Incorporation of baseline CTCs, with TLG level in the PET/CT-based volumetric assessment, enhances the stratification of patients with TIE-MM.
Compared with only R-ISS, our combined model improved the predictive performance as it allocated patients in the R-ISS II category.
Visual Abstract
We aimed to improve prognostic predictors in patients with transplant-ineligible multiple myeloma (TIE-MM) by combining baseline circulating clonal tumor cells (CTCs) and positron emission tomography/computed tomography (PET/CT) findings. The factors associated with prognosis were retrospectively investigated in 126 patients with TIE-MM who underwent CTC quantification by multiparameter flow cytometry and PET/CT at the initial presentation. The total lesion glycolysis (TLG) level was calculated using the Metavol software. The median percentage of CTC was 0.06% (range, 0%-4.82%), and 54 patients (42.9%) demonstrated high CTC levels. High CTC levels were associated with significantly poorer progression-free survival (PFS, 2-year 43.4% vs 68.1%; P < .001) and overall survival (OS, 5-year 39.0% vs 68.3%; P < .001). Similarly, high TLG levels significantly worsened the PFS (2-year, 41.2% vs 67.6%; P = .038) and OS (5-year, 37.7% vs 63.1%; P = .019). The multivariate analyses showed that Revised International Staging System (R-ISS) III, high CTC and TLG levels, and complete response were significant prognostic factors for PFS and OS. A novel predictive model was constructed using CTCs, TLG, and R-ISS III. The patients were stratified into 3 groups according to the number of risk factors, revealing an extremely high-risk group with a 2-year PFS of 0% and a 5-year OS of 20%. Patients without any high-risk features had better prognosis, with a 2-year PFS of 78.6% and a 5-year OS of 79.5%. The combination of CTCs and volumetric assessment of PET/CT at diagnosis augments the existing stratification systems and may pave the way for a risk-adapted treatment approach.
Introduction
Multiple myeloma (MM) is becoming increasingly common in older adults, with a median age of 69 years: ∼35% of the patients diagnosed with MM are aged ≥75 years, whereas 10% are aged ≥85 years.1 With the advent of novel therapies, many patients with MM are expected to achieve long-term survival.2 On the contrary, patients with transplant-ineligible (TIE) MM are likely to experience adverse outcomes from high-intensity treatments, necessitating a personalized approach based on appropriate risk assessments. The most commonly used prognostic system is the Revised International Staging System (R-ISS), which incorporates biological aspects into the laboratory parameter–based ISS.3 Although its prognostic relevance has been validated in numerous studies,4 the predictive performance is far from satisfactory because a substantial proportion of patients is consistently categorized as having stage II disease.
Circulating clonal tumor cells (CTCs) in the peripheral blood (PB) serve as a readily and noninvasive biomarker of various plasma cell disorders.5,6 The quantification of CTCs using sensitive multiparameter flow cytometry (MFC) has an independent prognostic impact on newly diagnosed MM (NDMM).7-14 However, as elucidated by 2 prospective trials published in 2022,10,11 the body of evidence regarding CTCs predominantly pertains to patients who are transplant eligible (TE) and individuals participating in clinical trials. Although Jelinek et al reported that a CTC level of >2% led to dismal outcomes similar to that observed in patients with TIE-MM with primary plasma cell leukemia (PCL), this 2% threshold seems suitable only for identifying patients who are at ultra-high risk rather than for broader stratification purposes.12
Furthermore, the complementary risk factors of CTC levels for refining risk models have not been fully explored, except for R-ISS.15,16 One of the candidate indicators is the total tumor burden of intra- and extramedullary myeloma lesions. Our research group and others previously reported the prognostic utility of the quantitative radiomic parameters derived from fluorine-18 fluorodeoxyglucose-positron emission tomography/computed tomography (18F-FDG-PET/CT)–based volumetric assessment, such as total metabolic tumor volume (TMTV) and total lesion glycolysis (TLG) level.17-19 However, studies examining the relationship between CTCs and these quantitative radiomics in patients with MM are lacking.
Therefore, this study aimed to (1) examine the prognostic impact of CTC levels in real-world patients with TIE-MM and (2) evaluate the performance of an integrated risk model that incorporates CTCs, TLG, and R-ISS.
Methods
Patient cohort and study design
We conducted a retrospective analysis of patients with TIE-NDMM with available PB-MFC and PET/CT data, who were treated at Kameda Medical Center between January 2011 and July 2021. According to recently updated diagnostic criteria,20 PCL was defined as having ≥5% circulating plasma cells in PB, and patients with this characteristic were excluded from the study. The diagnosis and response to treatment were evaluated based on the International Myeloma Working Group (IMWG) criteria.21 All PET/CT findings were interpreted in accordance with the IMWG consensus statement.22 t(4;14), t(14;16), and del(17p) identified by interphase fluorescence in situ hybridization were defined as high-risk cytogenetic abnormalities (HRCA). This study was conducted in accordance with the Declaration of Helsinki and was approved by our institutional review board (approval number: 22-061). The dataset was locked on 31 July 2023.
Quantification of CTCs
To quantify CTCs, mononuclear cells extracted from PB before treatment were collected in tubes containing ethylenediaminetetraacetic acid and promptly subjected to a red blood cell lysis procedure, followed by washes, cell suspension, and antibody staining. From 2011 to 2017, a 6-color panel of antibodies, CD38-PC7, CD138-APC, CD45-ECD, CD19-PE, and CD56-PC5.5, was used in accordance with the guidelines proposed by the European Myeloma Network during that period.23 Subsequently, the panel was revised to an 8-color DuraClone RE PC kit consisting of CD138-APC, CD38-PB, CD56-APC-A750, CD19-PC5.5, CD45-KrO, CD200-PC7, CD81–fluorescein isothiocyanate, and CD27-PE.24 After permeabilization, the samples were stained with cytoplasmic Igκ–fluorescein isothiocyanate and Igλ–phycoerythrin, along with CD38, CD45, CD138, and CD19 monoclonal antibodies, to substantiate the clonality of aberrant plasma cells. According to the findings of a previous study using a 6-color MFC,7 the minimum required number of clonal events per total collected cells was determined to be 20 out of 150 000 (0.013%). Consequently, the lower detection limit for CTCs was set at 0.01% throughout the study period. Any percentage below this sensitivity threshold was recorded as 0. Data were acquired on a Navios flow cytometer using Kaluza software (Beckman Coulter, CA).
Measurement of PET/CT–derived parameters
All PET/CT images were acquired using PET/CT scanners (Discovery ST Elite Performance, GE Medical Systems, Milwaukee, WI [January 2011-June 2017]; Discovery IQ ODYSSEY 5R, GE Medical Systems, Milwaukee, WI [June 2017-July 2021]) according to our institution’s standardized protocol; the detailed procedures are described elsewhere.17 As reported by Zamagni et al,25 PET/CT positivity was determined based on the following criteria: (1) the presence of focal areas with detectably increased tracer uptake within background bones, with or without an underlying lesion identified by CT; or (2) a maximum standardized uptake value (SUVmax) of ≥2.5 within osteolytic CT areas if >1 cm, and an SUVmax >1.5 if the size is between 0.5 cm and 1 cm. Focal lesions (FLs) were visibly identified regardless of the SUVmax within at least 2 contiguous CT slices. High-risk PET/CT findings were defined as the presence of >3 FLs, an SUVmax of >4.2, or detection of extramedullary disease (EMD).25 Similar to our previous study,17 TMTV was defined as the total volume of bone and EMD lesions with an SUV of ≥2.5. The TLG level was calculated using the following formula: TLG = TMTV × mean SUV, thereby incorporating tumor metabolic activity into the volumetric information. If no positive areas were detected on PET/CT scans, the TMTV and TLG level were recorded as 0. To delineate myeloma lesions and calculate the TMTV and TLG level, a semiautomatic computer-aided analysis of PET/CT was conducted using the open-source software Metavol (Hokkaido University, Sapporo, Japan) (supplemental Figure 1).
Statistical analysis
Continuous variables were analyzed using the Mann-Whitney U test, whereas categorical variables were compared using the Fisher exact test. Pearson correlation analysis was used to examine the relationships between continuous variables. The optimal cut-off values for 2-year progression-free survival (PFS) and 5-year overall survival (OS) were determined using receiver operating curves (ROCs) with the Youden index. The PFS and OS were estimated using the Kaplan-Meier methods and compared with the log-rank test. The Holm adjustment was used for multiple comparisons in survival analysis. Univariate and multivariate analyses were conducted using Cox proportional hazards models to assess the factors affecting PFS and OS. Akaike information criterion (AIC) and Harrell concordance index (c-index) were used to compare the predictive performance of various risk models. All statistical analyses were performed using R version 4.1.1 (R Foundation, Vienna, Austria). Statistical significance was defined as a 2-sided P value of <.05.
Results
Clinical characteristics of the entire cohort
Of the 266 patients with NDMM, 126 with TIE-MM were included in this analysis after excluding those who were TE, with PCL, and without available PET/CT or MFC data (supplemental Figure 2). The median age was 78 years (interquartile range [IQR], 73-83), and 48.4% of the patients had a European Cooperative Oncology Group (ECOG) performance status (PS) score of ≥2. Approximately 47.6% of the patients were classified as having ISS III. Twenty-four patients (19.0%) had HRCA, of whom 17 (70.8%) were t(4;14) and 7 (29.2%) had del(17p). Consequently, 22 patients (17.5%) were classified as having R-ISS III.
The most frequent induction regimen was a bortezomib/lenalidomide/dexamethasone triplet (64.3%), followed by a daratumumab-based regimen (18.3%) and a bortezomib/dexamethasone doublet (13.5%). Approximately 72.2% and 50.0% of the patients showed greater or equal to very good partial response and greater or equal to complete response (CR) before progression, respectively. Within the median observation period of 41 months (IQR, 24-69), 60 patients died, whereas 83 experienced a relapse or progression. The median estimated PFS and OS were 31 months (95% confidence interval [CI], 24-40) and 64 months (95% CI, 48-113), respectively (supplemental Figure 3).
Quantification of CTCs and their association with clinical parameters
CTCs were detected in 103 patients (80.5%) with a detection limit of 0.01%. The median percentage of CTCs was 0.06% (range, 0%-4.82%). Using ROC analysis, the optimal cut-off values for predicting 2-year PFS and 5-year OS were determined to be 0.08% (area under the curve [AUC], 0.64) and 0.09% (AUC, 0.61), respectively (supplemental Figure 4). For simplicity, 0.09% was selected as the cut-off value, identifying 54 patients (42.9%) with high CTC burden.
The baseline clinical features according to the CTC levels are shown in Table 1. Patients with high CTC levels were more likely to have high ECOG PS scores (61.1% vs 38.9%; P = .022) and be classified as having ISS III (59.3% vs 38.9%; P = .037). This may reflect the aggressive nature of the disease, including the higher levels of clonal bone marrow plasma cells (cBMPCs) (median %: 80 vs 55; P < .001), the involvement of free light chain (FLC) (median [mg/L], 949 vs 430; P = .029), and the presence of anemia (median hemoglobin level [g/dL], 8.9 vs 10; P = .003). In line with the findings of a previous study,11 the correlation of CTC burden with cBMPC and involvement of FLC was significant, albeit modest (R = 0.25, P = .004 for BMPC and R = 0.36, P < .001 for FLC, respectively). Furthermore, patients with HRCA had higher CTC levels than patients without HRCA (median, 0.2% vs 0.05%; P = .024), especially in those with t(4;14) (median, 0.25% vs 0.05%; P = .003) (supplemental Figure 5).
Clinical characteristics of included patients according to CTC burden
| . | Total (n = 126 [100%]) . | Patients with low CTCs (n = 72 [57.1%]) . | Patients with high CTCs (n = 54 [42.9%]) . | P value . |
|---|---|---|---|---|
| Age, y, median (IQR) | 78 (73-83) | 78 (71-83) | 80 (75-83) | .32 |
| Age ≥80 y, n (%) | 58 (46.0) | 31 (43.1) | 27 (51.9) | .34 |
| Female, n (%) | 67 (53.2) | 35 (48.6) | 32 (59.3) | .315 |
| ECOG-PS ≥2, n (%) | 61 (48.4) | 28 (38.9) | 33 (61.1) | .022 |
| Heavy chain, n (%) | ||||
| G | 71 (56.3) | 46 (63.9) | 25 (46.3) | .069 |
| A | 30 (23.8) | 16 (22.2) | 14 (25.9) | .676 |
| Light chain only | 20 (15.9) | 9 (12.5) | 11 (20.4) | .325 |
| Others | 5 (4.0) | 1 (1.4) | 4 (7.5) | .163 |
| Involved Ig (mg/dL), median (IQR) | 4356 (2680-5985) | 4084 (2577-5722) | 4922 (2850-6148) | .562 |
| Light chain, n (%) | ||||
| κ | 80 (63.5) | 44 (61.1) | 36 (66.7) | .578 |
| λ | 44 (34.9) | 28 (38.9) | 16 (29.6) | .346 |
| Involved FLC, (mg/L), median (IQR) | 540 (147-2670) | 430 (108-1812) | 949 (252-4112) | .029 |
| BMPC, % (IQR) | 70 (10-90) | 55 (35-70) | 80 (50-90) | <.001 |
| ISS, n (%) | ||||
| I | 19 (15.1) | 13 (18.1) | 6 (11.1) | .324 |
| II | 47 (37.3) | 31 (43.1) | 16 (29.6) | .14 |
| III | 60 (47.6) | 28 (38.9) | 32 (59.3) | .037 |
| R-ISS, n (%) | ||||
| I | 13 (10.3) | 11 (15.3) | 2 (3.7) | .04 |
| II | 91 (72.2) | 51 (70.8) | 40 (74.1) | .841 |
| III | 22 (17.5) | 10 (13.9) | 12 (22.2) | .326 |
| High risk cytogenetics, n (%) | 24 (19.0) | 9 (12.5) | 15 (27.8) | .053 |
| t(4;14) | 17 (13.5) | 5 (6.9) | 12 (22.2) | .026 |
| t(14;16) | 0 (0) | 0 (0) | 0 (0) | - |
| del17p | 7 (5.6) | 4 (5.6) | 3 (5.6) | 1 |
| Laboratory parameters, median (IQR) | ||||
| Alb (g/dL) | 3.2 (2.7-3.7) | 3.3 (2.8-3.7) | 3.0 (2.7-3.6) | .562 |
| β2MG (mg/dL) | 5.5 (3.0-8.2) | 4.5 (2.8-7.0) | 6 (3.7-12.5) | .019 |
| Corrected Ca (mg/dL) | 9.8 (9.2-10.5) | 9.8 (9.3-10.5) | 10 (9.2-10.5) | .826 |
| eGFR (mL/min per 1.73 m2)∗ | 51.5 (30.7-65.4) | 50.9 (33.9-63.6) | 52.4 (28.6-67.5) | .861 |
| Hb (g/dL) | 9.5 (8.3-11) | 10 (8.7-11.5) | 8.9 (8-10.1) | .003 |
| LDH (U/L) | 184 (149-227) | 184 (151-209) | 183 (146-237) | .648 |
| Induction regimen, n (%) | ||||
| PI only | 17 (13.5) | 11 (15.3) | 6 (11.1) | .679 |
| IMiDs only | 5 (4.0) | 4 (5.6) | 1 (1.9) | .553 |
| PI + IMiDs | 81 (64.3) | 44 (61.1) | 37 (68.5) | .502 |
| Anti-CD38 antibody–based therapy | 23 (18.3) | 13 (18.1) | 10 (18.5) | 1 |
| . | Total (n = 126 [100%]) . | Patients with low CTCs (n = 72 [57.1%]) . | Patients with high CTCs (n = 54 [42.9%]) . | P value . |
|---|---|---|---|---|
| Age, y, median (IQR) | 78 (73-83) | 78 (71-83) | 80 (75-83) | .32 |
| Age ≥80 y, n (%) | 58 (46.0) | 31 (43.1) | 27 (51.9) | .34 |
| Female, n (%) | 67 (53.2) | 35 (48.6) | 32 (59.3) | .315 |
| ECOG-PS ≥2, n (%) | 61 (48.4) | 28 (38.9) | 33 (61.1) | .022 |
| Heavy chain, n (%) | ||||
| G | 71 (56.3) | 46 (63.9) | 25 (46.3) | .069 |
| A | 30 (23.8) | 16 (22.2) | 14 (25.9) | .676 |
| Light chain only | 20 (15.9) | 9 (12.5) | 11 (20.4) | .325 |
| Others | 5 (4.0) | 1 (1.4) | 4 (7.5) | .163 |
| Involved Ig (mg/dL), median (IQR) | 4356 (2680-5985) | 4084 (2577-5722) | 4922 (2850-6148) | .562 |
| Light chain, n (%) | ||||
| κ | 80 (63.5) | 44 (61.1) | 36 (66.7) | .578 |
| λ | 44 (34.9) | 28 (38.9) | 16 (29.6) | .346 |
| Involved FLC, (mg/L), median (IQR) | 540 (147-2670) | 430 (108-1812) | 949 (252-4112) | .029 |
| BMPC, % (IQR) | 70 (10-90) | 55 (35-70) | 80 (50-90) | <.001 |
| ISS, n (%) | ||||
| I | 19 (15.1) | 13 (18.1) | 6 (11.1) | .324 |
| II | 47 (37.3) | 31 (43.1) | 16 (29.6) | .14 |
| III | 60 (47.6) | 28 (38.9) | 32 (59.3) | .037 |
| R-ISS, n (%) | ||||
| I | 13 (10.3) | 11 (15.3) | 2 (3.7) | .04 |
| II | 91 (72.2) | 51 (70.8) | 40 (74.1) | .841 |
| III | 22 (17.5) | 10 (13.9) | 12 (22.2) | .326 |
| High risk cytogenetics, n (%) | 24 (19.0) | 9 (12.5) | 15 (27.8) | .053 |
| t(4;14) | 17 (13.5) | 5 (6.9) | 12 (22.2) | .026 |
| t(14;16) | 0 (0) | 0 (0) | 0 (0) | - |
| del17p | 7 (5.6) | 4 (5.6) | 3 (5.6) | 1 |
| Laboratory parameters, median (IQR) | ||||
| Alb (g/dL) | 3.2 (2.7-3.7) | 3.3 (2.8-3.7) | 3.0 (2.7-3.6) | .562 |
| β2MG (mg/dL) | 5.5 (3.0-8.2) | 4.5 (2.8-7.0) | 6 (3.7-12.5) | .019 |
| Corrected Ca (mg/dL) | 9.8 (9.2-10.5) | 9.8 (9.3-10.5) | 10 (9.2-10.5) | .826 |
| eGFR (mL/min per 1.73 m2)∗ | 51.5 (30.7-65.4) | 50.9 (33.9-63.6) | 52.4 (28.6-67.5) | .861 |
| Hb (g/dL) | 9.5 (8.3-11) | 10 (8.7-11.5) | 8.9 (8-10.1) | .003 |
| LDH (U/L) | 184 (149-227) | 184 (151-209) | 183 (146-237) | .648 |
| Induction regimen, n (%) | ||||
| PI only | 17 (13.5) | 11 (15.3) | 6 (11.1) | .679 |
| IMiDs only | 5 (4.0) | 4 (5.6) | 1 (1.9) | .553 |
| PI + IMiDs | 81 (64.3) | 44 (61.1) | 37 (68.5) | .502 |
| Anti-CD38 antibody–based therapy | 23 (18.3) | 13 (18.1) | 10 (18.5) | 1 |
Alb, albumin; β2MG, beta-2 microglobulin; Ca, calcium; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; IMiDs, immunomodulatory drugs; LDH, lactate dehydrogenase; PI, proteasome inhibitor.
eGFR was calculated using the Japanese equation.
Calculation of TLG and its correlation with CTC burden
Thirty-seven patients (29.4%) had no FDG-avid lesions, a relatively higher percentage than the 24.0% reported in a previous study.25 Of those, 10 (27.0%) patients had at least 1 bone lesion detected on magnetic resonance imaging, suggesting false-negative PET/CT results.26,27 Among the remaining 89 patients (70.6%) who showed positive for PET/CT, 71 (79.8%) had >3 FLs, 53 (59.6%) had an SUVmax >4.2, and 6 (6.7%) had EMD as the high-risk PET/CT features. Specifically, for EMD, the median levels of CTCs were numerically higher in patients with EMD compared with those without (0.26 IQR, 0.07-0.27 vs 0.06 IQR, 0.01-0.21; P = .36), although they did not reach statistical significance. All other clinical and radiomic parameters were not significantly different between the presence or absence of EMD, except for younger age (median, 70 vs 79; P = .03) and higher SUVmax (median, 8.8 vs 3.6; P = .001) in patients with EMD.
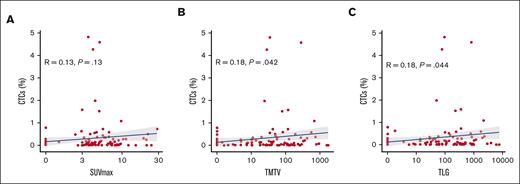
The median SUVmax, TMTV, and TLG were 3.7 (IQR, 0-5.5), 17 (IQR, 0-103), and 49 (IQR, 0-322), respectively. Regarding TLG, the ROC curve identified an optimal cut-off value of 267 for predicting both 2-year PFS and 5-year OS, with respective AUC of 0.61 and 0.66 (supplemental Figure 4). Using this cutoff value, 35 patients (27.7%) were categorized as having a high tumor burden. Of these, 29 patients (82.8%) had ≥2 high-risk PET/CT features. Remarkably, the correlation between CTC levels and the quantitative radiomic features were poor (SUVmax, R = 0.13, P = .13; TMTV, R = 0.18, P = .042; TLG, R =0.18, P = .044, respectively) (Figure 1). Consequently, only 16 of the 35 patients (45.7%) presented with both high TLG levels and CTC burden.
Correlation with CTCs and quantitative radiomic parameters. The blue line represents a linear regression showing the correlation between (A) SUVmax, (B) TMTV, and (C) TLG and CTC levels, with the shaded areas indicating the 95% CI.
Correlation with CTCs and quantitative radiomic parameters. The blue line represents a linear regression showing the correlation between (A) SUVmax, (B) TMTV, and (C) TLG and CTC levels, with the shaded areas indicating the 95% CI.
Outcomes according to CTCs, TLG, and R-ISS
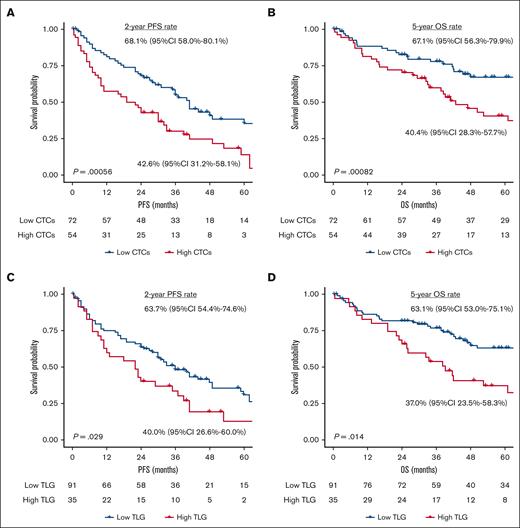
Figure 2 shows the PFS and OS according to levels of CTC and TLG. Patients with high CTC levels had significantly poorer PFS and OS than those with low CTC levels (2-year PFS rate = 43.4%, 95% CI, 31.9-59.0 vs 68.1%, 95% CI, 58.1-79.7; P < .001; 5-year OS rate = 39.0%, 95% CI, 26.9-56.7 vs 68.3%, 95% CI, 57.8-80.7; P < .001). Similarly, high TLG levels also significantly worsened the PFS (2-year PFS rate = 41.2%, 95% CI, 27.5-61.5 vs 67.6%, 95% CI, 53.6-85.3; P = .038) and OS (5-year OS rate = 37.7%, 95% CI, 23.9-59.4 vs 63.1%, 95% CI, 53.2-74.9; P = .019). Using the combination of high CTCs and TLG, the outcomes for patients with no risk factors, either risk factor, or both were almost equally divided with 2-year PFS rates of 74.0% (95% CI, 62.8-87.2), 50.9% (95% CI, 39.4-65.7), and 25.0% (95% CI, 10.7-58.4), and 5-year OS rates of 75.8% (95% CI, 64.0-89.6), 46.2% (95% CI, 34.2-62.5), and 27.3% (95% CI, 11.5-65.3), respectively (supplemental Figure 6). Furthermore, even when only 6 patients with EMD (4 with high CTCs and 2 with low CTCs) were analyzed, the CTC burden significantly affected both PFS and OS (both P < .05) (supplemental Figure 7).
Kaplan–Meier estimate of PFS and OS stratified by CTC and TLG levels. (A) PFS and (B) OS based on CTC levels, along with the (C) PFS and (D) OS based on TLG levels.
Kaplan–Meier estimate of PFS and OS stratified by CTC and TLG levels. (A) PFS and (B) OS based on CTC levels, along with the (C) PFS and (D) OS based on TLG levels.
In terms of R-ISS, patients with R-ISS I disease had excellent survival, with a 5-year OS rate of 100%. However, those with R-ISS II and R-ISS III diseases showed similar outcomes in our cohort: the 2-year PFS rates were 56.2% (95% CI, 46.8-67.5) and 42.9% (95% CI, 26.1-70.2), whereas the 5-year OS rates were 52.6% (95% CI, 42.6-64.9) and 43.2% (95% CI, 25.5-73.2), respectively (supplemental Figure 8). Even in patients with R-ISS II disease, the CTC levels significantly discriminated both PFS and OS, whereas high TLG levels showed a trend toward worse outcomes with marginal significance (supplemental Figure 9). Interestingly, a landmark analysis at 6-months from diagnosis showed similar outcomes between patients with low CTC levels who did not achieve CR and those with high CTC levels and achieved CR (2-year PFS rate = 50.0%, 95% CI, 34.0-73.4 vs 65.2%, 95% CI, 48.3-87.9; 5-year OS rate = 57.3%, 95% CI, 40.8-80.5 vs 56.3%, 95% CI, 36.6-86.4) (supplemental Figure 10).
Univariate and multivariate analyses predicting outcomes
Next, univariate and multivariate analyses were conducted to predict PFS and OS (Table 2). In the univariate analysis, R-ISS III, high levels of CTC and TLG, and CR achievement were significantly associated with PFS (R-ISS III: hazard ratio [HR], 2.04; 95% CI, 1.19-3.47; P = .009; high CTCs: HR, 2.12; 95% CI, 1.37-3.28; P = .001; high TLG: HR, 1.65; 95% CI, 1.04-2.62; P = .03; CR achievement: HR, 0.37; 95% CI, 0.23-0.58: P < .001) and OS (R-ISS III: HR, 1.88; 95% CI, 1.04-3.38; P = .035; high CTC levels: HR, 2.35; 95% CI, 1.4-3.95; P = .001; high TLG levels: HR, 1.91; 95% CI, 1.13-3.22; P = .015; CR achievement: HR, 0.43; 95% CI, 0.25-0.73; P = .002). All of these variables showed a prognostic significance on multivariate analysis (all P < .05 for PFS and OS).
Univariate and multivariate analyses for predicting PFS and OS
| . | PFS . | OS . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | Multivariate analysis . | |||||||||
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Age ≥80 y | 1.56 | 1.01-2.42 | .042 | - | - | - | 1.82 | 1.09-3.05 | .021 | - | - | - |
| ECOG-PS ≥2 | 1.51 | 0.98-2.33 | .061 | - | - | - | 2.06 | 1.23-3.46 | .006 | - | - | - |
| ISS | 1.63 | 1.18-2.24 | .003 | - | - | - | 1.89 | 1.27-2.8 | .002 | - | - | - |
| ISS III | 1.71 | 1.1-2.64 | .016 | - | - | - | 1.97 | 1.17-3.31 | .01 | - | - | - |
| R-ISS | 2.31 | 1.53-3.51 | <.001 | - | - | - | 2.28 | 1.44-3.62 | <.001 | - | - | - |
| R-ISS III | 2.04 | 1.19-3.47 | .009 | 2.8 | 1.58-4.98 | <.001 | 1.88 | 1.04-3.38 | .035 | 2.21 | 1.19-4.08 | .011 |
| High risk cytogenetics | 1.78 | 1.06-3 | .029 | - | - | - | 1.14 | 0.62-2.12 | .663 | - | - | - |
| High CTCs | 2.12 | 1.37-3.28 | .001 | 1.86 | 1.19-2.9 | .006 | 2.35 | 1.4-3.95 | .001 | 2.05 | 1.22-3.46 | .007 |
| Positive CTCs | 1.92 | 1.02-3.64 | .043 | - | - | - | 1.23 | 0.6-2.51 | .556 | - | - | - |
| High TLG | 1.65 | 1.04-2.62 | .03 | 1.78 | 1.12-2.83 | .015 | 1.91 | 1.13-3.22 | .015 | 2.03 | 1.2-3.45 | .008 |
| Number of FLs >3 | 1.21 | 0.77-1.89 | .394 | - | - | - | 1.49 | 0.87-2.56 | .142 | - | - | - |
| SUVmax >4.2 | 1.34 | 0.87-2.07 | .177 | - | - | - | 1.36 | 0.81-2.26 | .235 | - | - | - |
| EMD | 0.94 | 0.34-2.59 | .916 | - | - | - | 1.61 | 0.58-4.45 | .357 | - | - | - |
| CR achievement | 0.37 | 0.23-0.58 | <.001 | 0.32 | 0.2-0.52 | <.001 | 0.43 | 0.25-0.73 | .002 | 0.4 | 0.23-0.69 | .001 |
| . | PFS . | OS . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | Multivariate analysis . | |||||||||
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Age ≥80 y | 1.56 | 1.01-2.42 | .042 | - | - | - | 1.82 | 1.09-3.05 | .021 | - | - | - |
| ECOG-PS ≥2 | 1.51 | 0.98-2.33 | .061 | - | - | - | 2.06 | 1.23-3.46 | .006 | - | - | - |
| ISS | 1.63 | 1.18-2.24 | .003 | - | - | - | 1.89 | 1.27-2.8 | .002 | - | - | - |
| ISS III | 1.71 | 1.1-2.64 | .016 | - | - | - | 1.97 | 1.17-3.31 | .01 | - | - | - |
| R-ISS | 2.31 | 1.53-3.51 | <.001 | - | - | - | 2.28 | 1.44-3.62 | <.001 | - | - | - |
| R-ISS III | 2.04 | 1.19-3.47 | .009 | 2.8 | 1.58-4.98 | <.001 | 1.88 | 1.04-3.38 | .035 | 2.21 | 1.19-4.08 | .011 |
| High risk cytogenetics | 1.78 | 1.06-3 | .029 | - | - | - | 1.14 | 0.62-2.12 | .663 | - | - | - |
| High CTCs | 2.12 | 1.37-3.28 | .001 | 1.86 | 1.19-2.9 | .006 | 2.35 | 1.4-3.95 | .001 | 2.05 | 1.22-3.46 | .007 |
| Positive CTCs | 1.92 | 1.02-3.64 | .043 | - | - | - | 1.23 | 0.6-2.51 | .556 | - | - | - |
| High TLG | 1.65 | 1.04-2.62 | .03 | 1.78 | 1.12-2.83 | .015 | 1.91 | 1.13-3.22 | .015 | 2.03 | 1.2-3.45 | .008 |
| Number of FLs >3 | 1.21 | 0.77-1.89 | .394 | - | - | - | 1.49 | 0.87-2.56 | .142 | - | - | - |
| SUVmax >4.2 | 1.34 | 0.87-2.07 | .177 | - | - | - | 1.36 | 0.81-2.26 | .235 | - | - | - |
| EMD | 0.94 | 0.34-2.59 | .916 | - | - | - | 1.61 | 0.58-4.45 | .357 | - | - | - |
| CR achievement | 0.37 | 0.23-0.58 | <.001 | 0.32 | 0.2-0.52 | <.001 | 0.43 | 0.25-0.73 | .002 | 0.4 | 0.23-0.69 | .001 |
Furthermore, even after adjustment for older age or decreased PS, R-ISS III and high levels of CTC and TLG were independently associated with a worse outcome except R-ISS III for OS (supplemental Table 1). In the multivariate models, when replacing R-ISS with ISS, lactate dehydrogenase, and HRCA, both high CTCs and TLG remained statistically significant prognostic factors. In contrast, the detection of CTCs had limited predictive value, showing significance for predicting PFS (HR, 1.92; 95% CI, 1.02-3.64; P = .043) but not for OS (HR, 1.23; 95% CI, 0.6-2.51; P = .556). Moreover, all 3 PET-CT high-risk features were not significantly associated with worse outcomes in contrast to TLG.
Novel prognostic model integrating CTCs, TLG, and R-ISS
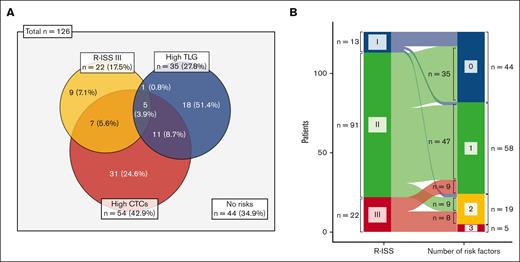
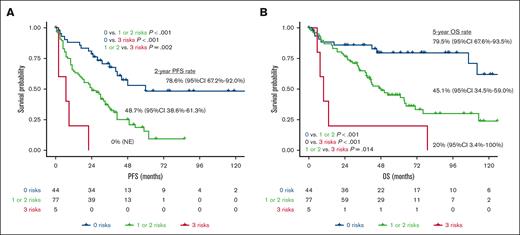
Based on the results of the multivariate analysis, we constructed a novel predictive model that incorporated the variables CTC level, TLG level, and R-ISS III. The distribution of these risk factors is presented in Figure 3. Overall, 82 patients (65.1%) had at least 1 high-risk feature, of whom 19 (15.1%) had 2 and 5 (4.0%) had 3 such features (Figure 3A). Among 91 patients classified as having an R-ISS II disease, 47 (51.6%) had either high CTC or high TLG levels, and 9 (9.9%) had both; in contrast, of the 22 patients as having an R-ISS III disease, 9 (40.9%) lacked any additional risk factors aside from their R-ISS category (Figure 3B). When patients were stratified according to the number of risk factors, those with 1 and 2 risk factors showed almost identical PFS and OS (supplemental Figure 11). Accordingly, we divided patients into those with no risk factors, those with 1 or 2, and those with 3 risk factors. This model differentiated all 3 risk groups (all P < .05; Figure 4). In particular, the patients with all risk factors had an extremely high risk of experiencing worse outcomes (2-year PFS rate: 0%, 95% CI: not evaluable; 5-year OS rate: 20%, 95% CI, 3.4-100), whereas those without any high-risk features had a substantially better prognosis, with a 2-year PFS rate of 78.6% (95% CI, 67.2-92.0) and a 5-year OS rate of 79.5% (95% CI, 67.6-93.5).
Distribution of high-risk features including CTC levels, TLG levels, and R-ISS category. (A) Venn diagram depicting the overlap of 3 high-risk features. (B) Alluvial diagram representing the transition of R-ISS to risk factor number–based classification.
Distribution of high-risk features including CTC levels, TLG levels, and R-ISS category. (A) Venn diagram depicting the overlap of 3 high-risk features. (B) Alluvial diagram representing the transition of R-ISS to risk factor number–based classification.
Kaplan–Meier estimate of PFS and OS according to the novel risk model. (A) PFS and (B) OS stratified by the number of high-risk features (CTC level, TLG level, and R-ISS category). NE, not evaluable.
Kaplan–Meier estimate of PFS and OS according to the novel risk model. (A) PFS and (B) OS stratified by the number of high-risk features (CTC level, TLG level, and R-ISS category). NE, not evaluable.
Finally, we quantitatively assessed this model’s performance using the c-index and AIC (supplemental Table 2). The model incorporating the CTC level, TLG level, and R-ISS category as categorical variables yielded the highest c-index (PFS: 0.637; OS: 0.643) and the lowest AIC (PFS: 685.48; OS: 500.14). These results indicated that the combined model offered superior predictive performance than each of the indices.
Discussion
This study demonstrated that the baseline CTC levels had a strong and independent prognostic impact on real-world patients with TIE-MM. The levels of CTC were associated with clinical parameters indicative of disease aggressiveness but were poorly correlated with quantitative radiomics. The integrated risk model, which incorporated the CTC levels, TLG levels, and R-ISS category, improved the accuracy of outcome prediction.
In the past decade, since the introduction of triplet therapy and with the advent of daratumumab-based induction, significant progress has been made in achieving prompt and profound responses, even among patients who are TIE. The recent update of the MAIA trial showed that the addition of daratumumab to lenalidomide/dexamethasone nearly doubled the rates of achieving CR or better (51%) and those of minimal residual disease negativity (31%) in patients with TIE-MM.21 However, the development of a more sophisticated risk model has not kept pace with the rapid expansion of the treatment landscape. Within the TIE-MM cohorts, a higher degree of patient heterogeneity is observed than initially anticipated, based solely on chronological age. Outcomes in a clinical trial were favorable for those categorized frail owing to age alone compared with those considered frail for additional frailty parameters.28 In conjunction with the assessment of aging-associated vulnerabilities, the identification of genuinely patients who are at high risk who would derive substantial benefits from intensive therapy assumes particular significance.
CTCs represent a biologically distinct subpopulation of cBMPC, characterized by the enrichment of quiescent and clonogenic cells and the downregulation of adhesion markers.29 In this study, CTCs were detected in 80.5% of the patients with a median percent of 0.06, which was higher than that in the recent pivotal TIE-MM cohort using more sensitive next-generation flow cytometry (75% and 0.03%).12 Given the observed association between high CTC levels and both higher ECOG PS scores and ISS, the higher prevalence of CTC in our study may reflect the inclusion of a greater number of older adults (median ages, 77 vs 72 years) and more patients with advanced-stage disease (ISS III, 49.5% vs 42.4%).12 Consistent with the reports of previous studies, CTC levels were significantly increased in patients with HRCA, especially t(4;14) (median, 0.25% vs 0.05%), with a modest positive correlation observed between CTCs and cBMPC.11 In relation to the determined cutoff value, the lower sensitivity of the MFC used may have resulted in the higher threshold (0.09%) for CTCs, compared with the 0.01% and 0.07% proposed in 2 recent prospective trials.10,11 The quantification of CTCs facilitated the detection of concealed patients within the R-ISS II category who are at high risk, thereby mitigating the primary limitations associated with the R-ISS. Interestingly, the attainment of CR proved to be an advantageous factor in achieving the desired outcomes, irrespective of the baseline CTC burden. In this regard, the 2 abovementioned trials demonstrated that minimal residual disease negativity abrogated the negative prognostic impact of CTCs.10,11 Taken together, our data emphasize the importance of risk-adapted therapy and the routine inclusion of baseline CTC measurements into the diagnostic workup.
The unique contribution of this study lies in its evaluation of whether quantifying CTC enhances the prognostic value offered by tumor burden estimation through PET/CT imaging. Contrary to the results of a previous study25 that proposed high-risk PET/CT features and our previous research,30 none of these qualitative markers showed prognostic significance in our TIE-MM cohort. This discrepancy may be partly because of the difference in the study populations; the previous studies evaluated cohorts that included patients with TE-MM. Instead, the quantification of TLG provided a more precise assessment of the tumor burden with independent prognostic value. Importantly, poor correlation was observed between TLG and CTC levels, resulting in fewer than half of the patients having a combination of these 2 risk factors. The combination of CTCs and TLG improved the prognostic assessment, especially in the R-ISS II group. By incorporating CTC and TLG into the R-ISS, 35 out of 91 patients (38.5%) with R-ISS II disease and 17 out of 22 patients (77.3%) with R-ISS III disease were reclassified in the new risk model. Although only 4.0% of the patients were carrying all 3 risks, these individuals experienced extremely unfavorable outcomes, with a 2-year PFS rate of 0% and a 5-year OS rate of 20%. Conversely, patients without any risk factors had excellent survival rates, with ∼80% achieving a 5-year OS, although the majority of them were initially categorized as having an R-ISS II disease. Compared with the standalone use of R-ISS, this combined model improved the predictive performance, albeit with a modest c-index value of 0.63, primarily because of the appropriate allocation of patients in the R-ISS II category. To our knowledge, this study is the first to confirm the complementarity of CTC level, TLG level, and R-ISS category in patients with MM.
This study had several limitations. First, the retrospective nature of the study, the heterogeneity in treatment approaches, and the relatively limited sample size collectively posed limitations that hindered the formulation of unequivocal conclusions. The high CR rate observed (50%), despite the majority of patients having received bortezomib, lenalidomide, and dexamethasone–induction therapy, could potentially be attributed to treatment modifications made before the definition of progression according to the IMWG criteria. Second, the established CTC cutoff value requires validation through a more sensitive and widely adopted method, such as the next-generation EuroFlow MFC method,31 although our previous research has demonstrated excellent correlation between EuroFlow and our 8-color DuraClone method.32 In addition, thresholds were applied to CTC measurements at levels >10−4; sensitivity <10−5 was not a requirement for this study. Similarly, the optimal threshold for calculating TLG has not yet been established. Based on our previous study, an absolute SUV of ≥2.5 was adopted to identify positive myeloma lesions. Further studies using alternative methods, such as the SUV 41% method19 or the recently proposed Deauville scale,33 may contribute to identifying the most suitable methodology for integrating TLG in clinical practice. Finally, false-negative PET results, which primarily contributed to the low levels of hexokinase,26,27 may lead to an underestimation of tumor burden. Based on our previous findings linking older age with a higher frequency of PET/CT false negativity,26 the imaging data should be interpreted more carefully in TIE-MM cohorts. Alternatively, in future studies, whole-body magnetic resonance imaging–based tumor-volume quantification, such as total diffusion volume,34 might substitute for TLG.
In conclusion, the incorporation of baseline CTCs, in conjunction with TLG level in the PET/CT–based volumetric assessment, can enhance the stratification of patients with TIE-MM.
Acknowledgments
The authors thank Hajime Senjo (Department of Hematology, Hokkaido University) and Kenji Hirata (Department of Nuclear Medicine, Hokkaido University) for their assistance in using the Metavol software. The authors also thank Editage for providing excellent English language editing assistance.
Authorship
Contributions: D.I. and K.M. designed the study, interpreted the data, performed the statistical analysis, provided patient care, and wrote the manuscript; D.I. and T.T. calculated the quantitative radiomic features; Y.M. interpreted the imaging findings; M.O., A.U., R.T., K.N., and M.T. provided the patient care; and all authors critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daisuke Ikeda, Division of Hematology/Oncology, Department of Medicine, Kameda Medical Center, 929 Higashi-chou, Kamogawa-shi, Chiba 296-8602, Japan; email: dskikd.2409@gmail.com.
References
Author notes
The datasets generated and analyzed during the current study are available upon reasonable request from the corresponding author, Daisuke Ikeda (dskikd.2409@gmail.com).
The full-text version of this article contains a data supplement.