Teclistamab induces severe defects in humoral immunity with decreased polyclonal immunoglobulin levels and impaired vaccination responses.
IVIG use was associated with a significantly lower risk of serious infections among patients receiving teclistamab treatment.
Visual Abstract
Teclistamab and other B-cell maturation antigen (BCMA)–targeting bispecific antibodies (BsAbs) have substantial activity in patients with heavily pretreated multiple myeloma (MM) but are associated with a high rate of infections. BCMA is also expressed on normal plasma cells and mature B cells, which are essential for the generation of a humoral immune response. The aim of this study was to improve the understanding of the impact of BCMA-targeting BsAbs on humoral immunity. The impact of teclistamab on polyclonal immunoglobulins and B cell counts was evaluated in patients with MM who received once-weekly teclistamab 1.5 mg/kg subcutaneously. Vaccination responses were assessed in a subset of patients. Teclistamabinduced rapid depletion of peripheral blood B cells in patients with MM and eliminated normal plasma cells in ex vivo assays. In addition, teclistamab reduced the levels of polyclonal immunoglobulins (immunoglobulin G [IgG], IgA, IgE, and IgM), without recovery over time while receiving teclistamab therapy. Furthermore, response to vaccines against Streptococcus pneumoniae, Haemophilus influenzae type B, and severe acute respiratory syndrome coronavirus 2 was severely impaired in patients treated with teclistamab compared with vaccination responses observed in patients with newly diagnosed MM or relapsed/refractory MM. Intravenous immunoglobulin (IVIG) use was associated with a significantly lower risk of serious infections among patients treated with teclistamab (cumulative incidence of infections at 6 months: 5.3% with IVIG vs 54.8% with observation only [P < .001]). In conclusion, our data show severe defects in humoral immunity induced by teclistamab, the impact of which can be mitigated by the use of immunoglobulin supplementation. This trial was registered at www.ClinicalTrials.gov as #NCT04557098.
Introduction
Teclistamab is the first approved B-cell maturation antigen (BCMA)–targeting T cell redirecting bispecific antibody (BsAb) with pronounced activity in patients with heavily pretreated multiple myeloma (MM).1-3 At a median follow-up of 22.8 months, the overall response rate with teclistamab was 63.0%, with (stringent) complete response in 45.5%, which translated into durable responses (median of 21.6 months), progression-free survival (median of 11.3 months), and overall survival (median of 21.9 months).3
The downside is the high incidence of infections (all grade: 80.0%; grade ≥ 3: 55.2%). Other BCMA-targeting BsAbs have comparable high activity in heavily pretreated MM but are also accompanied by frequent occurrence of severe infections.4-9 The types of infections in patients treated with BsAbs are wide ranging, with upper and lower respiratory infections being most common.1,10 Opportunistic infections have also been described (eg, Pneumocystis jirovecii pneumonia [PJP] and cytomegalovirus-related disease).1,3-9
The high rate of infections during treatment with a BCMA-targeting BsAb may be explained by global immunoparesis, which is, in part, disease related but also due to long-term exposure to immunosuppressive therapies before BsAb treatment.11-13 In addition, chronic activation of T cells with a BsAb may lead to T-cell exhaustion, which reduces the ability of T cells to kill cancer cells or cells infected with virus.14 Moreover, because BCMA is also expressed on normal plasma cells and on a subset of B cells, a negative effect of BCMA BsAbs on these immune cells may also play a role.15,16 There are only limited data available on the impact of BCMA-targeting BsAbs on B cell counts, polyclonal immunoglobulins, and vaccination responses. Because infections are causing substantial morbidity and mortality in this patient group, and are associated with substantial health care costs, it is crucial to better understand underlying causes for infections. In this study, we aimed to define the impact of teclistamab on humoral immunity to provide improved guidance on infectious prophylaxis and vaccination strategy.
Materials and methods
Study design and patients
The study design and methods of the MajesTEC-1 study have been previously published.1 Briefly, this was an open-label, multicenter phase 1 (NCT03145181) and phase 2 (NCT04557098) study in patients with relapsed/refractory MM (RRMM). Eligible patients were adults with a diagnosis of RRMM; progressive, measurable disease per International Myeloma Working Group criteria; and with an Eastern Cooperative Oncology Group performance status score of 0 or 1. Patients should also have received ≥ 3 prior lines of therapy, including a proteasome inhibitor, an immunomodulatory drug, and an anti-CD38 mAb. Patients who had received prior BCMA-targeted therapy were excluded. In the pivotal recommended phase 2 dose (RP2D) cohort, patients received teclistamab as a weekly subcutaneous dose of 1.5 mg/kg preceded by 2 step-up (priming) doses of 0.06 and 0.3 mg/kg.
The protocol and other relevant documents were approved by institutional review boards of all participating institutions. All patients provided written informed consent.
Enumeration of B cells and T cells
For 135 patients treated with teclistamab at the RP2D in the MajesTEC-1 study, B cells and T cells were enumerated in the peripheral blood by flow cytometry, explained in detail in supplemental Methods.
Polyclonal immunoglobulin assessment
Serial peripheral blood serum samples were obtained from patients treated with teclistamab at the RP2D in the MajesTEC-1 study. Uninvolved, polyclonal immunoglobulin levels were assessed using an immunoturbidimetric assay (Labcorp). Additional information can be found in supplemental Methods.
Antibody response to vaccination
Vaccination responses against Streptococcus pneumoniae, Haemophilus influenzae type B, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were evaluated in a subset of patients who were treated with teclistamab at Amsterdam University Medical Center and who achieved partial response (PR) or better after day 1 of cycle 3 (17 patients were evaluable for S. pneumoniae and H.influenzae vaccination, and 13 patients were evaluable for SARS-CoV-2 vaccination). These vaccinations were administered in the outpatient clinic, which allowed for systematic, serial monitoring of the antibody response. In addition, patients received influenza vaccination via their general practitioner at variable time points, and therefore response to this vaccine could not be systematically assessed. As control groups, we also vaccinated patients receiving maintenance treatment after autologous stem cell transplantation in first remission (n = 22), patients with daratumumab-naive RRMM (n = 11), and patients with RRMM receiving daratumumab-containing therapy (n = 20). A subset of the patients with RRMM has been described previously.17
Data on vaccination response were collected until patients received intravenous immunoglobulin (IVIG) treatment (either as primary or secondary prophylaxis of bacterial infections in patients with immunoglobulin G (IgG) of < 4 g/L) or another anti-MM treatment. Patients with a history of prior vaccination against S. pneumoniae or H. influenzae type B were ineligible for vaccination response assessment. Patients were evaluable for antibody response to S. pneumoniae or H. influenzae type B vaccination if baseline and postvaccination titers were available, and those with protective titers at baseline (because of prior infection) were excluded from response evaluation.
The S. pneumoniae vaccination schedule consisted of the conjugated PCV-13 vaccine (Prevenar; Pfizer, New York, NY) followed by the polysaccharide PPV-23 vaccine (Pneumovax; Merck Sharp & Dohme, Kenilworth, NJ), which were administered by intramuscular injection with an 8-week interval.18 Specific antibody (IgG) titers were measured using an enzyme-linked immunosorbent assay at baseline, 4 and 8 weeks after PCV-13 vaccination, as well as 4 and 8 weeks after PPV-23 vaccination. Response was defined as an absolute titer of ≥ 2 μg/mL or a twofold or greater increase in 6 of 9 analyzed pneumococcal subtypes (6B, 8, 9, 14, 15B, 19F, 20, 23F, and 33F) according to the criteria proposed by Palazzo.19 To assess fold increase in antibody titer, titers below the lower limit of detection (LLOD; 0.04 μg/mL) were set to 50% of the LLOD, as described previously.20
H. influenzae type B vaccination consisted of a single intramuscular injection of the Act-Hib vaccine (Sanofi, Paris, France). Measurement of specific IgG was performed using an enzyme-linked immunosorbent assay at baseline, and 4 and 8 weeks after vaccination. Response was defined as an absolute titer of ≥ 1 μg/mL, or a fourfold or greater increase if the peak titer was between 0.15 and 0.99 μg/mL.19 To assess fold increase in antibody titer, titers below the LLOD (0.11 μg/mL) were set to 50% of the LLOD, as described previously.21
Antibody response after SARS-CoV-2 vaccination with messenger RNA vaccines (mRNA1273 [Moderna] or BNT162b2 [Pfizer]) was evaluated in patients who received teclistamab treatment. Approximately 5 months after completing the standard 2-dose schedule, patients received a third booster vaccination. Patients were excluded from analyses in case of (prior) SARS-CoV-2 infection (detected by real-time reverse transcription polymerase chain reaction or antigen test). Although rare, a limitation of this strategy is the failure to identify patients who were infected but who did not develop symptoms that prompted further testing. Patients were also excluded in case of passive immunotherapy with anti–SARS-CoV-2 antibodies. Serologic response to the vaccine was measured with a chemiluminescence immunoassay (Liason, DiaSorin, Italy) to detect antibodies against SARS-CoV-2 spike protein. Seroconversion was defined as obtaining a S1-IgG concentration of > 33.8 binding antibody units per mL. Serum samples were obtained ∼4 weeks after the third vaccination.
IVIG supplementation
The impact of IVIG supplementation on the frequency of severe infections was investigated in all patients who received teclistamab at Amsterdam University Medical Center. Use of IVIG (Nanogam; Prothya Biosolutions, Amsterdam, The Netherlands) and development of infections were monitored throughout teclistamab treatment until disease progression or death, whichever occurred first. IVIG was prescribed as primary prophylaxis of bacterial infections in cases with polyclonal IgG of < 4 g/L, or as secondary prophylaxis in patients who developed a severe infection with IgG of < 4 g/L (at individual physicians’ discretion). IVIG was administered every 4 weeks with a 10-gram starting dose, whereby the dose was subsequently adjusted to achieve IgG levels of > 4 g/L. IVIG was administered outside of the cytokine release syndrome risk window, because IVIG supplementation can be associated with similar signs and symptoms as observed during cytokine release syndrome (eg, chills). Patients also received herpes zoster (valacyclovir) and PJP prophylaxis (co-trimoxazole, or pentamidine in case of co-trimoxazole allergy). Patients did not receive other antibacterial or antifungal prophylaxis. An infection was defined as a microbiologically confirmed infection, or clinical documentation of suspected infection and either treatment with new antimicrobials or fever without localizing symptoms not thought to be from MM itself.22 Infections were designated as bacterial, viral, or fungal based on microbiological confirmation only.22
Infections were graded according to the Common Terminology Criteria for Adverse Events version 4.03.
Flow cytometry–based ex vivo cytotoxicity assays
Teclistamab-mediated lysis of normal plasma cells was evaluated in ex vivo killing assays using bone marrow (BM) from healthy controls. Detailed description is provided in supplemental Methods.
Assessment of BCMA expression levels
Surface BCMA expression was examined in BM samples using flow cytometry. More details are reported in supplemental Methods.
Statistics
Polyclonal immunoglobulin and flow cytometry data from the MajesTEC-1 study were analyzed as per the clinical cutoff date of 9 December 2022. The 2-sided, unpaired, Wilcoxon rank-sum test was used to compare each on-treatment time point to the pretreatment, baseline time point (priming dose 1).
In other analyses, comparisons between variables were performed using 2-tailed (paired) Student t test, and Mann-Whitney U test or Wilcoxon matched-pairs signed-rank test when data did not follow normal distribution. Vaccination response rates were compared using Pearson χ2 test or Fisher exact test.
The impact of IVIG supplementation on frequency of infections was analyzed in patients who received IVIG as primary prophylaxis (with polyclonal IgG of < 4 g/L) or as secondary prophylaxis (observation group; IVIG supplementation in severe infection and with IgG of < 4 g/L) as per the clinical cutoff date of 1 June 2023. Incidence-rate ratios with 95% confidence intervals (CIs) were calculated using Poisson regression. Cumulative incidence of serious infections was estimated using the Kaplan-Meier method. Patients who did not experience a serious infection were censored at the date of last follow-up. In addition, patients in the observation group, who started delayed IVIG treatment without development of a serious infection, were censored at the date of IVIG initiation. Univariate Cox regression was used to identify prognostic factors of severe infections. The factors that showed a significance of P ≤ .10 were included in a multivariate Cox regression model (backward analysis) to identify independent prognostic factors. P values < .05 were considered significant.
Data were analyzed in SPSS (version 26; Armonk, NY), GraphPad Prism (version 9.4.0; Boston, MA), Stata (version 17.0; StataCorp, College Station, TX), and R language (R Foundation for Statistical Computing, Vienna, Austria; version 4.2.1 or higher).
Results
Impact of teclistamab on normal plasma cells
We have previously shown that normal and malignant plasma cells have comparable BCMA expression.16 To evaluate the impact of teclistamab on normal plasma cells, we incubated BM mononuclear cells obtained from 4 healthy controls with control antibody or teclistamab for 48 hours. In these experiments, there were minimal effects of the control antibody (mean lysis: 4.3%), whereas there was substantial teclistamab-induced lysis of normal plasma cells (mean lysis at 0.8 μg/mL: 58.0% [P < .01]; mean lysis at 4 μg/mL: 68.6% [P < .001]; supplemental Figure 1).
Impact of teclistamab on normal B cells and T cells
In addition to expression of BCMA on normal mature B cells,23,24 we show here that a fraction of B cell progenitors in the BM from healthy donors or patients with MM also express BCMA on the cell surface (supplemental Figure 2). We therefore analyzed the effect of teclistamab on B cell frequency in sequential peripheral blood samples, obtained from 135 unique patients treated with teclistamab at the RP2D in the MajesTEC-1 study. The characteristics of the patients at baseline have been described previously (median 5 prior lines of therapy; 77.6% triple-class refractory).1 Teclistamab treatment resulted in a rapid and almost complete elimination of circulating B cells (CD45+CD19+; baseline: median B cell count = 10.6 × 106/L; cycle 2, day 1: median B cell count = 0.2 × 106/L; P < .001), which persisted during treatment (C7D1: median B cell count = 0.0 × 106/L; P < .001; Figure 1A). In contrast, the frequencies of both CD4+ and CD8+ T cells initially decreased but subsequently recovered to baseline levels or higher over time (Figure 1B-C).
Teclistamab treatment reduces peripheral blood B cells and decreases serum IgG levels. (A-C) Longitudinal data representation of (A) absolute B cell counts, (B) CD4+ T cell counts, and (C) CD8+ T cell counts over time in the peripheral blood. Peripheral blood was obtained from 135 unique patients during treatment with teclistamab. There is a reduction of sample numbers over time because of study discontinuation. Most patients discontinued study treatment because of progressive disease (n = 58; 43.0%), and only 5 patients (3.7%) discontinued because of adverse events, including 2 (1.5%) due to infections. (D) Serum levels of uninvolved, polyclonal IgG were analyzed using an immunoturbidimetric assay, at baseline (priming dose 1), before the first full dose, and directly before initiation of each subsequent treatment cycle in 44 patients with non-IgG RRMM who received teclistamab monotherapy (RP2D). Serum samples were obtained directly before the teclistamab administration. Time points after patients received IVIG supplementation were removed. The black dotted line represents the lower limit of normal (7 g/L); the gray dotted line represents the LLOD (0.7 g/L). Data are depicted as box plots, indicating the distribution, including median and interquartile range. Wilcoxon rank sum test was used to compare each mean with priming dose 1. ns, not significant. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
Teclistamab treatment reduces peripheral blood B cells and decreases serum IgG levels. (A-C) Longitudinal data representation of (A) absolute B cell counts, (B) CD4+ T cell counts, and (C) CD8+ T cell counts over time in the peripheral blood. Peripheral blood was obtained from 135 unique patients during treatment with teclistamab. There is a reduction of sample numbers over time because of study discontinuation. Most patients discontinued study treatment because of progressive disease (n = 58; 43.0%), and only 5 patients (3.7%) discontinued because of adverse events, including 2 (1.5%) due to infections. (D) Serum levels of uninvolved, polyclonal IgG were analyzed using an immunoturbidimetric assay, at baseline (priming dose 1), before the first full dose, and directly before initiation of each subsequent treatment cycle in 44 patients with non-IgG RRMM who received teclistamab monotherapy (RP2D). Serum samples were obtained directly before the teclistamab administration. Time points after patients received IVIG supplementation were removed. The black dotted line represents the lower limit of normal (7 g/L); the gray dotted line represents the LLOD (0.7 g/L). Data are depicted as box plots, indicating the distribution, including median and interquartile range. Wilcoxon rank sum test was used to compare each mean with priming dose 1. ns, not significant. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
Impact of teclistamab on polyclonal immunoglobulin levels
Next, we assessed the effect of teclistamab treatment on serum polyclonal immunoglobulin levels in sequential peripheral blood serum samples, obtained from patients treated at the RP2D in the MajesTEC-1 study, in which patients who started IVIG treatment were censored for IgG assessments. As expected in these patients who were heavily pretreated, polyclonal immunoglobulins were suppressed at baseline (IgG, IgA, and IgM below lower level of normal in 93%, 99%, and 96% of patients, respectively). After the start of teclistamab therapy, there was a significant additional decrease in polyclonal IgG (median baseline level: 1.39 g/L; cycle 4, day 1: 0.81 g/L; and from cycle 5, day 1 the majority of measurements were below the LLOD; Figure 1D). Moreover, IgA, IgE, and IgM levels decreased rapidly after initiation of teclistamab treatment (supplemental Figure 3). Furthermore, patients with measurable IgD at baseline exhibited a reduction of IgD to the LLOD after initiation of teclistamab treatment (supplemental Figure 3). Notably, for all immunoglobulin isotypes, longitudinal analysis throughout teclistamab treatment showed no recovery of polyclonal immunoglobulin levels over time. In addition, the levels of uninvolved free light chains decreased significantly after teclistamab initiation (supplemental Figure 3).
Antibody response after S pneumoniae and H influenzae type B vaccination
To evaluate the effect of teclistamab on antigen-specific antibody responses, we vaccinated 17 patients against S. pneumoniae and H. influenzae type B during teclistamab monotherapy. All these patients had achieved ≥PR at the time of vaccination. As control groups, we also vaccinated 22 patients with newly diagnosed MM (NDMM) receiving maintenance therapy after autologous stem cell transplantation, 11 patients with RRMM who were not exposed to daratumumab or BsAb (daratumumab-naive RRMM; most patients [82%] were treated with an immunomodulatory drug–based regimen), and 20 patients with RRMM who received a daratumumab-containing regimen (supplemental Table 1). Of note, regardless of control group, the remission status was stable disease or better in all individuals, with the majority of patients with ≥ PR at the time of first vaccination (≥ PR: 100% in patients with NDMM; 73% in patients with daratumumab-naive RRMM; 60% in patients treated with daratumumab; and 100% in patients treated with teclistamab; supplemental Table 1).
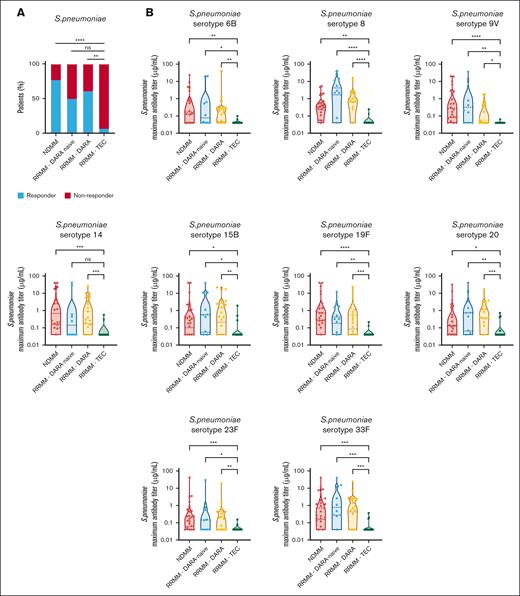
Teclistamab treatment was associated with impaired response (response rate: 7.1%) to S. pneumoniae vaccination compared with the response rate in patients with NDMM (77.3%; P < .0001), patients with daratumumab-naive RRMM (50.0%; P = .05), and patients with daratumumab-treated RRMM (61.1%; P = .002; Figure 2A). The peak antibody titer was also significantly lower for each evaluated pneumococcal subtype in patients treated with teclistamab, than in patients with NDMM and those with daratumumab-naive and daratumumab-treated RRMM (Figure 2B).
Teclistamab impairs vaccine response to S. pneumoniae. (A) Response after vaccination against S. pneumoniae in patients treated with teclistamab (n = 17). Control groups were patients with NDMM on maintenance therapy after autologous stem cell transplantation (n = 22), patients with daratumumab-naive RRMM (n = 11), and patients with RRMM treated with a daratumumab-containing regimen (n = 20). Response rates were compared using Pearson χ2 test or Fisher exact test. (B) Peak specific IgG titers (μg/mL) against pneumococcal serotypes 6B, 8, 9V, 14, 15B, 19F, 20, 23F, and 33F, assessed by enzyme-linked immunosorbent assay, after PCV-13 and PPV-23 vaccination in the teclistamab-treated and control groups. Data are depicted as violin plots, indicating the distribution, including the median and interquartile range. Groups were compared using Kruskal-Wallis test with Dunns correction for multiple comparisons. RRMM-DARA naive, patients with daratumumab-naive RRMM; RRMM-DARA, patients with RRMM treated with a daratumumab-containing regimen; RRMM-TEC, patients with RRMM treated with teclistamab; ns, not significant. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001.
Teclistamab impairs vaccine response to S. pneumoniae. (A) Response after vaccination against S. pneumoniae in patients treated with teclistamab (n = 17). Control groups were patients with NDMM on maintenance therapy after autologous stem cell transplantation (n = 22), patients with daratumumab-naive RRMM (n = 11), and patients with RRMM treated with a daratumumab-containing regimen (n = 20). Response rates were compared using Pearson χ2 test or Fisher exact test. (B) Peak specific IgG titers (μg/mL) against pneumococcal serotypes 6B, 8, 9V, 14, 15B, 19F, 20, 23F, and 33F, assessed by enzyme-linked immunosorbent assay, after PCV-13 and PPV-23 vaccination in the teclistamab-treated and control groups. Data are depicted as violin plots, indicating the distribution, including the median and interquartile range. Groups were compared using Kruskal-Wallis test with Dunns correction for multiple comparisons. RRMM-DARA naive, patients with daratumumab-naive RRMM; RRMM-DARA, patients with RRMM treated with a daratumumab-containing regimen; RRMM-TEC, patients with RRMM treated with teclistamab; ns, not significant. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001.
Similarly, the response rate after H. influenzae vaccination was significantly lower in patients treated with teclistamab (response rate: 5.9%) than in patients with NDMM (87.5%; P < .0001) as well as those with daratumumab-naive RRMM (77.8%; P < .0001) and patients with RRMM who received daratumumab-based therapy (82.4%; P < .0001; Figure 3A). The peak antibody titer was also significantly lower in patients treated with teclistamab (median peak titer, 0.11 μg/mL), than in either control group (median peak titer, 9.0 μg/mL for patients with NDMM; 4.88 μg/mL for patients with daratumumab-naive RRMM; and 2.95 μg/mL for patients with daratumumab-treated RRMM; Figure 3B).
Teclistamab impairs vaccine response to H. influenzae. (A) Response after vaccination against H. influenzae type B in patients treated with teclistamab (n = 17). Control groups were patients with NNMM on maintenance therapy after autologous stem cell transplantation (n = 22), patients with daratumumab-naive RRMM (n = 11), and patients with RRMM treated with a daratumumab-containing regimen (n = 20). Response rates were compared using Pearson χ2 test or Fisher exact test. (B) Peak specific IgG titers (μg/mL), assessed by enzyme-linked immunosorbent assay, after H. influenzae type B vaccination in the teclistamab-treated and control groups. Data are depicted as violin plots, indicating the distribution, including the median and interquartile range. Groups were compared using Kruskal-Wallis test with Dunns correction for multiple comparisons. RRMM-DARA naive, patients with daratumumab-naive RRMM; RRMM-DARA, patients with RRMM treated with a daratumumab-containing regimen; RRMM-TEC, patients with RRMM treated with teclistamab; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001.
Teclistamab impairs vaccine response to H. influenzae. (A) Response after vaccination against H. influenzae type B in patients treated with teclistamab (n = 17). Control groups were patients with NNMM on maintenance therapy after autologous stem cell transplantation (n = 22), patients with daratumumab-naive RRMM (n = 11), and patients with RRMM treated with a daratumumab-containing regimen (n = 20). Response rates were compared using Pearson χ2 test or Fisher exact test. (B) Peak specific IgG titers (μg/mL), assessed by enzyme-linked immunosorbent assay, after H. influenzae type B vaccination in the teclistamab-treated and control groups. Data are depicted as violin plots, indicating the distribution, including the median and interquartile range. Groups were compared using Kruskal-Wallis test with Dunns correction for multiple comparisons. RRMM-DARA naive, patients with daratumumab-naive RRMM; RRMM-DARA, patients with RRMM treated with a daratumumab-containing regimen; RRMM-TEC, patients with RRMM treated with teclistamab; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001.
SARS-CoV-2 vaccination
Thirteen patients without documented prior SARS-CoV-2 infection started SARS-CoV-2 vaccination with mRNA vaccines (mRNA1273 [Moderna] or BNT162b2 [Pfizer]). Approximately 5 months after completing the standard 2-dose schedule, patients received a third vaccination (booster shot). Vaccine-induced spike S1 domain-reactive antibodies were quantified ∼4 weeks after the third vaccination. Throughout the vaccination period, all patients continued to receive teclistamab, and none received passive immunotherapy with anti–SARS-CoV-2 antibody products as prophylaxis. None of them developed COVID-19 infection before response assessment. After 3 mRNA vaccinations against SARS-CoV-2, none of the patients had a vaccine-induced anti-spike IgG response (antibody levels were below the LLOD in all 13 patients tested).
Impact of IVIG supplementation
Because teclistamab treatment reduced polyclonal IgG and impaired the generation of new antibodies because of elimination of normal B cells and normal plasma cells, we analyzed the impact of IVIG supplementation on risk of infections in all 52 patients treated with teclistamab at Amsterdam University Medical Center. Twenty of these patients received IVIG supplementation to prevent development of serious infections (4 patients were already receiving IVIG before teclistamab treatment because of history of serious infections, and 16 started IVIG supplementation early after initiating teclistamab therapy if polyclonal IgG level was < 4 g/L [within 1-2 months; primary prophylaxis]). None of these 20 patients discontinued IVIG supplementation during teclistamab treatment. The remaining 32 patients in the observation group only received IVIG supplementation when they experienced a severe infection and had polyclonal IgG of < 4 g/L to prevent new episodes of severe infections (secondary prophylaxis). Patient characteristics and disease-related factors were comparable between both groups (Table 1).
Baseline characteristics of patients treated with teclistamab combined with IVIG supplementation, or without IVIG supplementation
| . | IVIG as primary prophylaxis, n = 20 . | No IVIG (IVIG only after serious infection), n = 32 . | ||
|---|---|---|---|---|
| Age (y), median (range) | 64 (47 – 80) | 64 (43 – 79) | ||
| Male sex, n (%) | 11 (55) | 18 (56) | ||
| M-protein, n (%) | ||||
| IgG | 12 (60) | 21 (66) | ||
| IgA | 0 | 4 (13) | ||
| IgD | 0 | 1 (3) | ||
| Light chain only | 8 (40) | 6 (19) | ||
| Time (mos) since diagnosis, median (range) | 89 (23 – 211) | 72 (15 – 281) | ||
| Duration (mos) of follow-up since start of teclistamab, median (range) | 5 (1 – 31) | 5 (0.8 – 51) | ||
| Number of prior lines of treatment, median (range) | 5 (2 – 11) | 6 (2 – 14) | ||
| Prior stem cell transplantation, n (%) | ||||
| Autologous SCT | 14 (70) | 27 (84) | ||
| Allogeneic SCT | 2 (10) | 5 (16) | ||
| Refractory disease, n (%) | ||||
| IMiD refractory | 19 (95) | 31 (97) | ||
| PI refractory | 15 (75) | 26 (81) | ||
| CD38-targeting antibody refractory | 20 (100) | 30 (94) | ||
| Triple-class refractory∗ | 14 (70) | 23 (72) | ||
| Penta-drug refractory† | 3 (15) | 10 (31) | ||
| Prior IMiD treatment, n (%) | Exposed | Refractory‡ | Exposed | Refractory‡ |
| Thalidomide | 12 (60) | 2 (10) | 13 (41) | 5 (16) |
| Lenalidomide | 19 (95) | 18 (90) | 31 (97) | 30 (94) |
| Pomalidomide | 14 (70) | 14 (70) | 24 (75) | 24 (75) |
| Iberdomide | 4 (20) | 4 (20) | 11 (34) | 11 (34) |
| Prior PI treatment, n (%) | Exposed | Refractory‡ | Exposed | Refractory‡ |
| Bortezomib | 16 (80) | 12 (60) | 31 (97) | 21 (66) |
| Carfilzomib | 11 (55) | 4 (20) | 22 (69) | 17 (53) |
| Ixazomib | 3 (15) | 3 (15) | 1 (3) | 1 (3) |
| Prior CD38-targeting antibody treatment, n (%) | Exposed | Refractory‡ | Exposed | Refractory‡ |
| Daratumumab | 20 (100) | 20 (100) | 29 (91) | 29 (91) |
| Isatuximab | 0 | 0 | 1 (3) | 1 (3) |
| Prior bispecific antibody treatment, n (%) | Exposed | Refractory‡ | Exposed | Refractory‡ |
| Teclistamab (BCMAxCD3) | 0 | 0 | 0 | 0 |
| Talquetamab (GPRC5dxCD3) | 1 (5) | 1 (5) | 3 (9) | 3 (9) |
| Other prior myeloma treatment, n (%) | Exposed | Refractory‡ | Exposed | Refractory‡ |
| Elotuzumab | 2 (10) | 2 (10) | 3 (9) | 3 (9) |
| Durvalumab | 1 (5) | 1 (5) | 3 (9) | 3 (9) |
| Nivolumab | 2 (10) | 2 (10) | 4 (13) | 3 (9) |
| . | IVIG as primary prophylaxis, n = 20 . | No IVIG (IVIG only after serious infection), n = 32 . | ||
|---|---|---|---|---|
| Age (y), median (range) | 64 (47 – 80) | 64 (43 – 79) | ||
| Male sex, n (%) | 11 (55) | 18 (56) | ||
| M-protein, n (%) | ||||
| IgG | 12 (60) | 21 (66) | ||
| IgA | 0 | 4 (13) | ||
| IgD | 0 | 1 (3) | ||
| Light chain only | 8 (40) | 6 (19) | ||
| Time (mos) since diagnosis, median (range) | 89 (23 – 211) | 72 (15 – 281) | ||
| Duration (mos) of follow-up since start of teclistamab, median (range) | 5 (1 – 31) | 5 (0.8 – 51) | ||
| Number of prior lines of treatment, median (range) | 5 (2 – 11) | 6 (2 – 14) | ||
| Prior stem cell transplantation, n (%) | ||||
| Autologous SCT | 14 (70) | 27 (84) | ||
| Allogeneic SCT | 2 (10) | 5 (16) | ||
| Refractory disease, n (%) | ||||
| IMiD refractory | 19 (95) | 31 (97) | ||
| PI refractory | 15 (75) | 26 (81) | ||
| CD38-targeting antibody refractory | 20 (100) | 30 (94) | ||
| Triple-class refractory∗ | 14 (70) | 23 (72) | ||
| Penta-drug refractory† | 3 (15) | 10 (31) | ||
| Prior IMiD treatment, n (%) | Exposed | Refractory‡ | Exposed | Refractory‡ |
| Thalidomide | 12 (60) | 2 (10) | 13 (41) | 5 (16) |
| Lenalidomide | 19 (95) | 18 (90) | 31 (97) | 30 (94) |
| Pomalidomide | 14 (70) | 14 (70) | 24 (75) | 24 (75) |
| Iberdomide | 4 (20) | 4 (20) | 11 (34) | 11 (34) |
| Prior PI treatment, n (%) | Exposed | Refractory‡ | Exposed | Refractory‡ |
| Bortezomib | 16 (80) | 12 (60) | 31 (97) | 21 (66) |
| Carfilzomib | 11 (55) | 4 (20) | 22 (69) | 17 (53) |
| Ixazomib | 3 (15) | 3 (15) | 1 (3) | 1 (3) |
| Prior CD38-targeting antibody treatment, n (%) | Exposed | Refractory‡ | Exposed | Refractory‡ |
| Daratumumab | 20 (100) | 20 (100) | 29 (91) | 29 (91) |
| Isatuximab | 0 | 0 | 1 (3) | 1 (3) |
| Prior bispecific antibody treatment, n (%) | Exposed | Refractory‡ | Exposed | Refractory‡ |
| Teclistamab (BCMAxCD3) | 0 | 0 | 0 | 0 |
| Talquetamab (GPRC5dxCD3) | 1 (5) | 1 (5) | 3 (9) | 3 (9) |
| Other prior myeloma treatment, n (%) | Exposed | Refractory‡ | Exposed | Refractory‡ |
| Elotuzumab | 2 (10) | 2 (10) | 3 (9) | 3 (9) |
| Durvalumab | 1 (5) | 1 (5) | 3 (9) | 3 (9) |
| Nivolumab | 2 (10) | 2 (10) | 4 (13) | 3 (9) |
GPRC5D, G protein–coupled receptor family C group 5 member D; IMiD, immunomodulatory drug; PI, proteasome inhibitor; SCT, stem cell transplantation.
triple-class refractory means refractory to an IMiD, a PI, and a CD38-targeting antibody;
penta-drug refractory means refractory to lenalidomide, pomalidomide, bortezomib, carfilzomib, and a CD38-targeting antibody;
refractory disease is defined as progressive disease during therapy, no response (less than PR), or progressive disease within 60 days of stopping treatment, according to the International Uniform Response Criteria for Multiple Myeloma.
First, we analyzed the incidence of infections during observation (no IVIG) vs during IVIG treatment (primary prophylaxis). The incidence rate was 1.36 per patient-year (95% CI, 0.84-2.03) in the observation group vs 0.12 per patient-year (95% CI, 0.014-0.42) in the IVIG group (incidence rate ratio, 11.6; 95% CI, 2.70-50.0l; P = .001; Figure 4A]). Also, time-to-event analysis showed a significantly greater cumulative incidence of serious infections in the observation group than in the IVIG group (cumulative incidence of serious infections at 6 months: 54.8% vs 5.3%; P < .001; Figure 4B). There were only 2 episodes of serious infections in 2 patients during 204.5 months of IVIG treatment (1 patient with stable disease and history of recurrent urinary tract infections related to benign prostatic hyperplasia, developed a urinary tract infection [Escherichia coli], while his IgG level was in the target range [5.1 g/L]; the second patient in complete remission developed a pneumonia and breast abscess [Pseudomonas aeruginosa] when IgG was below the target level (2.16 g/L; monthly IVIG dose too low). In contrast, there were 20 infectious episodes in 18 patients not receiving IVIG during 176.8 months of observation. The most common type of grade ≥3 infections reported in the observation group were lower respiratory tract infections (13 of 20 infections [65.0%]; Table 2). A total of 18 of 20 infections had microbiological confirmation, with 13 (72.2%) bacterial, 4 (22.2%) viral, and 1 (5.6%) a combination of viral and bacterial isolates. P. aeruginosa was the most common pathogen (isolated in 8 infections; Table 2). All patients in the observation group had IgG of < 4 g/L at the time of serious infections (median IgG level: 1.67 g/L). At the time of infection, 17 of 18 patients (94.4%) had achieved ≥ PR with at least very good partial response in 11 patients (61.1%), whereas 1 patient (5.6%) had stable disease. In a multivariate analysis, IVIG supplementation was independently associated with decreased risk of severe infection (P = .009), with a trend of higher risk for patients with low baseline serum albumin level (P = .062). Other features had no impact on infection risk (age; baseline neutrophil and lymphocyte count; and baseline level of polyclonal IgG, LDH, and β2-microglobulin).
IVIG supplementation reduces the frequency of serious infections in patients treated with teclistamab. (A) Serious (grade ≥ 3) infectious events per patient-year in patients treated with teclistamab according to treatment with IVIG (primary prophylaxis) or without IVIG (observation group). (B) Cumulative incidence plot of time to first serious infection in patients treated with teclistamab according to treatment with IVIG (primary prophylaxis) or without IVIG (observation group). IRR, incidence rate ratio.
IVIG supplementation reduces the frequency of serious infections in patients treated with teclistamab. (A) Serious (grade ≥ 3) infectious events per patient-year in patients treated with teclistamab according to treatment with IVIG (primary prophylaxis) or without IVIG (observation group). (B) Cumulative incidence plot of time to first serious infection in patients treated with teclistamab according to treatment with IVIG (primary prophylaxis) or without IVIG (observation group). IRR, incidence rate ratio.
Serious infections by type and pathogen according to treatment with and without IVIG
| . | IVIG as primary prophylaxis . | No IVIG . |
|---|---|---|
| Pneumonia/pneumosepsis | 1 episode (also with breast abscess) • P. aeruginosa (n = 1) | 11 episodes • P. aeruginosa (n = 5) • P. aeruginosa and Klebsiella pneumoniae (n = 1) • Enterobacter cloacae (n = 1) • Infuenza A + P. aeruginosa (n = 1) • Moraxella catarrhalis (n = 1) • No pathogen (n = 2) |
| Pneumonia and empyema | 0 | 2 episodes • P aeruginosa (n = 1) • Moraxella catarrhalis (n = 1) |
| Urosepsis | 1 episode • E. coli (n = 1) | 3 episodes • E. coli (n = 2) • Citrobacter freundii (n = 1) |
| COVID-19 | 0 | 4 episodes |
| . | IVIG as primary prophylaxis . | No IVIG . |
|---|---|---|
| Pneumonia/pneumosepsis | 1 episode (also with breast abscess) • P. aeruginosa (n = 1) | 11 episodes • P. aeruginosa (n = 5) • P. aeruginosa and Klebsiella pneumoniae (n = 1) • Enterobacter cloacae (n = 1) • Infuenza A + P. aeruginosa (n = 1) • Moraxella catarrhalis (n = 1) • No pathogen (n = 2) |
| Pneumonia and empyema | 0 | 2 episodes • P aeruginosa (n = 1) • Moraxella catarrhalis (n = 1) |
| Urosepsis | 1 episode • E. coli (n = 1) | 3 episodes • E. coli (n = 2) • Citrobacter freundii (n = 1) |
| COVID-19 | 0 | 4 episodes |
Fourteen of 18 patients in the observation group who developed a serious infection subsequently initiated IVIG treatment (secondary prophylaxis; 77.8%), whereas 3 died as a consequence of the infection (16.7%), and 1 went off study because of disease progression (5.6%). Only 1 of these 14 patients developed a serious infection (Campylobacter infection; IgG, 5.8 g/L) after initiation of IVIG treatment during 144.8 months of follow-up (incidence rate: 0.083 per patient-year; 95% CI, 0.0021-0.45), which is significantly lower than what was observed in the absence of IVIG supplementation (incidence rate ratio = 16.4; 95% CI, 2.22-125; P = .006). The cumulative frequency of serious infections after initiation of delayed IVIG treatment (secondary prophylaxis) was 0% after 6 months (supplemental Figure 4). None of the patients discontinued IVIG treatment because of adverse events.
Discussion
Our data show that teclistamab reduces the levels of polyclonal immunoglobulins and impairs humoral immune response after vaccination, which is consistent with the depletion of normal plasma cells in ex vivo assays, and with profound decrease of B cells during therapy. Based on our data showing a substantial reduction in serious infections, we recommend immunoglobulin supplementation to prevent serious infections in patients treated with teclistamab or other BCMA-targeting BsAbs as soon as polyclonal IgG levels drop below 4 g/L.
BCMA is expressed on mature B cells and normal and malignant plasma cells, and plays a critical role in regulation of B-cell proliferation and survival, as well as differentiation of B cells into plasma cells.24 We also detected BCMA protein on the surface of a fraction of B cell progenitors in the BM. Normal plasma cells have similar BCMA expression to that of malignant plasma cells,15,16 which explains why teclistamab effectively depletes BCMA-positive normal plasma cells. We also show, to our knowledge, for the first time, that teclistamab treatment rapidly reduces peripheral blood B-cell counts by > 99%. Because both B cells and plasma cells are critical to the generation of humoral immune response, it is not surprising that teclistamab substantially reduced polyclonal immunoglobulin levels. In addition to confirming impaired humoral immune response after SARS-CoV-2 vaccination in patients receiving BCMA-directed therapies (antibody–drug conjugates, chimeric antigen receptor T cells, and BsAbs),25-32 we show compromised vaccination responses against S. pneumoniae and H. influenzae in patients treated with teclistamab compared with responses observed in patients with NDMM or RRMM, including those receiving treatment with anti-CD38 antibodies. Overall, this illustrates the profound dampening effect of teclistamab on humoral immune responses, which may, in part, explain the high rate of infections.
Although larger studies are needed to confirm our findings, we recommend starting IVIG directly when patients develop hypogammaglobulinemia (IgG < 4.0 g/L) because of the high rate of serious infections (including infectious deaths) in the absence of a clear plateau in the observation group. Notably, in the subset of patients without IVIG supplementation there was a high incidence of gram-negative bacterial infections, especially P. aeruginosa. There is preclinical evidence that IVIG, which also contains specific anti-Pseudomonas antibodies, confers protection against severe Pseudomonas infections in mouse models.33-35 Although patient numbers are small, there was also a lower frequency of severe COVID-19 infections in the primary prophylaxis group. To what extent IVIG protects against COVID-19 is unclear, but current IVIG products may protect against severe COVID-19 infections because these contain high titers of neutralizing IgG SARS-CoV-2 antibodies from vaccination and natural infection.36,37 The low incidence of gram-positive bacterial infections in both groups could be explained, in part, by the use of co-trimoxazole in the majority of patients to prevent PJP, because co-trimoxazole also has a prophylactic antibacterial effect (eg, against S. pneumoniae).38 Although patient characteristics and disease-related factors were comparable between patients receiving IVIG as primary prophylaxis vs those in the observation group, a limitation of our analysis is the potential for selection bias because of the absence of randomization and its retrospective nature. However, we observed a comparable protective effect when IVIG was initiated after a grade ≥ 3 infection in patients who initially received observation only, which suggests that selection probably had no or only limited impact on observed infection rates. In addition, multivariate analysis, adjusting for potentially confounding factors, showed that IVIG supplementation was an independent prognostic factor for severe infections. Furthermore, our data are in agreement with another retrospective single-center study with 37 patients treated with BCMA-targeting BsAbs.39 In that study, IVIG was used in 92% of the patients who achieved a disease response, but these patients were on IVIG treatment only 56% of the time.39 There was a 10-fold reduction in serious infections while patients were on IVIG, compared with periods without IVIG supplementation.39 Limitations of IVIG use include high cost, limited availability, and, although typically well tolerated, some patients develop infusion-related reactions.11
Although correction of low IgG levels with IVIG prophylaxis resulted into a substantial decline in the occurrence of serious infections, there were a small number of breakthrough infections. Secretory IgA plays a crucial role in protecting mucosal membranes against pathogens40 and its persisting deficiency (IVIG contains only trace amounts of non-IgG antibodies) may contribute to increased risk of infections. Furthermore, there is increasing evidence that long-term exposure to T-cell redirecting BsAbs results in T-cell exhaustion, which probably increases the risk of viral infection.14 In addition, development of neutropenia, especially during the first 1 to 2 cycles, may contribute to increased infection risk.1,41 Alongside prophylactic IVIG treatment in case of low IgG concentrations (< 4 g/L), additional strategies to prevent infections in patients treated with BCMA-targeting BsAbs are essential to reduce the burden of infectious complications, including the prophylactic use of granulocyte colony-stimulating factor, herpes zoster prophylaxis, and PJP prophyaxis.2,11,13,42 IVIG supplementation may also be considered in patients with higher IgG levels and recurrent bacterial infections (especially due to encapsulated bacteria) despite antibiotic prophylaxis, because these patients may not be able to mount an adequate immune response.13,42 Before starting teclistamab, patients should be screened for hepatitis B virus, hepatitis C virus, and HIV, and patients at risk of hepatitis B virus reactivation should receive appropriate prophylaxis.13,42 Patients should also be carefully monitored for signs and symptoms of infection, with prompt treatment of infection, during which teclistamab treatment should be postponed. Patients treated with BCMA BsAbs with early COVID-19 can be offered antiviral therapy to prevent hospitalization or death.43,44 Furthermore, in the future, these patients may also benefit from new monoclonal antibodies with efficacy against circulating SARS-CoV-2 variants in case of breakthrough infection or for primary prevention.44 Continuous therapy with BsAbs is associated with persistent immune paresis and therefore new studies should explore fixed-duration treatment or administration of lower/less-frequent maintenance doses upon achieving a deep response to allow for immune recovery.
Although humoral immunity is a key measure of SARS-CoV-2 vaccine response, the development of inadequate antibody titers does not necessarily mean absence of vaccine benefit because vaccination may also provide protection against SARS-CoV-2 through the generation of a T cell response.45,46 We therefore recommend the vaccination of all patients receiving BsAb treatment with (emerging variant-specific) SARS-CoV-2 vaccines. However, T cell responses may be compromised in these patients, because of disease-related T-cell dysfunction and BsAb-mediated T-cell exhaustion.14 Therefore, studies are warranted to investigate the impact of BsAbs on cellular immune response after SARS-CoV2 vaccination, and also after other vaccinations. The value of vaccination against S. pneumoniae and H. influenzae in patients receiving BCMA-specific BsAbs is probably limited, because vaccine-induced antibodies play a crucial role in protecting against these encapsulated bacteria. Importantly, the best setting in which to vaccinate patients with MM is probably during periods of well controlled disease, early in the disease course when prior exposure to immunosuppressive therapy is minimal, compared with the relapsed/refractory disease setting.13,47
In conclusion, patients treated with teclistamab have a high rate of serious infections, which is attributable, at least in part, to the development of hypogammaglobulinemia and failure to generate new humoral immune responses. Use of IVIG supplementation was associated with a significantly lower risk of serious infections, underlining the severity of teclistamab-induced defects in humoral immunity. Our data support the use of immunoglobulin supplementation as primary prophylaxis in patients receiving a BCMA-targeting BsAb who develop severe hypogammaglobulinemia.
Acknowledgments
The MajesTEC-1 study was supported by Janssen Pharmaceuticals. The funder of the study had a role in study design (MajesTEC-1 study), data collection, data analysis, data interpretation, and review of the report.
Authorship
Contribution: K.A.F., S.Z. R.I.V., and N.W.C.J.v.d.D. designed the study; M.V.M., T.G.M., C.R., A.N., S.K., M.T.K., K.G., H.L.Z., S.Z., and N.W.C.J.v.d.D. enrolled patients, contributed data, and interpreted data; K.A.F., C.P.M.V., A.B., K.C., A.P.-P., T.S., C.U., R.K., B.v.d.H., D.V., S.S., D.C.-S., and M.D. designed and performed statistical analyses; R.I.V. and N.W.C.J.v.d.D. drafted the first version of the manuscript; and all authors had access to all the data reported in the study, and revised and approved the final version of the manuscript.
Conflict-of-interest disclosure: M.V.M. reports honoraria from, and membership on a board of directors/advisory committee for, Amgen, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Janssen Cilag, Pfizer, Sanofi, and Takeda; is a speaker bureau member for Janssen Cilag; and is a board of directors/advisory committee member for Oncopeptides. T.G.M. reports research funding (institutional) from Amgen, Janssen, Sanofi, and Seattle Genetics; and consultancy for GlaxoSmithKline and Roche. C.R. reports personal fees from Amgen, Bristol Myers Squibb, and Takeda. A.N. has served on advisory boards of, and received honorarium from, Adaptive Biotechnologies, Amgen, BeyondSpring, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Karyopharm, Oncopeptides, Pfizer, Sanofi, Secura Bio, and Takeda; has received grant/research support (institutional) from Aduro Biotech, Amgen, Arch Oncology, Bristol Myers Squibb, Genentech, GlaxoSmithKline, Janssen, Karyopharm, Kite Pharma, Pfizer, and Takeda; and received grant/research support for investigator-initiated studies from Amgen, GlaxoSmithKline, Janssen, Merck, and Takeda. A.B. is an employee of Janssen and is a stockholder in Janssen. K.C. is an employee of Janssen and is a stockholder in Janssen. A.P.-P. is an employee of Janssen and is a stockholder in Janssen. T.S. is an employee of Janssen and is a stockholder in Janssen. C.U. is an employee of Janssen and is a stockholder in Janssen. R.K. is an employee of Janssen and is a stockholder in Janssen. B.v.d.H. reports honoraria for data safety monitoring board membership from the Intergroupe Francophone du Myélome. S.K. serves on advisory boards for Janssen Pharmaceuticals. D.V. is an employee of Janssen and is a stockholder in Janssen. S.S. is an employee of Janssen and is a stockholder in Janssen. D.C.-S. is an employee of Janssen and is a stockholder in Janssen. M.D. is an employee of Janssen and is a stockholder in Janssen. S.Z. has received research funding from Celgene, Takeda, and Janssen; and serves on advisory boards for Janssen, Takeda, Bristol Myers Squibb, Oncopeptides, and Sanofi, all paid to institution. R.I.V. is an employee of Janssen and is a stockholder in Janssen. N.W.C.J.v.d.D. has received research support from Janssen Pharmaceuticals, Amgen, Celgene, Novartis, Cellectis, and Bristol Myers Squibb; and serves on advisory boards for Janssen Pharmaceuticals, AMGEN, Celgene, Bristol Myers Squibb, Takeda, Roche, Novartis, Bayer, Adaptive, Pfizer, AbbVie, and Servier, all paid to institution. The remaining authors declare no competing financial interest.
Correspondence: Niels W.C.J. van de Donk, Department of Hematology, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; email: n.vandedonk@amsterdamumc.nl.
References
Author notes
Presented, in part, at the annual meeting of the European Hematology Association, Frankfurt, Germany, 8 June 2023.
Data are available on request from the corresponding author, Niels W. C. J. van de Donk (n.vandedonk@amsterdamumc.nl).
The full-text version of this article contains a data supplement.