 274 of 422 (64.9%) patients aged ≥65 years, 280 of 425 (65.9%) vs 295 of 422 (69.9%) were male, and 348 of 425 (81.9%) vs 357 of 422 (84.6%) were White in the zanubrutinib vs ibrutinib arms, respectively (Table 1).
Baseline demographics and clinical characteristics
| Characteristic . | Pooled ASPEN cohort 1 and ALPINE (ASPEN/ALPINE) . | Pooled all patients treated with Zanubrutinib (N = 1550) . | |
|---|---|---|---|
| Zanubrutinib (N = 425) . | Ibrutinib (N = 422) . | ||
| Median age (range), y | 68 (35-90) | 68 (35-90) | 67 (20-95) |
| Age group, n (%) | |||
| ≥60 y | 331 (77.9) | 347 (82.2) | 1186 (76.5) |
| ≥65 y | 265 (62.4) | 274 (64.9) | 950 (61.3) |
| ≥65 and ˂75 y | 155 (36.5) | 181 (42.9) | 615 (39.7) |
| ≥75 y | 110 (25.9) | 93 (22.0) | 335 (21.6) |
| Sex, n (%) | |||
| Male | 280 (65.9) | 295 (69.9) | 1027 (66.3) |
| Female | 145 (34.1) | 127 (30.1) | 523 (33.7) |
| Race, n (%) | |||
| White | 348 (81.9) | 357 (84.6) | 1032 (66.6) |
| Asian | 49 (11.5) | 44 (10.4) | 424 (27.4) |
| Not reported | 17 (4.0) | 17 (4.0) | 42 (2.7) |
| Other | 11 (2.6) | 4 (0.9) | 51 (3.3) |
| Missing | 0 | 0 | 1 (0.1) |
| Geographic region, n (%)∗ | |||
| Europe | 259 (60.9) | 250 (59.2) | 551 (35.5) |
| Australia/New Zealand | 60 (14.1) | 60 (14.2) | 414 (26.7) |
| Asia | 45 (10.6) | 43 (10.2) | 406 (26.2) |
| North America | 61 (14.4) | 69 (16.4) | 179 (11.5) |
| Median BMI (range), kg/m2 | 26.1 (15.2-53.1) | 26.3 (18.0-44.6) | 25.5 (15.2-54.6) |
| ECOG PS, n (%) | |||
| 0 | 174 (40.9) | 164 (38.9) | 692 (44.6) |
| 1 | 239 (56.2) | 238 (56.4) | 763 (49.2) |
| 2 | 12 (2.8) | 20 (4.7) | 95 (6.1) |
| Diagnosis, n (%) | |||
| CLL/SLL | 324 (76.2) | 324 (76.8) | 938 (60.5) |
| WM | 101 (23.8) | 98 (23.2) | 249 (16.1) |
| MCL | - | - | 140 (9.0) |
| MZL | - | - | 93 (6.0) |
| FL | - | - | 59 (3.8) |
| DLBCL | - | - | 45 (2.9) |
| Other† | - | - | 26 (1.7) |
| Characteristic . | Pooled ASPEN cohort 1 and ALPINE (ASPEN/ALPINE) . | Pooled all patients treated with Zanubrutinib (N = 1550) . | |
|---|---|---|---|
| Zanubrutinib (N = 425) . | Ibrutinib (N = 422) . | ||
| Median age (range), y | 68 (35-90) | 68 (35-90) | 67 (20-95) |
| Age group, n (%) | |||
| ≥60 y | 331 (77.9) | 347 (82.2) | 1186 (76.5) |
| ≥65 y | 265 (62.4) | 274 (64.9) | 950 (61.3) |
| ≥65 and ˂75 y | 155 (36.5) | 181 (42.9) | 615 (39.7) |
| ≥75 y | 110 (25.9) | 93 (22.0) | 335 (21.6) |
| Sex, n (%) | |||
| Male | 280 (65.9) | 295 (69.9) | 1027 (66.3) |
| Female | 145 (34.1) | 127 (30.1) | 523 (33.7) |
| Race, n (%) | |||
| White | 348 (81.9) | 357 (84.6) | 1032 (66.6) |
| Asian | 49 (11.5) | 44 (10.4) | 424 (27.4) |
| Not reported | 17 (4.0) | 17 (4.0) | 42 (2.7) |
| Other | 11 (2.6) | 4 (0.9) | 51 (3.3) |
| Missing | 0 | 0 | 1 (0.1) |
| Geographic region, n (%)∗ | |||
| Europe | 259 (60.9) | 250 (59.2) | 551 (35.5) |
| Australia/New Zealand | 60 (14.1) | 60 (14.2) | 414 (26.7) |
| Asia | 45 (10.6) | 43 (10.2) | 406 (26.2) |
| North America | 61 (14.4) | 69 (16.4) | 179 (11.5) |
| Median BMI (range), kg/m2 | 26.1 (15.2-53.1) | 26.3 (18.0-44.6) | 25.5 (15.2-54.6) |
| ECOG PS, n (%) | |||
| 0 | 174 (40.9) | 164 (38.9) | 692 (44.6) |
| 1 | 239 (56.2) | 238 (56.4) | 763 (49.2) |
| 2 | 12 (2.8) | 20 (4.7) | 95 (6.1) |
| Diagnosis, n (%) | |||
| CLL/SLL | 324 (76.2) | 324 (76.8) | 938 (60.5) |
| WM | 101 (23.8) | 98 (23.2) | 249 (16.1) |
| MCL | - | - | 140 (9.0) |
| MZL | - | - | 93 (6.0) |
| FL | - | - | 59 (3.8) |
| DLBCL | - | - | 45 (2.9) |
| Other† | - | - | 26 (1.7) |
BMI, body mass index; DLBCL, diffuse large B-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; FL, follicular lymphoma; MZL, marginal zone lymphoma.
Asia includes China (Mainland and Taiwan) and South Korea; Europe includes Austria, Belgium, Belarus, Bulgaria, Czech Republic, France, Germany, Greece, Italy, The Netherlands, Russian Federation, Poland, Spain, Sweden, Turkey, and United Kingdom; and North America includes the United States and Canada.
Includes 13 patients with Richter transformation, 11 patients with hairy cell leukemia, 1 patient with B-lineage lymphoma, and 1 patient with indolent lymphoma.
Medical history of cardiovascular events
In a pooled analysis of 1550 patients treated with zanubrutinib, 101 (6.5%) had a medical history of atrial fibrillation/flutter, 14 (0.9%) had a medical history of ventricular arrhythmia, and 668 (43.1%) had a medical history of hypertension.
In ASPEN/ALPINE, the proportion of patients with a medical history of atrial fibrillation/flutter (zanubrutinib, 29/425 [6.8%]; ibrutinib, 26/422 [6.2%]) or hypertension (zanubrutinib, 204/425 [48.0%]; ibrutinib, 207/422 [49.1%]) was similar between the zanubrutinib and ibrutinib arms and comparable with that in the pooled analysis of 1550 patients. In ASPEN/ALPINE, a medical history of ventricular arrhythmia was present in 3 of 425 (0.7%) patients treated with zanubrutinib and 1 of 422 (0.2%) patients treated with ibrutinib (Table 2).
Medical history of cardiovascular events
| . | ASPEN cohort 1 WM . | ALPINE R/R CLL/SLL . | Pooled analysis ASPEN/ALPINE . | Pooled analysis B-cell malignancies . | |||
|---|---|---|---|---|---|---|---|
| Zanubrutinib (n = 101) . | Ibrutinib (n = 98) . | Zanubrutinib (n = 324) . | Ibrutinib (n = 324) . | Zanubrutinib (N = 425) . | Ibrutinib (N = 422) . | Zanubrutinib (N = 1550) . | |
| Medical history of cardiovascular events, n (%) | |||||||
| Atrial fibrillation/flutter | 10 (9.9) | 8 (8.2) | 19 (5.9) | 18 (5.6) | 29 (6.8) | 26 (6.2) | 101 (6.5) |
| Ventricular arrhythmia∗ | 1 (1.0) | 0 | 2 (0.6) | 1 (0.3) | 3 (0.7)† | 1 (0.2)‡ | 14 (0.9) |
| Hypertension§ | 39 (38.6) | 45 (45.9) | 165 (50.9) | 162 (50.0) | 204 (48.0) | 207 (49.1) | 668 (43.1) |
| . | ASPEN cohort 1 WM . | ALPINE R/R CLL/SLL . | Pooled analysis ASPEN/ALPINE . | Pooled analysis B-cell malignancies . | |||
|---|---|---|---|---|---|---|---|
| Zanubrutinib (n = 101) . | Ibrutinib (n = 98) . | Zanubrutinib (n = 324) . | Ibrutinib (n = 324) . | Zanubrutinib (N = 425) . | Ibrutinib (N = 422) . | Zanubrutinib (N = 1550) . | |
| Medical history of cardiovascular events, n (%) | |||||||
| Atrial fibrillation/flutter | 10 (9.9) | 8 (8.2) | 19 (5.9) | 18 (5.6) | 29 (6.8) | 26 (6.2) | 101 (6.5) |
| Ventricular arrhythmia∗ | 1 (1.0) | 0 | 2 (0.6) | 1 (0.3) | 3 (0.7)† | 1 (0.2)‡ | 14 (0.9) |
| Hypertension§ | 39 (38.6) | 45 (45.9) | 165 (50.9) | 162 (50.0) | 204 (48.0) | 207 (49.1) | 668 (43.1) |
Any-grade ventricular arrhythmia.
Includes 2 events of ventricular extrasystoles and 1 event of ventricular tachycardia.
Includes 1 event of ventricular extrasystoles.
Includes hypertension, essential hypertension, preeclampsia, hypertensive cardiomyopathy, hypertensive retinopathy, hypertension neonatal, blood pressure increased, orthostatic hypertension, and hypertensive heart disease.
Treatment-emergent cardiovascular events in pooled analysis of patients receiving zanubrutinib
In a pooled analysis of 1550 patients who received zanubrutinib at a median treatment duration of 34.4 months (range, 0.1-90.0), treatment–emergent cardiovascular events were reported in 324 (20.9%) patients, with 161 (10.4%) patients reporting grade ≥3 events (Table 3). Atrial fibrillation/flutter was reported in 75 (4.8%) patients, with a prevalence of 1.8% to 2.6% per year in the first 4 years, and hypertension was reported in 259 (16.7%) patients, with a prevalence of 9.7% to 15.2% per year in the first 4 years. The incidence rate of symptomatic ventricular arrhythmia was 0.7% (11/1550), with a prevalence of 0.1% to 0.4% per year (Figure 1A). The EAIR was 0.15 persons per 100 person-months for atrial fibrillation, 0.57 persons per 100 person-months for hypertension, and 0.02 persons per 100 person-months for symptomatic ventricular arrhythmia (Figure 2A-C). Treatment-emergent cardiac disorders led to dose interruption in 64 (4.1%), dose reduction in 13 (0.8%), and treatment discontinuation in 16 (1.0%) of the 1550 patients (supplemental Tables 2-4). Cardiac disorder led to death in 12 of the 1550 (0.8%) patients, 3 of which were due to ventricular arrhythmias and 1 of which was due to hypertensive heart disease (supplemental Table 5). In the latter case, the patient had baseline hypertension and diabetes mellitus but no treatment-emergent worsening of hypertension during the study. The patient died of heart failure due to chronic hypertension in the context of COVID-19, and the investigator considered death unrelated to zanubrutinib.
Treatment-emergent cardiovascular events
| Category (preferred term), n (%) . | ASPEN cohort 1 WM . | ALPINE R/R CLL/SLL . | Pooled ASPEN/ALPINE . | Pooled analysis B-cell malignancies . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zanubrutinib (n = 101) . | Ibrutinib (n = 98) . | Zanubrutinib (n = 324) . | Ibrutinib (n = 324) . | Zanubrutinib (N = 425) . | Ibrutinib (N = 422) . | Zanubrutinib (N = 1550) . | ||||||||
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Any cardiovascular event | 23 (22.8) | 12 (11.9) | 41 (41.8) | 26 (26.5) | 91 (28.1) | 56 (17.3) | 106 (32.7) | 59 (18.2) | 114 (26.8) | 68 (16.0) | 147 (34.8) | 85 (20.1) | 324 (20.9) | 161 (10.4) |
| Atrial fibrillation/ flutter | 9 (8.9) | 2 (2.0) | 23 (23.5) | 8 (8.2) | 17 (5.2) | 8 (2.5) | 43 (13.3) | 13 (4.0) | 26 (6.1) | 10 (2.4) | 66 (15.6) | 21 (5.0) | 75 (4.8) | 31 (2.0) |
| Atrial fibrillation | 8 (7.9) | 2 (2.0) | 21 (21.4) | 6 (6.1) | 15 (4.6) | 6 (1.9) | 40 (12.3) | 12 (3.7) | 23 (5.4) | 8 (1.9) | 61 (14.5) | 18 (4.3) | 69 (4.5) | 27 (1.7) |
| Atrial flutter | 1 (1.0) | 0 | 4 (4.1) | 2 (2.0) | 2 (0.6) | 2 (0.6) | 3 (0.9) | 1 (0.3) | 3 (0.7) | 2 (0.5) | 7 (1.7) | 3 (0.7) | 7 (0.5) | 5 (0.3) |
| Symptomatic ventricular arrhythmia∗ | 0 | 0 | 1 (1.0) | 0 | 3 (0.9) | 0 | 6 (1.9)† | 4 (1.2) | 3 (0.7) | 0 | 7 (1.7)† | 4 (0.9) | 11 (0.7) | 6 (0.4) |
| Ventricular arrhythmia | 0 | 0 | 1 (1.0) | 0 | 2 (0.6) | 0 | 1 (0.3) | 0 | 2 (0.5) | 0 | 2 (0.5) | 0 | 4 (0.3) | 1 (0.1) |
| Ventricular extrasystoles | 0 | 0 | 0 | 0 | 1 (0.3) | 0 | 1 (0.3) | 0 | 1 (0.2) | 0 | 1 (0.2) | 0 | 3 (0.2) | 1 (0.1) |
| Cardiac arrest | 0 | 0 | 0 | 0 | 0 | 0 | 3 (0.9) | 3 (0.9)‡ | 0 | 0 | 3 (0.7) | 3 (0.7)‡ | 2 (0.1) | 2 (0.1) |
| Pulseless electrical activity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 1 (0.1) |
| Ventricular tachycardia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 1 (0.1) |
| Ventricular fibrillation | 0 | 0 | 0 | 0 | 0 | 0 | 2 (0.6) | 2 (0.6) | 0 | 0 | 2 (0.5) | 2 (0.5) | 0 | 0 |
| Hypertension | 17 (16.8) | 10 (9.9) | 25 (25.5) | 20 (20.4) | 76 (23.5) | 49 (15.1) | 74 (22.8) | 44 (13.6) | 93 (21.9) | 59 (13.9) | 99 (23.5) | 64 (15.2) | 259 (16.7) | 129 (8.3) |
| Hypertension | 17 (16.8) | 10 (9.9) | 24 (24.5) | 19 (19.4) | 71 (21.9) | 48 (14.8) | 64 (19.8) | 36 (11.1) | 88 (20.7) | 58 (13.6) | 88 (20.9) | 55 (13.0) | 242 (15.6) | 125 (8.1) |
| Blood pressure increased | 0 | 0 | 1 (1.0) | 1 (1.0) | 7 (2.2) | 4 (1.2) | 14 (4.3) | 10 (3.1) | 7 (1.6) | 4 (0.9) | 15 (3.6) | 11 (2.6) | 16 (1.0) | 6 (0.4) |
| Hypertensive crisis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (0.3) | 1 (0.1) |
| Essential hypertension | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 0 |
| Prehypertension | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 0 |
| Systolic hypertension | 0 | 0 | 0 | 0 | 1 (0.3) | 0 | 0 | 0 | 1 (0.2) | 0 | 0 | 0 | 1 (0.1) | 0 |
| Hypertensive heart disease | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 1 (0.1) |
| Category (preferred term), n (%) . | ASPEN cohort 1 WM . | ALPINE R/R CLL/SLL . | Pooled ASPEN/ALPINE . | Pooled analysis B-cell malignancies . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zanubrutinib (n = 101) . | Ibrutinib (n = 98) . | Zanubrutinib (n = 324) . | Ibrutinib (n = 324) . | Zanubrutinib (N = 425) . | Ibrutinib (N = 422) . | Zanubrutinib (N = 1550) . | ||||||||
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Any cardiovascular event | 23 (22.8) | 12 (11.9) | 41 (41.8) | 26 (26.5) | 91 (28.1) | 56 (17.3) | 106 (32.7) | 59 (18.2) | 114 (26.8) | 68 (16.0) | 147 (34.8) | 85 (20.1) | 324 (20.9) | 161 (10.4) |
| Atrial fibrillation/ flutter | 9 (8.9) | 2 (2.0) | 23 (23.5) | 8 (8.2) | 17 (5.2) | 8 (2.5) | 43 (13.3) | 13 (4.0) | 26 (6.1) | 10 (2.4) | 66 (15.6) | 21 (5.0) | 75 (4.8) | 31 (2.0) |
| Atrial fibrillation | 8 (7.9) | 2 (2.0) | 21 (21.4) | 6 (6.1) | 15 (4.6) | 6 (1.9) | 40 (12.3) | 12 (3.7) | 23 (5.4) | 8 (1.9) | 61 (14.5) | 18 (4.3) | 69 (4.5) | 27 (1.7) |
| Atrial flutter | 1 (1.0) | 0 | 4 (4.1) | 2 (2.0) | 2 (0.6) | 2 (0.6) | 3 (0.9) | 1 (0.3) | 3 (0.7) | 2 (0.5) | 7 (1.7) | 3 (0.7) | 7 (0.5) | 5 (0.3) |
| Symptomatic ventricular arrhythmia∗ | 0 | 0 | 1 (1.0) | 0 | 3 (0.9) | 0 | 6 (1.9)† | 4 (1.2) | 3 (0.7) | 0 | 7 (1.7)† | 4 (0.9) | 11 (0.7) | 6 (0.4) |
| Ventricular arrhythmia | 0 | 0 | 1 (1.0) | 0 | 2 (0.6) | 0 | 1 (0.3) | 0 | 2 (0.5) | 0 | 2 (0.5) | 0 | 4 (0.3) | 1 (0.1) |
| Ventricular extrasystoles | 0 | 0 | 0 | 0 | 1 (0.3) | 0 | 1 (0.3) | 0 | 1 (0.2) | 0 | 1 (0.2) | 0 | 3 (0.2) | 1 (0.1) |
| Cardiac arrest | 0 | 0 | 0 | 0 | 0 | 0 | 3 (0.9) | 3 (0.9)‡ | 0 | 0 | 3 (0.7) | 3 (0.7)‡ | 2 (0.1) | 2 (0.1) |
| Pulseless electrical activity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 1 (0.1) |
| Ventricular tachycardia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 1 (0.1) |
| Ventricular fibrillation | 0 | 0 | 0 | 0 | 0 | 0 | 2 (0.6) | 2 (0.6) | 0 | 0 | 2 (0.5) | 2 (0.5) | 0 | 0 |
| Hypertension | 17 (16.8) | 10 (9.9) | 25 (25.5) | 20 (20.4) | 76 (23.5) | 49 (15.1) | 74 (22.8) | 44 (13.6) | 93 (21.9) | 59 (13.9) | 99 (23.5) | 64 (15.2) | 259 (16.7) | 129 (8.3) |
| Hypertension | 17 (16.8) | 10 (9.9) | 24 (24.5) | 19 (19.4) | 71 (21.9) | 48 (14.8) | 64 (19.8) | 36 (11.1) | 88 (20.7) | 58 (13.6) | 88 (20.9) | 55 (13.0) | 242 (15.6) | 125 (8.1) |
| Blood pressure increased | 0 | 0 | 1 (1.0) | 1 (1.0) | 7 (2.2) | 4 (1.2) | 14 (4.3) | 10 (3.1) | 7 (1.6) | 4 (0.9) | 15 (3.6) | 11 (2.6) | 16 (1.0) | 6 (0.4) |
| Hypertensive crisis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (0.3) | 1 (0.1) |
| Essential hypertension | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 0 |
| Prehypertension | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 0 |
| Systolic hypertension | 0 | 0 | 0 | 0 | 1 (0.3) | 0 | 0 | 0 | 1 (0.2) | 0 | 0 | 0 | 1 (0.1) | 0 |
| Hypertensive heart disease | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 1 (0.1) |
Data are shown for symptomatic ventricular arrhythmia, only grade ≥2 events are included in the “any grade” column.
One patient reported 2 ventricular arrhythmia events: ventricular fibrillation and cardiac arrest (both grade 4).
Including 2 grade 5 cardiac arrest events.
Prevalence of treatment-emergent cardiovascular events over time. Pooled analysis of 1550 patients (A) and analysis of ASPEN/ALPINE (B). Atrial fibrillation includes atrial fibrillation and flutter.
Prevalence of treatment-emergent cardiovascular events over time. Pooled analysis of 1550 patients (A) and analysis of ASPEN/ALPINE (B). Atrial fibrillation includes atrial fibrillation and flutter.
EAIR. Atrial fibrillation, including atrial fibrillation and flutter (A), symptomatic ventricular arrhythmia (B), and hypertension in patients treated with zanubrutinib or ibrutinib (C) in ASPEN cohort 1, ALPINE, pooled analysis of the 2 studies (ASPEN/ALPINE), and pooled analysis of 1550 patients with B-cell malignancies. Hypertension in patients treated with zanubrutinib in 9 clinical studies (D). Studies BGB-3111-210 and BGB-3111-1002 were pooled for this analysis to increase the number of patients, and BGB-3111-LTE studies were pooled into the corresponding parental studies. The dashed line indicates the EAIR of hypertension in the pooled analysis of 1550 patients with B-cell malignancies (0.57 persons per 100 person-months). LTE, long-term extension; NA, not applicable; NC, not calculated.
EAIR. Atrial fibrillation, including atrial fibrillation and flutter (A), symptomatic ventricular arrhythmia (B), and hypertension in patients treated with zanubrutinib or ibrutinib (C) in ASPEN cohort 1, ALPINE, pooled analysis of the 2 studies (ASPEN/ALPINE), and pooled analysis of 1550 patients with B-cell malignancies. Hypertension in patients treated with zanubrutinib in 9 clinical studies (D). Studies BGB-3111-210 and BGB-3111-1002 were pooled for this analysis to increase the number of patients, and BGB-3111-LTE studies were pooled into the corresponding parental studies. The dashed line indicates the EAIR of hypertension in the pooled analysis of 1550 patients with B-cell malignancies (0.57 persons per 100 person-months). LTE, long-term extension; NA, not applicable; NC, not calculated.
Treatment-emergent cardiovascular events with zanubrutinib vs ibrutinib
The incidence of treatment-emergent cardiovascular events with zanubrutinib and ibrutinib was evaluated using the pooled ASPEN/ALPINE data. At a median treatment exposure of 32.6 months (range, 0.4-68.7) for zanubrutinib and 25.7 months (range, 0.1-59.3) for ibrutinib, the incidence rates of treatment-emergent cardiovascular events of any grade (114/425 [26.8%] vs 147/422 [34.8%]) and grade ≥3 (68/425 [16.0%] vs 85/422 [20.1%]) were lower with zanubrutinib vs ibrutinib, respectively (Table 3). Fewer patients treated with zanubrutinib had treatment–emergent cardiovascular events that led to dose modifications or treatment discontinuation than patients treated with ibrutinib (supplemental Table 6). Treatment-emergent cardiac disorders leading to death occurred in 1 of the 425 (0.2%) patients treated with zanubrutinib and in 7 of the 422 (1.7%) patients treated with ibrutinib (supplemental Table 5).
Treatment-emergent atrial fibrillation/flutter with zanubrutinib vs ibrutinib
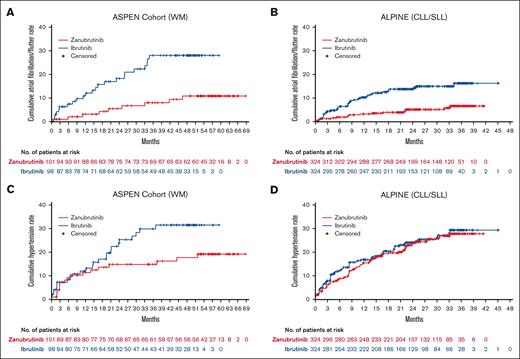
Among patients in ASPEN/ALPINE, the incidence rate of treatment-emergent atrial fibrillation/flutter was significantly lower with zanubrutinib than with ibrutinib (26/425 [6.1%] vs 66/422 [15.6%]; P < .0001; Table 3) and its prevalence remained low (Figure 1B). The cumulative rate of atrial fibrillation/flutter was lower with zanubrutinib than with ibrutinib in both ASPEN cohort 1 and ALPINE (Figure 3A-B). The EAIR of atrial fibrillation/flutter was also significantly lower in patients treated with zanubrutinib vs ibrutinib (0.20 vs 0.64 persons per 100 person-months) in the pooled analysis of these studies, with an EAIR ratio of ∼0.31 between zanubrutinib and ibrutinib (P < .0001) indicating a 69% reduction in the risk for atrial fibrillation/flutter when corrected for duration on therapy (Figure 2A). Among the patients with treatment-emergent atrial fibrillation/flutter, 5 of 26 (19.2%) zanubrutinib-treated patients vs 23 of 66 (34.8%) ibrutinib-treated patients required dose interruption (supplemental Table 7), and 3 of 26 (11.5%) vs 8 of 66 (12.1%) required dose reduction (supplemental Table 8) due to atrial fibrillation/flutter with zanubrutinib vs ibrutinib, respectively. Although no patient discontinued zanubrutinib owing to atrial fibrillation/flutter, 7 of 66 (10.6%) patients discontinued ibrutinib (supplemental Table 9). No deaths due to atrial fibrillation or flutter occurred in either arm.
Time-to-first atrial fibrillation/flutter or hypertension event. Time-to-first atrial fibrillation/flutter event in ASPEN cohort 1 (A) and ALPINE (B), and time-to-first hypertension event in ASPEN cohort 1 (C) and ALPINE (D).
Time-to-first atrial fibrillation/flutter or hypertension event. Time-to-first atrial fibrillation/flutter event in ASPEN cohort 1 (A) and ALPINE (B), and time-to-first hypertension event in ASPEN cohort 1 (C) and ALPINE (D).
Treatment-emergent ventricular arrhythmias with zanubrutinib vs ibrutinib
In ASPEN/ALPINE, treatment-emergent symptomatic ventricular arrhythmia events occurred with zanubrutinib in 3 of 425 (0.7%) patients vs 7 of 422 (1.7%) ibrutinib-treated patients (P = .1992). In the zanubrutinib arm, the prevalence of symptomatic ventricular arrhythmia was 0.3% to 0.7% per year during the first 3 years and 0% thereafter. In the ibrutinib arm, the prevalence was 0.4% to 1.9% per year during the first 4 years and 2.3% after >4 years (Figure 1B). The EAIR was 0.02 persons per 100 person-months with zanubrutinib vs ibrutinib at 0.06 persons per 100 person-months (P = .1449; Figure 2B). In ASPEN cohort 1, there were no symptomatic ventricular arrhythmia events among patients treated with zanubrutinib at a median treatment exposure of 46.8 months (range, 0.8-59.9). In ALPINE, at a median treatment exposure of 28.4 months (range, 0.4-41.6), 3 of 324 (0.9%) patients treated with zanubrutinib reported symptomatic ventricular arrhythmia (ventricular arrhythmia [n = 2] and ventricular extrasystoles [n = 1]); all were grade 2 events (Table 3). Among patients treated with ibrutinib (median treatment exposure: ASPEN cohort 1, 44.7 months [range, 0.3-59.3]; ALPINE, 24.3 months [range, 0.1-45.1]), 1 of 98 (1.0%) patients in ASPEN cohort 1 and 6 of 324 (1.9%) patients in ALPINE reported symptomatic ventricular arrhythmia events (cardiac arrest [n = 3], grade 5 in 2 patients and grade 4 in 1 patient; ventricular fibrillation [n = 2], both grade 4; ventricular arrhythmia [n = 1], grade 2; ventricular extrasystoles [n = 1], grade 2). Of the patients in ALPINE, 1 patient reported both grade 4 cardiac arrest and grade 4 ventricular fibrillation.
Among patients with symptomatic ventricular arrhythmia in ASPEN/ALPINE, 3 patients discontinued ibrutinib (cardiac arrest [n = 2] and ventricular fibrillation [n = 1]), and no patients discontinued zanubrutinib (supplemental Table 9). One patient in the zanubrutinib arm discontinued per the investigator decision due to grade 1 (asymptomatic) ventricular extrasystole. In ASPEN/ALPINE, no deaths due to symptomatic ventricular arrhythmia occurred with zanubrutinib vs 2 deaths with ibrutinib (both cardiac arrest; supplemental Table 5).
Treatment-emergent hypertension with zanubrutinib vs ibrutinib
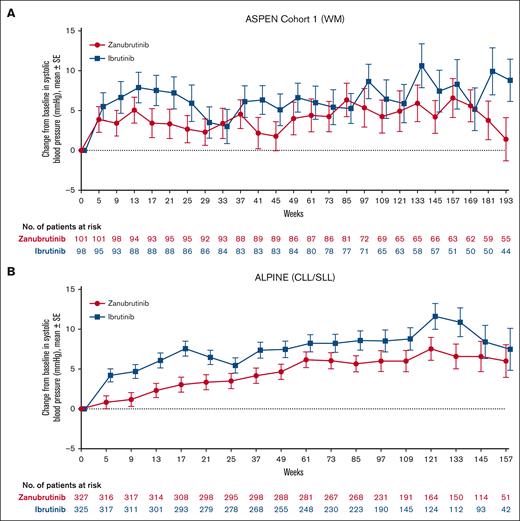
Mean changes from baseline over time in systolic blood pressure were generally lower in patients treated with zanubrutinib vs ibrutinib in both ASPEN cohort 1 and ALPINE, with the difference between treatment arms being greatest in ALPINE (Figure 4). In ASPEN/ALPINE, rates of treatment-emergent hypertension events were reported in 93 of 425 patients (21.9%) in the zanubrutinib arm vs 99 of 422 patients (23.5%) in the ibrutinib arm (P = .5835; Table 3). The hypertension time-to-event curves for ibrutinib were similar in ASPEN and ALPINE (Figures 3C-D). The curves for zanubrutinib in both studies followed those for ibrutinib in the first 15 to 18 months. However, after this time, the zanubrutinib curve diverged from ibrutinib and flattened in ASPEN (Figure 3C) but aligned with ibrutinib for ALPINE (Figure 3D). When looking at the head-to-head studies separately, the EAIR of hypertension was significantly (P = .0211) lower with zanubrutinib than with ibrutinib in ASPEN cohort 1 but similar between treatment arms in ALPINE (Figure 2C). The EAIR for ALPINE (1.04 persons per 100 person-months) was not consistent with those observed in the other zanubrutinib studies (range [excluding ALPINE], 0.27-0.62 persons per 100 person-months) (Figure 2D). Including ALPINE, the median EAIR for all the zanubrutinib studies was 0.57. Despite the similar proportion of patients with hypertension in ALPINE in the 2 treatment arms (Table 3), cardiac disorders led to only 1 discontinuation in patients treated with zanubrutinib compared with 14 in patients treated with ibrutinib (supplemental Table 4). One patient discontinued treatment because of hypertension in the ibrutinib arm of ALPINE and none in the zanubrutinib arm (supplemental Table 9).
Systolic blood pressure over time in patients treated with zanubrutinib or ibrutinib. ASPEN cohort 1 (A). ALPINE (B).
Systolic blood pressure over time in patients treated with zanubrutinib or ibrutinib. ASPEN cohort 1 (A). ALPINE (B).
To identify whether there was a relationship between hypertension and atrial fibrillation incidence, we performed a correlation analysis in each treatment arm of the ASPEN/ALPINE populations and the total pooled zanubrutinib population. All kappa coefficients were < 0.1 (supplemental Table 10), indicating a lack of correlation between atrial fibrillation and hypertension. Additionally, using regression modeling to evaluate whether hypertension, treatment, or the potential interaction between hypertension and treatment was predictive of atrial fibrillation status, we found that only treatment was significant (P < .0001) (supplemental Table 11).
Treatment-emergent cardiac death with zanubrutinib vs ibrutinib
Fewer fatal cardiac disorders occurred with zanubrutinib than with ibrutinib in ALPINE: no fatal cardiac disorder occurred with zanubrutinib vs 6 with ibrutinib (cardiac arrest [n = 2], myocardial infarction [n = 2], acute cardiac failure [n = 1], and congestive cardiomyopathy [n = 1]; supplemental Table 5). In ASPEN cohort 1, a fatal cardiac disorder was reported in 1 patient in each arm (cardiomegaly [n = 1] with zanubrutinib and acute cardiac failure [n = 1] with ibrutinib). No deaths related to hypertension occurred in ASPEN/ALPINE (supplemental Table 12).
Discussion
Treatment-emergent cardiovascular events, including atrial fibrillation, ventricular arrhythmias, and hypertension, can limit the use of ibrutinib. Head-to-head comparisons are needed to determine whether a cardiac disorder is drug-specific or a class effect of covalent BTK inhibitors. Zanubrutinib, a next-generation BTK inhibitor, was developed to improve BTK specificity and minimize off-target binding.24 Here, we assessed the cardiovascular event profile of zanubrutinib and compared it with the profile of ibrutinib retrospectively using data from 2 head-to-head studies (ASPEN and ALPINE). We found that the cardiovascular event profile improved with zanubrutinib vs ibrutinib and was consistent with a pooled analysis of 10 studies with zanubrutinib monotherapy in patients with B-cell malignancies. Additional research to better understand the etiology of cardiac events observed with BTK inhibitors will be important to further minimize their occurrence.
The risk of atrial fibrillation increases not only with age but also with diabetes, high blood pressure, and heart disease, all of which are risk factors for stroke and heart failure.30,31 A retrospective study of newly diagnosed patients with TN CLL (median age, 65 years at diagnosis) from the Mayo Clinic Database reported that 6% of 2444 patients with newly diagnosed, untreated CLL had a prior history of atrial fibrillation at the time of diagnosis.32 In our analysis of ASPEN/ALPINE, we observed a similar rate, with 6.8% of patients in the zanubrutinib arm and 6.2% of patients in the ibrutinib arm having a medical history of atrial fibrillation. Despite a comparable proportion of patients with prior atrial fibrillation in each treatment arm of this combined ASPEN/ALPINE analysis, fewer patients experienced treatment-emergent atrial fibrillation/flutter with zanubrutinib than with ibrutinib. In examining the slopes of the time-to-first incident of atrial fibrillation curves, the slope for the ibrutinib curve appears steeper during the first 6 months than that for zanubrutinib. After 6 months, the slopes of the curves appear more similar but a steeper slope for ibrutinib remains. Consistent with a prior study,33 these data suggest that those at risk will develop atrial fibrillation on ibrutinib early in the course of treatment, with the later parts of the curve being closer to the background. The slope of the zanubrutinib curve remained low and stable throughout the course of the study. Thus, these data suggest that ibrutinib is associated with an increased risk of atrial fibrillation compared with zanubrutinib.
Ventricular arrhythmias are serious cardiac disorders that can lead to sudden death. Several reports of cardiac arrhythmias and failure with ibrutinib have prompted an update to the ibrutinib label in 2022.34,35 Retrospective analysis using the United States–based Comprehensive Cancer Center registry reported that 6 of 582 (1.0%) patients with hematologic malignancies without prior history of coronary artery disease developed symptomatic ventricular arrythmia with ibrutinib over a median follow-up of 32 months (range, 0.7-73).14 In a study using the international pharmacovigilance database, VigiBase, significantly higher rates of ventricular arrhythmias were reported in patients treated with ibrutinib (70/13 572 [0.5%]) than in other patients in the database from 2013 to 2018 (9220/8 318 890 [0.1%]), with a reporting odds ratio (OR), 4.7; 95% confidence interval [CI], 3.7-5.9; P < .0001.15 The median time to onset of ventricular arrhythmia in these patients was 70 days (interquartile range [IQR], 28.5-152.5) after ibrutinib administration, and the associated outcome in 7 of these 70 (10%) patients was death.15 An updated analysis of VigiBase in January 2019 confirmed that ibrutinib was significantly associated with ventricular arrhythmia (99 reports observed in 21 110 ibrutinib reports [0.5%]; reporting OR, 5.4; 95% CI, 4.4-6.6) and sudden death (126/21 110 [0.6%]; reporting OR, 1.9; 95% CI, 1.6-2.3), but not with drug–induced QT prolongation.35
In ASPEN/ALPINE, the risk of symptomatic (grade ≥2) ventricular arrhythmia was lower with zanubrutinib (0.7%) than that with ibrutinib (1.7%). Although the incidence of symptomatic ventricular arrhythmias was low, the EAIR with zanubrutinib in the pooled analysis of 10 clinical studies was consistent with that seen with zanubrutinib in ASPEN/ALPINE and one-third of that seen with ibrutinib. In ASPEN/ALPINE, 2 deaths in the ibrutinib arms and no deaths in the zanubrutinib arms were associated with ventricular arrhythmias.
Treatment-emergent hypertension rates associated with ibrutinib have led to a recommendation for monitoring blood pressure in patients treated with BTK inhibitors.8,15,35,36 The rate of hypertension in ASPEN cohort 1 was lower with zanubrutinib (17/101 [16.8%]; median treatment exposure, 46.8 months) than ibrutinib (25/98 [25.5%]; median treatment exposure, 44.7 months) and similar to that in the pooled analysis of 10 clinical studies with zanubrutinib (259/1550 [16.7%]). In ALPINE, hypertension rates were similar with zanubrutinib (76/324 [23.5%]; median treatment exposure, 28.4 months) and ibrutinib (74/324 [22.8%]; median treatment exposure, 24.3 months). When analyzing the EAIR of hypertension across individual studies with zanubrutinib, most had an EAIR between 0.3 to 0.6 persons per 100 person-months except for ALPINE, which was markedly higher (1.04 persons per 100 person-months). The rate of hypertension in this study was not consistent with that in other clinical studies of zanubrutinib and will be the subject of further investigation. The low EAIR for hypertension in the CLL population in the SEQUOIA trial (0.54 persons per 100 person-months) further supports this claim. It is also noteworthy that in the ASPEN trial, a lower incidence of hypertension reported with zanubrutinib compared with ibrutinib was observed only after at least 12 months.
Although hypertension can lead to cardiovascular events, despite the similar rates of hypertension between arms in ALPINE, the overall incidence of cardiac disorders (21.3% vs 29.6%) and discontinuations due to cardiac disorders (0.3% vs 4.3%) were lower with zanubrutinib compared with ibrutinib, respectively.29 Additionally, the rate of any-grade (5.2% vs 13.3%) and grade ≥3 (2.5% vs 4.0%) atrial fibrillation or flutter was lower with zanubrutinib than with ibrutinib.29 None of the zanubrutinib-treated patients died due to cardiac disorders; however, 6 deaths due to cardiac disorders were reported among patients receiving ibrutinib.29 Notably, the rates of grade ≥3 treatment-emergent hypertension with zanubrutinib have been shown to decrease with longer follow-up.37 Similarly, at a 44.4-month median follow-up, the rate of hypertension in ASPEN cohort 1 was 14.9% (15/101) with zanubrutinib but 25.5% (25/98) with ibrutinib.38 A lack of correlation between atrial fibrillation and hypertension was observed in each treatment arm of the ASPEN/ALPINE populations and the total pooled zanubrutinib population, suggesting that the mechanisms inducing atrial fibrillation and hypertension with BTK inhibitors may be distinct.
This study was subject to several limitations because of its retrospective nature. Cardiovascular events were not preplanned end points in most of the assessed studies. Additionally, the timing of blood pressure measurements and electrocardiograms (ECGs) was not the same between the studies. For example, although blood pressure measurements and ECGs were part of the scheduled safety assessments for both ALPINE and ASPEN, ECGs were performed more frequently in ALPINE (day 1 of cycles 1-4 and then every 3 cycles thereafter vs day 1 of cycles 1 and 2 and then every 4 cycles thereafter in ASPEN), whereas blood pressure measurements were done more frequently in ASPEN (day 1 of cycles 1-13 and every 3 cycles thereafter) vs in ALPINE (day 1 of cycles 1-7 and every 3 cycles thereafter). Thus, asymptomatic and transient blood pressure elevation and cardiac events only detectable on scheduled assessment may not have always been captured. As the phase 3 trials included in this analysis were conducted during the COVID-19 pandemic, the possible effects of SARS-CoV-2 infection on cardiovascular events cannot be excluded. Furthermore, the pooled analysis included a range of B-cell malignancies, various lines of therapy, and various treatment exposure times, which may limit the interpretation of the analysis. The variable treatment exposure times were accounted for using the EAIR analysis. Despite the range of B-cell malignancies, the patient disposition and medical history of cardiac disorders generally appeared similar among the studies included in this pooled analysis. In addition, the overall rates of cardiovascular events in the individual studies were similar to those in the pooled population, suggesting a consistent and favorable cardiac safety profile of zanubrutinib.
This retrospective analysis demonstrated that the rates of atrial fibrillation, symptomatic ventricular arrhythmias, and hypertension with zanubrutinib were low and generally occured less frequently than with ibrutinib. These data support the use of zanubrutinib as a treatment option with an improved cardiovascular events profile for patients with B-cell malignancies.
Acknowledgments
The authors thank the patients who participated in the study, their supporters, and the investigators and clinical research staff from the study centers.
This study was supported by research funding from BeiGene Co, Ltd, (Beijing, China). Medical writing and editorial assistance were funded by BeiGene and provided by Miriam Cohen of Bio Connections LLC (Chicago, IL) and Jenna M. Gaska of Nucleus Global, an Inizio company.
Authorship
Contribution: J.J.M., M.Z., and H.M. devised the analysis; J.J.M. was involved in the adjudication of cardiac disorders; J.Z. and L.C. analyzed the data; and all authors interpreted the data, wrote, reviewed, and approved the manuscript and are accountable for all aspects of the work.
Conflict-of-interest disclosure: J.J.M. reports financial support from Bristol Myers Squibb (BMS), Deciphera, Takeda, AstraZeneca, Regeneron, Janssen, Myovant, Silverback Therapeutics, Kurome Therapeutics, Kiniksa Pharmaceuticals, Daiichi Sankyo, CRC Oncology, BeiGene, Pharmacyclics, Prelude Therapeutics, TransThera Sciences, Antev Ltd, IQVIA, Incyte, AskBio, Labcorp, Paladin, Quell Therapeutics, Voyager Therapeutics, CRC Oncology, Bitterroot Bio, Repare Therapeutics, Teva, and Cytokinetics and is supported by grants from the National Institutes of Health (grants R01HL141466, R01HL155990, R01HL156021, and R01HL160688). R.R.F. reports a consulting or advisory role with AbbVie, AstraZeneca, BeiGene, Genentech, Janssen, Lilly Oncology, Sanofi, MEI Pharma, and X4 Pharmaceuticals; and reports speaker fees from AstraZeneca and Janssen. C.S.T. reports honoraria from Janssen, AbbVie, BeiGene, Loxo Oncology, Novartis; research funding from Janssen, AbbVie, BeiGene. J.E-S reports research funding from Novartis; consulting fees from CRC Oncology, Repare Therapeutics, and BMS; honoraria from Servier, Eiasi, BeiGene, and IPSN; meeting support from BeiGene; and equipment, materials, or other services support from BMS. C.R.F. reports a consulting or advisory role with Bayer, Gilead, Spectrum, AbbVie, Celgene, Denovo Biopharma, BeiGene, Karyopharm Therapeutics, Pharmacyclics/Janssen, Genentech/Roche, Epizyme, Genmab, Seagen, Foresight Diagnostics, BMS/Celgene, Curio Science, AstraZeneca, and MorphoSys; and stock ownership with Foresight Diagnostics and NPower. A.C., H.M., M.Z., J.Z., and L.C. report employment and stock ownership with BeiGene. J.R.B. reports research funding from BeiGene, Gilead, iOnctura, Loxo/Lilly, MEI Pharma, Sun, Verastem Oncology/Secura Bio, and TG Therapeutics; consulting fees from AbbVie, Acerta/AstraZeneca, BeiGene, BMS/Juno/Celgene, Catapult, Eli Lilly, Genentech/Roche, Grifols Worldwide Operations, HUTCHMED, iOnctura, Janssen, Loxo, MEI Pharma, MorphoSys AG, Nextcea, Novartis, Pfizer, Pharmacyclics, and Rigel; and served on data safety monitoring Committees for Invectys.
Correspondence: Javid J. Moslehi, Smith Cardiovascular Research Building, 555 Mission Bay Blvd., South, Mail Code 3118, San Francisco, CA 94143-3118; email: javid.moslehi@ucsf.edu.
References
Author notes
BeiGene voluntarily shares anonymous data on completed studies responsibly and provides qualified scientific and medical researchers access to anonymous data and supporting clinical trial documentation for clinical trials in dossiers for medicines and indications after submission and approval in the United States, China, and Europe. Clinical trials supporting subsequent local approvals, new indications, or combination products are eligible for sharing once corresponding regulatory approvals are achieved. BeiGene shares data only when permitted by applicable data privacy and security laws and regulations. In addition, data can only be shared when it is feasible to do so without compromising the privacy of study participants.
Qualified researchers may submit data requests/research proposals for BeiGene review and consideration through BeiGene’s clinical trial webpage at https://www.beigene.com/our-science-and-medicines/our-clinical-trials/.
The full-text version of this article contains a data supplement.