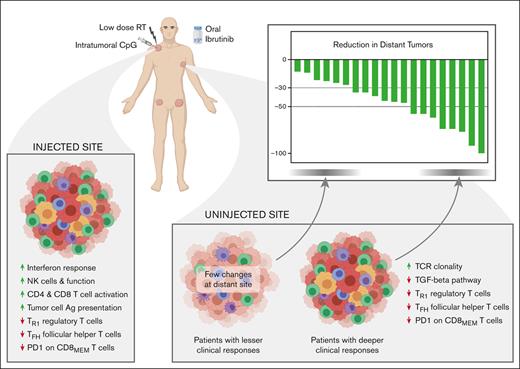
ISV with intratumoral CpG, local low-dose radiation, and systemic ibrutinib was safe and effective in lymphoma.
Both activation of immune effectors and dampening of immune suppressors were important in generating favorable outcomes.
Visual Abstract
In situ vaccination (ISV) triggers an immune response to tumor-associated antigens at 1 tumor site, which can then tackle the disease throughout the body. Here, we report clinical and biological results of a phase 1/2 ISV trial in patients with low-grade lymphoma, combining an intratumoral toll-like receptor 9 (TLR9) agonist with local low-dose radiation and ibrutinib (an inhibitor of B- and T-cell kinases). Adverse events were predominately low grade. The overall response rate was 50%, including 1 complete response. All patients experienced tumor reduction at distant sites. Single-cell analyses of serial fine needle aspirates from injected and uninjected tumors revealed correlates of clinical response, such as lower CD47 and higher major histocompatibility complex class II expression on tumor cells, enhanced T-cell and natural killer cell effector function, and reduced immune suppression from transforming growth factor β and inhibitory T regulatory 1 cells. Although changes at the local injected site were more pronounced, changes at distant uninjected sites were more often associated with clinical responses. Functional immune response assays and tracking of T-cell receptor sequences provided evidence of treatment-induced tumor-specific T-cell responses. Induction of immune effectors and reversal of negative regulators were both important in producing clinically meaningful tumor responses. The trial was registered at www.clinicaltrials.gov as #NCT02927964.
Introduction
The recent success of checkpoint blockade and chimeric antigen receptor–based adoptive cell therapies has brought remarkable benefits to patients and provided impetus for the pursuit of novel immunotherapeutic strategies. In situ vaccination (ISV), in which immune stimulating agents are delivered directly to a tumor site where relevant tumor antigens are present, can generate antitumor immune responses that then act systemically to control distant disease. ISV has the potential to induce durable tumor-specific responses with limited side effects.
Intratumoral delivery of TLR9 agonists has been effective in clinical trials in multiple tumor types.1-3 We have previously shown that ibrutinib, a multikinase inhibitor, significantly enhances the effect of intratumoral TLR9 in generating systemic antitumor T-cell responses in a preclinical model of lymphoma.4 This effect is lost upon depletion of either CD4 or CD8 T-cells, and T-cells transferred from treated mice prevent tumor growth in adoptive hosts. Ibrutinib irreversibly inhibits Bruton tyrosine kinase (BTK), expressed by B-cells, and several kinases expressed by T-cells, including interleukin-2 (IL-2)–inducible T-cell kinase (ITK), lymphocyte cell-specific kinase (LCK), and Janus kinase 3.5-8 We, therefore, designed a clinical trial to test whether T-cell–mediated effects of ibrutinib could provide similar synergistic effects in patients. We treated patients with low-grade B-cell non-Hodgkin lymphoma with intratumoral SD101, a class C CpG oligodeoxynucleotide TLR9 agonist (hereafter, CpG), together with local low-dose radiotherapy (LDRT) at 1 tumor site, followed by oral ibrutinib. LDRT was incorporated to build upon prior results3 and to expose a broader array of tumor antigens.
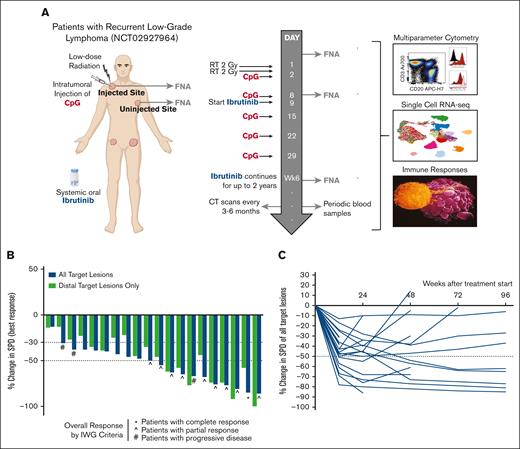
Our understanding of how immunotherapy results in clinical responses has been limited by an inability to know what is taking place inside tumors during treatment. Here, we used minimally invasive serial fine needle tumor aspirates that, coupled with modern high throughput technologies, provide a wealth of data on both the tumor and its microenvironment.9 We collected samples from the locally injected tumor site and a distant uninjected tumor site before treatment and at 2 timepoints during treatment (Figure 1A). We analyzed these using multiparameter flow cytometry and single-cell RNA sequencing (scRNA-seq). We report the clinical efficacy and safety of this trial, along with critical insights into the underpinnings of successful antitumor responses.
Study design and clinical outcome. (A) Schematic representation of the clinical trial design, treatments, and biopsy schedule and overview of assays performed on the obtained specimens. (B) Waterfall plot depicting best response in SPD of either all target lesions (blue bars) or uninjected, distant target lesions only (green bars). All 3 patients with progressive disease progressed at a single site of disease but experienced tumor reduction elsewhere. (C) Spider plot depicting change in SPD of all target lesions over time from treatment initiation. CT, computed tomography; FNA, fine needle aspirate; RT, radiotherapy; SPD, sum of product of diameters; IWG, international working group.
Study design and clinical outcome. (A) Schematic representation of the clinical trial design, treatments, and biopsy schedule and overview of assays performed on the obtained specimens. (B) Waterfall plot depicting best response in SPD of either all target lesions (blue bars) or uninjected, distant target lesions only (green bars). All 3 patients with progressive disease progressed at a single site of disease but experienced tumor reduction elsewhere. (C) Spider plot depicting change in SPD of all target lesions over time from treatment initiation. CT, computed tomography; FNA, fine needle aspirate; RT, radiotherapy; SPD, sum of product of diameters; IWG, international working group.
Methods
Study design
This was a single-center, open label, phase 1/2 study (NCT02927964) designed to test the safety and preliminary efficacy of the combination of low-dose radiation, intratumoral SD-101 (CpG), and ibrutinib. We enrolled patients with recurrent low-grade lymphoma and at least 1 site of disease accessible for direct injection. The study protocol was approved and regulated by the Stanford Institutional Review Board and the Stanford Cancer Institute’s Scientific Review and Data Safety and Monitoring committees. All participants provided written informed consent before any study procedures. All investigations were performed in accordance with good clinical practice as defined in the International Conference on Harmonization guidelines and the US Code of Federal Regulations, which, in turn, are consistent with the Declaration of Helsinki.
Study treatments and assessments
Eligible enrolled participants received 2 Gy radiation on 2 consecutive days (for a total of 4 Gy) to the chosen treatment site, termed “injected site.” After the second and final radiation treatment, patients received the first of 5 weekly doses of 3 mg of intratumoral SD-101 (CpG) at the same site of disease. On day 9, a dosage of 560 mg of oral ibrutinib daily was initiated. Ibrutinib was continued for up to 96 weeks, the duration of the study.
Safety and efficacy assessments
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). All grade ≥4 treatment-related toxicities and most grade 3 treatment-related toxicities were considered dose-limiting toxicities (DLTs). Serious AEs were defined as AEs that were life-threatening or fatal, required hospitalization, were disabling, or required medical or surgical intervention to prevent a serious adverse outcome. Disease assessment included imaging at screening, at 3 and 6 months after treatment, and every 6 months thereafter. Measurement and reporting of shrinkage in uninjected sites is standard in trials of intratumoral therapy,10 and this informative continuous measure was used as the clinical outcome for correlative biomarker analyses.
Sample acquisition
Fine needle aspiration (FNA) specimens were collected before initiation of therapy (Pre Tx”), just before the second weekly intratumoral injection (“Day 8”), and 1 week after completion of all 5 intratumoral injections (“Week 6”) from the injected site and a prespecified distant uninjected site outside the radiation field and in a different nodal group from the injected site. FNA cell suspensions were subjected, typically within an hour after acquisition, to scRNA-seq and multiparameter flow cytometry.
Single-cell sequencing: sample and data processing and analysis
Single-cell libraries were prepared using the Chromium Single-cell 5′ Library and Gel Bead Kit and the Chromium Single-cell variable diversity joining Enrichment Kit (10x Genomics). Sequencing was performed on Illumina NovaSeq for scRNA-seq libraries and on Illumina NextSeq and/or NovaSeq for T-cell receptor (TCR) libraries. scRNA-seq data were processed using cellranger (version 3; 10x Genomics). Single-cell TCR-sequencing data were processed by cellranger vdj (version 3.1; 10x Genomics) using the “cellranger mkfastq” and “cellranger vdj” commands with the prebuilt reference. Additional quality control steps are described in the supplemental Material.
The final data set was analyzed using the R package Seurat (version 3.1.1; RRID:SCR_016341),11 using standard workflows (see supplemental Material for details). We calculated the adjusted Rand index for overlap between batch identity and cluster identity for all T-cells from all samples and found that cluster assignments were near random with respect to batch identity (adjusted Rand index, 0.05), suggesting no significant batch effects.
Flow cytometry
Single-cell suspensions were stained with Live/Dead Aqua Fixable Stain (Thermo Fisher), followed by 3 panels of fluorochrome-conjugated antibodies (supplemental Table 1), fixed and permeabilized using the FoxP3 Fix/Perm solution (Thermo Fisher), and then stained for intracellular proteins. Flow cytometry was performed using an LSRII cytometer (BD Immunocytometry Systems), and the data were analyzed using Cytobank Software (version 9.1; RRID:SCR_014043).
Statistical analysis
Statistical analysis was performed with the R stats package (version 3.4.4). For global differential gene expression analysis, we used an unpaired Wilcoxon rank-sum test with Bonferroni correction, unless otherwise specified. Statistical significance between pretreatment and on-treatment analyses on a patient-averaged level was determined with the paired Wilcoxon rank-sum test. For correlation analyses, Spearman correlation coefficients and associated 2-sided P values were computed. In box plot graphs, each box represents the interquartile range (the range between the 25th and 75th percentile) with the median of the data; whiskers indicate the upper and lower value within 1.5 times the interquartile range. Data beyond the end of the whiskers are plotted individually.
Results
Patient characteristics
Twenty-one patients were enrolled. One was taken off the study before receiving any intratumoral injections because of suspected infection. The median age of evaluable patients was 64 years (range, 35-79 years); 55% were male (Table 1). Nineteen had a diagnosis of follicular lymphoma (FL), whereas 1 had marginal zone lymphoma. Eighty-five percent had presented with stage III to IV disease at diagnosis. All had received at least 1, and up to 5, prior lines of therapy for lymphoma.
Baseline patient characteristics
| . | . | Median/number . | Range/percent . |
|---|---|---|---|
| Age (y) | 64 | 35-79 | |
| Sex | Male | 11 | 55% |
| Female | 9 | 45% | |
| Lymphoma histology | FL | 19 | 95% |
| MZL | 1 | 5% | |
| Stage at diagnosis | I | 0 | 0% |
| II | 3 | 15% | |
| III | 6 | 30% | |
| IV | 11 | 55% | |
| Grade at diagnosis (for patients with FL, n = 19) | 1-2 | 15 | 75% |
| 3A | 4 | 20% | |
| FLIPI at diagnosis (for patients with FL, n = 19) | 0-2 | 13 | 65% |
| 3+ | 6 | 30% | |
| Prior lines of therapy | 0 | 0 | 0% |
| 1 | 13 | 65% | |
| 2 | 3 | 15% | |
| 3 | 1 | 5% | |
| 4 | 1 | 5% | |
| 5 | 2 | 10% | |
| Prior therapy | Anti-CD20 | 14 | 70% |
| Chemotherapy | 10 | 50% | |
| PI3K inhibitor | 1 | 5% | |
| Lenalidomide | 1 | 5% | |
| Radiation | 5 | 25% | |
| Investigational agents | 6 | 30% |
| . | . | Median/number . | Range/percent . |
|---|---|---|---|
| Age (y) | 64 | 35-79 | |
| Sex | Male | 11 | 55% |
| Female | 9 | 45% | |
| Lymphoma histology | FL | 19 | 95% |
| MZL | 1 | 5% | |
| Stage at diagnosis | I | 0 | 0% |
| II | 3 | 15% | |
| III | 6 | 30% | |
| IV | 11 | 55% | |
| Grade at diagnosis (for patients with FL, n = 19) | 1-2 | 15 | 75% |
| 3A | 4 | 20% | |
| FLIPI at diagnosis (for patients with FL, n = 19) | 0-2 | 13 | 65% |
| 3+ | 6 | 30% | |
| Prior lines of therapy | 0 | 0 | 0% |
| 1 | 13 | 65% | |
| 2 | 3 | 15% | |
| 3 | 1 | 5% | |
| 4 | 1 | 5% | |
| 5 | 2 | 10% | |
| Prior therapy | Anti-CD20 | 14 | 70% |
| Chemotherapy | 10 | 50% | |
| PI3K inhibitor | 1 | 5% | |
| Lenalidomide | 1 | 5% | |
| Radiation | 5 | 25% | |
| Investigational agents | 6 | 30% |
Baseline characteristics of the 20 patients evaluable for safety and efficacy. Median and range (for age) or count and percent of all patients (for all other characteristics) are reported.
FLIPI, follicular lymphoma international prognostic index; MZL, marginal zone lymphoma.
Clinical safety
Among the 20 patients evaluable for safety, the most frequent treatment-related AEs of any grade were fatigue, myalgia, diarrhea, chills, headache, injection site reactions, bruising, and nausea, and most were grade 1 to 2 (supplemental Table 2). Serious AEs occurred in 4 patients and included infections, atrial fibrillation, bleeding, and rash. No grade 4 events or deaths were observed. One patient did not receive the fifth dose of intratumoral CpG due to a treatment-related grade 3 rash. Three patients discontinued ibrutinib due to treatment-related grade 3 events.
Clinical efficacy
Of the 20 patients evaluable for efficacy, 50% achieved an overall response, including 1 patient with a complete response (Figure 1B; supplemental Figure 1A; supplemental Table 3). To focus on systemic responses, we also evaluated response at uninjected tumor sites only (termed as “distant response”), as is recommended for ISV studies.10 Fourteen patients (70%) experienced at least a 30% distant response (Figure 1B). Most responding patients achieved their response by 12 weeks, and several experienced deepening of response over time (Figure 1C). Three patients had progressive disease due to discordant growth at a single site (supplemental Figure 1B). With a median follow-up time of 12.5 months, the median progression-free survival was 11 months (supplemental Figure 1C). Shrinkage at the sampled uninjected site consistently mirrored shrinkage at all other distant sites (P < .0001; supplemental Figure 1D), suggesting that correlative analyses at this site would well represent other distant sites.
Tumor-resident cell phenotypes
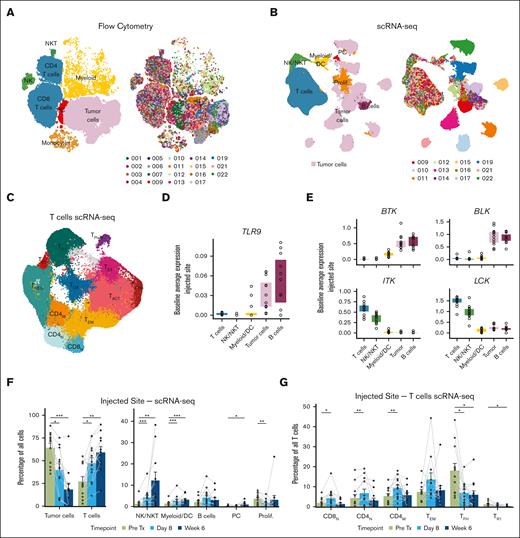
Tumor samples were analyzed by flow cytometry for 19 patients and by single-cell transcriptome, TCR, and B-cell receptor sequencing for 12 patients (supplemental Tables 3 and 4). Distant tumor burden reduction for the 12-patient scRNA-seq cohort was representative of the overall 20-patient cohort (supplemental Figure 2A). Graph-based clustering and cluster annotation revealed similar populations in each data set (Figure 2A-B). We identified tumor cells in the flow cytometry data set by clonal surface light chain expression (supplemental Figure 2B;supplemental Tables 1 and 5) and in the scRNA-seq data set by (1) clonal light chain expression and (2) distinct patient-specific clustering (supplemental Figure 3A-B; Figure 2B). There was excellent agreement on population frequencies between the orthogonal data sets (Spearman coefficient = 0.80; P < .001; supplemental Figure 3C).
ISV reshapes the composition of the tumor microenvironment. (A-B) Uniform manifold approximation and projections (UMAPs) of flow cytometry (A) or scRNA-seq (B) data from all tumor samples, colored by cell type (left) or patient (right). Note: patient colors are consistent throughout the article. (C) Reclustering of T-cells from the scRNA-seq data set. Clusters are denoted by color and labeled according to assigned phenotype. (D-E) Baseline gene expression of TLR9 (D) and selected ibrutinib targets (E) for each patient for indicated cell populations. (F) Relative abundance of indicated cell types in the scRNA-seq data set at the injected site in the pretreatment and on-treatment samples. (G) Relative abundance of selected T-cell subpopulations in the scRNA-seq data at the injected tumor site, pretreatment, and on treatment. P values were calculated by paired 2-sided Wilcoxon rank-sum tests for pretreatment (Pre Tx) vs on treatment (Day 8 or Week 6): ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. P values are only shown if P ≤ .05. DC, dendritic cell; PC, plasma cell.
ISV reshapes the composition of the tumor microenvironment. (A-B) Uniform manifold approximation and projections (UMAPs) of flow cytometry (A) or scRNA-seq (B) data from all tumor samples, colored by cell type (left) or patient (right). Note: patient colors are consistent throughout the article. (C) Reclustering of T-cells from the scRNA-seq data set. Clusters are denoted by color and labeled according to assigned phenotype. (D-E) Baseline gene expression of TLR9 (D) and selected ibrutinib targets (E) for each patient for indicated cell populations. (F) Relative abundance of indicated cell types in the scRNA-seq data set at the injected site in the pretreatment and on-treatment samples. (G) Relative abundance of selected T-cell subpopulations in the scRNA-seq data at the injected tumor site, pretreatment, and on treatment. P values were calculated by paired 2-sided Wilcoxon rank-sum tests for pretreatment (Pre Tx) vs on treatment (Day 8 or Week 6): ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. P values are only shown if P ≤ .05. DC, dendritic cell; PC, plasma cell.
For further analysis of T-cells and other immune cells, we subsetted and reclustered these populations. We identified 12 distinct T-cell subpopulations: naïve CD4 and CD8 T-cells (CD4N, CD8N), memory CD4 and effector memory T-cells (CD4M and TEM), activated/exhausted T-cells (TACT, TIFNG, and TEX), proliferating T-cells (TPROLIF), regulatory T-cells (TREGs), T follicular helper cells (TFHs), T regulatory type 1 cells (TR1s), and a population with high expression of oxidative phosphorylation genes (TOX) (Figure 2C; supplemental Figure 3D). We also identified 3 distinct natural killer (NK) cell populations, 2 monocyte/macrophage populations, 3 dendritic cell populations, a small population of stromal cells, and nonmalignant B- and plasma cell populations (supplemental Figure 3E).
Baseline expression of drug targets
We investigated pretreatment expression levels of proteins targeted by CpG and ibrutinib. TLR9 (the target of CpG) was expressed in tumor and in nonmalignant B-cells and, to a variable degree, in myeloid cells (Figure 2D). To explore ibrutinib targets, we cross-referenced kinases inhibited by ibrutinib (KINOMEscan6) against those with known expression in human hematopoietic cells. Although BTK and BLK expression were seen predominately in malignant and nonmalignant B-cells, ITK and LCK were primarily expressed in T and NK cells (Figure 2E).
ISV reshapes the tumor microenvironment
In both the flow cytometry and scRNA-seq data sets, tumor cells decreased significantly at the injected site, whereas T-cells, NK/NK T-cells, and myeloid/dendritic cells increased after treatment (Figure 2F; supplemental Figure 4A). Within T-cells, there was a treatment-induced shift in favor of CD8s over CD4s (supplemental Figure 4B). Naïve and memory T-cell subsets, both CD4 and CD8, increased after treatment, whereas there were dramatic reductions in TFH and TR1 populations (Figure 2G; supplemental Figure 4C-D). Minimal changes were observed across patients in these populations at the uninjected site (supplemental Figure 5).
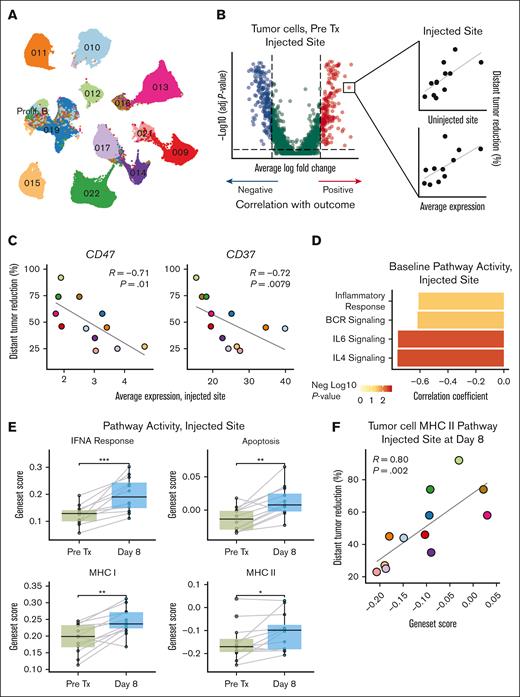
Baseline tumor cell phenotypes predict response to treatment
To identify baseline correlates of response, we reclustered tumor B-cells (Figure 3A) and searched for genes significantly differentially expressed between patients with greater vs lesser distant responses (Figure 3B; supplemental Figure 6A). To focus on the most robust predictors, we filtered this pool of candidate genes by requiring that patient-level baseline average expression at both sites correlate with distant response. The vast majority of identified genes were inversely correlated with distant response (supplemental Table 6). CD47 and CD37, both clinically targetable cell surface receptors, were among the top hits and associated with worse clinical outcome (Figure 3C; supplemental Figure 6B). Additional genes with inverse outcome correlation belonged to key lymphoma signaling pathways (eg, BANK1, IKZF3, and IL4R). Calculating scores for gene sets representing these pathways for each tumor cell, we confirmed this inverse relationship between clinical outcome and B-cell receptor signaling, inflammatory response, and signaling via the IL-4 and IL-6 receptors (Figure 3D; supplemental Figure 6C). Baseline proportions of tumor or immune cells did not predict response (supplemental Figure 6D).
Tumor cell phenotypes predict response to treatment. (A) UMAP of reclustered tumor cells from the scRNA-seq data set. (B) Strategy used to identify baseline biomarkers. Genes differentially expressed between tumor cells from patients with higher (n = 6) vs lower (n = 6) distant response at the injected tumor site were selected. Genes whose patient-level averages at both sites correlated with outcome were further evaluated. (C) Correlation between CD47 (left) and CD37 (right) expression in tumor cells at baseline and percentage distant tumor reduction at the injected tumor site. See supplemental Figure 5B for uninjected site data. (D) Bar plot of correlation coefficients between selected pathways at baseline and percentage distant tumor reduction. Bars are colored by negative log10 of the P value. See supplemental Figure 5C for uninjected site data. (E) Comparison of pretreatment (pre Tx) and on-treatment (day 8) scores for the indicated gene sets at the injected tumor site. (F) Tumor cell MHCII gene set score on day 8 at the injected site correlates with percentage distant tumor reduction. Correlations in (C), (D), and (F) were assessed using the Spearman test. P values in (E) were calculated using 2-sided paired Wilcoxon rank-sum tests: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. P values are only shown if P ≤ .05. BCR, B-cell receptor; FC, fold change; MHC I, antigen presentation via MHCI; MHC II, antigen presentation via MHCII. UMAP colors in (A) and data point colors in (C) and (F) correspond to individual patients as in (A) and in Figure 2(A-B).
Tumor cell phenotypes predict response to treatment. (A) UMAP of reclustered tumor cells from the scRNA-seq data set. (B) Strategy used to identify baseline biomarkers. Genes differentially expressed between tumor cells from patients with higher (n = 6) vs lower (n = 6) distant response at the injected tumor site were selected. Genes whose patient-level averages at both sites correlated with outcome were further evaluated. (C) Correlation between CD47 (left) and CD37 (right) expression in tumor cells at baseline and percentage distant tumor reduction at the injected tumor site. See supplemental Figure 5B for uninjected site data. (D) Bar plot of correlation coefficients between selected pathways at baseline and percentage distant tumor reduction. Bars are colored by negative log10 of the P value. See supplemental Figure 5C for uninjected site data. (E) Comparison of pretreatment (pre Tx) and on-treatment (day 8) scores for the indicated gene sets at the injected tumor site. (F) Tumor cell MHCII gene set score on day 8 at the injected site correlates with percentage distant tumor reduction. Correlations in (C), (D), and (F) were assessed using the Spearman test. P values in (E) were calculated using 2-sided paired Wilcoxon rank-sum tests: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. P values are only shown if P ≤ .05. BCR, B-cell receptor; FC, fold change; MHC I, antigen presentation via MHCI; MHC II, antigen presentation via MHCII. UMAP colors in (A) and data point colors in (C) and (F) correspond to individual patients as in (A) and in Figure 2(A-B).
ISV induces IFN responses and antigen presentation in tumor B-cells
Next, we investigated effects induced by treatment at the injected site in tumor cells. Genes involved in interferon (IFN) responses (IRF1 and MX1), apoptosis (FAS and BAX), and antigen presentation (HLA-A, HLA-B, and HLA-DRB1) were up-regulated on day 8 (supplemental Figure 6E). Scoring of gene sets representing these pathways confirmed these findings (Figure 3E). Intriguingly, tumor cell major histocompatibility complex class II (MHCII) expression after treatment correlated positively with subsequent clinical response (R = 0.80, P = .002; Figure 3F), suggesting that antigen presentation by tumor B-cells, likely a direct effect of TLR9 agonism, may be important for training the ensuing immune response. Cell surface HLA-DR expression detected by flow cytometry trended in the same direction (supplemental Figure 6F). Genes associated with antigen presentation also increased in myeloid and nonmalignant B-cells, but this did not relate to clinical responses (data not shown).
ISV induces IFN responses in microenvironmental cells
An important effect of TLR9 agonism is the production of type 1 IFNs by innate immune cells, which in turn induce the expression of IFN-stimulated genes (ISGs) in responsive cells. We detected induced expression of ISGs across all patients, on day 8 in all cell types and sustained at week 6 in some (Figure 4A; supplemental Figure 7A). This occurred at the injected site only, indicating that the intratumorally delivered TLR9 agonist acted predominately locally. Degree of ISG induction, at either timepoint, did not correlate with distant clinical response (supplemental Figure 7B).
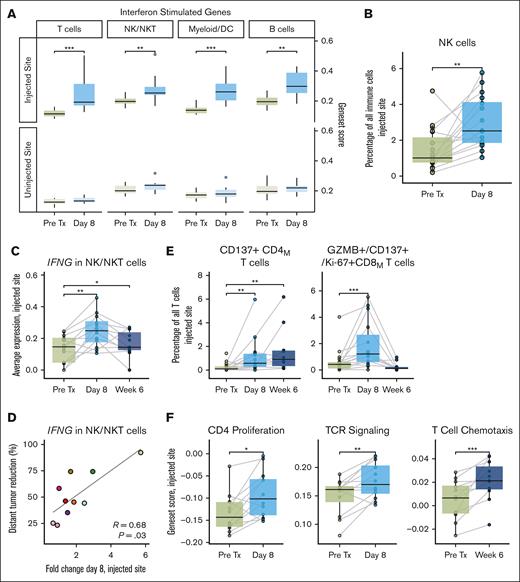
ISV induces interferon responses, antigen presentation and effector cell activation. (A) Comparing gene set scores for interferon-stimulated genes between pretreatment (pre Tx) and on-treatment (day 8) timepoints for the injected and uninjected tumor sites, averaged per sample per patient. (B) NK cell abundance by flow cytometry at the injected tumor site. (C) IFNG expression at the injected tumor site in NK/NKT cells across timepoints. (D) Fold change in NK/NKT expression of IFNG at day 8 at the injected site vs percentage distant tumor reduction. Dots are colored by patient, as in Figure 2B. (E) Treatment-induced changed in activated CD137+ memory CD4 T-cells (right) and CD137+/granzyme B–positive memory CD8 T-cells (left) at the injected tumor site, measured by flow cytometry. (F) Changes in indicated pathways in T-cells across patients during therapy at the injected tumor site. P values were calculated by 2-sided Wilcoxon rank-sum tests for paired samples: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. P values are only shown if P ≤ .05.
ISV induces interferon responses, antigen presentation and effector cell activation. (A) Comparing gene set scores for interferon-stimulated genes between pretreatment (pre Tx) and on-treatment (day 8) timepoints for the injected and uninjected tumor sites, averaged per sample per patient. (B) NK cell abundance by flow cytometry at the injected tumor site. (C) IFNG expression at the injected tumor site in NK/NKT cells across timepoints. (D) Fold change in NK/NKT expression of IFNG at day 8 at the injected site vs percentage distant tumor reduction. Dots are colored by patient, as in Figure 2B. (E) Treatment-induced changed in activated CD137+ memory CD4 T-cells (right) and CD137+/granzyme B–positive memory CD8 T-cells (left) at the injected tumor site, measured by flow cytometry. (F) Changes in indicated pathways in T-cells across patients during therapy at the injected tumor site. P values were calculated by 2-sided Wilcoxon rank-sum tests for paired samples: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. P values are only shown if P ≤ .05.
ISV increases and activates NK and T-cells
Type 1 IFN responses can recruit and activate NK cells and increase their production of IFNγ.12,13 NK cells expanded significantly in tumors after treatment (Figure 4B), and we observed intense local NK cell stimulation with significant upregulation of genes related to NK activation and cytotoxicity (supplemental Figure 7C) as well as increased production of cytokines (CCL4 and CCL5) and cytotoxicity markers (GZMB, GZMA, and PRF1; supplemental Figure 7D). IFNG expression increased in NK/NK T-cells (Figure 4C), and posttreatment IFNG levels correlated with positive clinical outcomes (Figure 4D). Activation and cytotoxicity markers also increased in CD4 and CD8 T-cells after treatment (by flow cytometry, Figure 4E; by scRNA-seq, supplemental Figure 7E). Gene set analysis revealed treatment-induced upregulation of pathways related to TCR signaling, CD4 T-cell proliferation, and T-cell chemotaxis during therapy (Figure 4F).
ISV relieves barriers to immunotherapy success
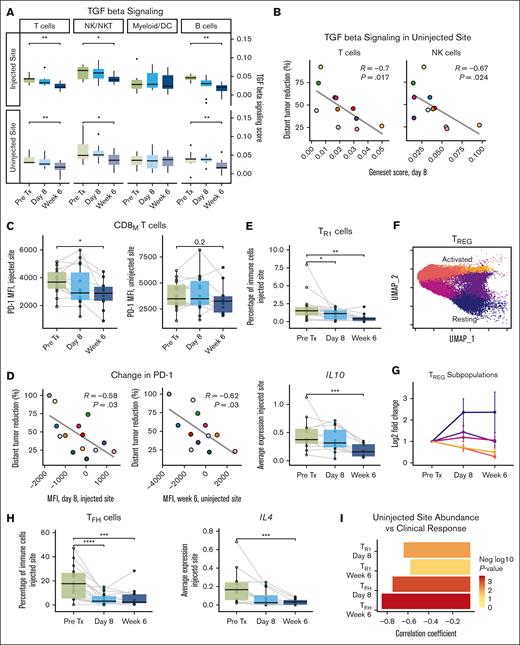
For ISV to be effective, locally primed T-cells must be able to function at distant tumor sites with potentially unfavorable environments. Transforming growth factor β (TGF-β) restrains the development of effective immune responses against developing cancers and during immunotherapy.14 We, therefore, assessed TGF-β signaling in microenvironmental cells before and after treatment. Among T-cells, NK/NK T-cells, and nonmalignant B-cells, TGF-β–associated genes decreased at both sites at week 6 (Figure 5A). In T-cells and NK cells, posttreatment levels of TGF-β–associated genes at the uninjected site correlated with clinical outcomes (Figure 5B). Inhibitory checkpoints also restrain immune responses. Levels of cell surface programmed cell death protein 1 (PD-1) on antigen-experienced (CD45RO+) CD8 T-cells decreased significantly after treatment at the injected site and, in some patients, at the uninjected site (Figure 5C). Reduction in cell surface PD-1 at both sites correlated with clinical responses (Figure 5D).
ISV relieves global and local immunosuppression. (A) TGF-β signaling scores (by patient) at the injected and uninjected sites for the indicated cell types. (B) Scatter plots depicting relationship between TGF-β signaling scores at day 8 at the uninjected site and percentage distant tumor reduction for T and NK cells. (C) PD-1 mean fluorescence intensity on memory CD8 T-cells across time points at the injected site and uninjected site. (D) Scatter plots depicting relationship between CD8 T-cell PD-1 mean fluorescence intensity on day 8 at the injected and uninjected sites and percentage distant tumor reduction. (E) Treatment-induced changes in TR1s (top) by flow cytometry and IL10 expression (bottom) by scRNA-seq at the injected site. (F) UMAP of TREGs, colored as per the subpopulations identified by unsupervised clustering. (G) Fold change in abundance of TREG subpopulations during therapy at the injected tumor site. (H) Treatment-induced changes in TFHs (left) by flow cytometry and IL4 expression (right) by scRNA-seq at the injected site. (I) Bar plot of correlation coefficients relating change in TFH and TR1 abundance by flow cytometry at the uninjected site and percentage distant tumor reduction. Correlations assessed using Spearman test. P values were calculated by 2-sided Wilcoxon rank-sum tests for paired samples pretreatment (pre Tx) vs on treatment (day 8/week 6): ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. P values are only shown if P ≤ .05. Data point colors in (B) and (D) correspond to individual patients as in Figure 2(A-B).
ISV relieves global and local immunosuppression. (A) TGF-β signaling scores (by patient) at the injected and uninjected sites for the indicated cell types. (B) Scatter plots depicting relationship between TGF-β signaling scores at day 8 at the uninjected site and percentage distant tumor reduction for T and NK cells. (C) PD-1 mean fluorescence intensity on memory CD8 T-cells across time points at the injected site and uninjected site. (D) Scatter plots depicting relationship between CD8 T-cell PD-1 mean fluorescence intensity on day 8 at the injected and uninjected sites and percentage distant tumor reduction. (E) Treatment-induced changes in TR1s (top) by flow cytometry and IL10 expression (bottom) by scRNA-seq at the injected site. (F) UMAP of TREGs, colored as per the subpopulations identified by unsupervised clustering. (G) Fold change in abundance of TREG subpopulations during therapy at the injected tumor site. (H) Treatment-induced changes in TFHs (left) by flow cytometry and IL4 expression (right) by scRNA-seq at the injected site. (I) Bar plot of correlation coefficients relating change in TFH and TR1 abundance by flow cytometry at the uninjected site and percentage distant tumor reduction. Correlations assessed using Spearman test. P values were calculated by 2-sided Wilcoxon rank-sum tests for paired samples pretreatment (pre Tx) vs on treatment (day 8/week 6): ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. P values are only shown if P ≤ .05. Data point colors in (B) and (D) correspond to individual patients as in Figure 2(A-B).
Immunosuppression in the TME can also originate from professional inhibitory cells. TR1s are highly suppressive to effector T-cells and have been found in solid and hematologic tumors.15 In our study, TR1s decreased significantly at the injected site over time, as did IL10, a key immunosuppressive cytokine elaborated by these cells (Figure 5E). Lower posttreatment IL10 on day 8 correlated with better clinical outcomes (supplemental Figure 8A). Abundance of conventional FOXP3+ TREGs did not change (supplemental Figures 4C-D and 5C-D). However, TREGs comprised several subpopulations, ranging from more quiescent (resting) to highly activated (Figure 5F; supplemental Figure 8B), and TREG composition shifted toward the resting phenotype during treatment (Figure 5G). This was accompanied by significant decreases in markers of TREG suppressive function: CTLA4, IL2RA (CD25), LAG3, and TNFRSF1B (TNFR2) (supplemental Figure 8C).
Immunotherapy efficacy can also be limited by the presence of tumor-favorable elements. TFHs are thought to support lymphoma cell growth, and this population decreased significantly at the injected site, as did IL4, a key cytokine implicated in B-cell–TFH interactions (Figure 5H). Patients who experienced reduction in TFH and TR1 populations at the uninjected site had better subsequent clinical outcomes (Figure 5I). Taken together, these results suggest that our treatment removed multiple barriers to immunotherapy and that treatment-induced relief of systemic immune suppression was important for immunotherapy efficacy.
Integrated TCR and transcriptome analysis demonstrates selective clonotypic expansion
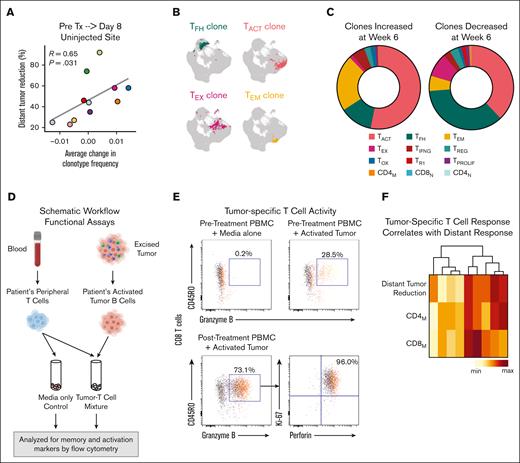
We analyzed paired single-cell TCR-sequencing data from tumors for clonotype abundance. Degree of TCR clonality at baseline did not relate to clinical responses (data not shown); however, the average change in clonotype frequencies by day 8 at the uninjected site did correlate with distant response (Figure 6A). Of the 163 183 T-cells in the transcriptome data set, we could give 145 449 (89%) T-cells a paired chain-based clonotype assignment (examples in Figure 6B). Using this TCR-transcriptome integrated data set, we identified clones that expanded or contracted with treatment and compared the distribution of phenotypes in each group. T-cells belonging to expanding clones were predominantly of activated and memory phenotypes, whereas T-cells belonging to contracting clones were more often of TFH and exhausted phenotypes (Figure 6C).
TCR clonotype expansion and tumor-specific T-cell responses are induced during therapy. (A) Scatterplot relating average change in frequency of multiplet TCR clones from pretreatment (pre Tx) to day 8 (both sites combined) to percentage distant tumor reduction. (B) Examples of 4 TCR clonotypes and the location of cells with those clonotypes in the T-cell UMAP. (C) Phenotype distribution of TCR clones that increased by week 6 after treatment (left) and those that decreased by week 6 (right). (D) Schematic workflow of experimental in vitro immune response assay. T-cell reactivity against tumors were measured before treatment and on treatment (weeks 1, 3, 6, and 12). (E) Representative flow cytometry plots measuring autologous tumor-reactive granzyme B–positive memory (CD45RO+) CD8 T-cells under the indicated conditions. Most granzyme B–positive/CD45RO+ CD8M T-cells were also Ki-67-positive and perforin-positive (mean, 67%; range, 2.3%-96%). (F) Unsupervised clustering of patients by percentage distant tumor reduction and percentage change in tumor-reactive activated memory CD4 and CD8 T-cells at week 3. PBMC, peripheral blood mononuclear cells. max, maximum; min, minimum.
TCR clonotype expansion and tumor-specific T-cell responses are induced during therapy. (A) Scatterplot relating average change in frequency of multiplet TCR clones from pretreatment (pre Tx) to day 8 (both sites combined) to percentage distant tumor reduction. (B) Examples of 4 TCR clonotypes and the location of cells with those clonotypes in the T-cell UMAP. (C) Phenotype distribution of TCR clones that increased by week 6 after treatment (left) and those that decreased by week 6 (right). (D) Schematic workflow of experimental in vitro immune response assay. T-cell reactivity against tumors were measured before treatment and on treatment (weeks 1, 3, 6, and 12). (E) Representative flow cytometry plots measuring autologous tumor-reactive granzyme B–positive memory (CD45RO+) CD8 T-cells under the indicated conditions. Most granzyme B–positive/CD45RO+ CD8M T-cells were also Ki-67-positive and perforin-positive (mean, 67%; range, 2.3%-96%). (F) Unsupervised clustering of patients by percentage distant tumor reduction and percentage change in tumor-reactive activated memory CD4 and CD8 T-cells at week 3. PBMC, peripheral blood mononuclear cells. max, maximum; min, minimum.
ISV elicits functional tumor-specific T-cell responses
Given the expansion of TEM clonotypes during therapy (Figure 6C), we hypothesized that our treatment had induced tumor-specific T-cell responses. To test this hypothesis, we performed functional in vitro immune response experiments for 9 patients from whom we had sufficient tumor material from before treatment. We cocultured peripheral blood T-cells from before and during treatment with autologous tumor cells and identified tumor-specific activation of memory CD4 and CD8 T-cells (Figure 6D-E; supplemental Table 7; supplemental Figure 9A). Tumor-reactive activated cytolytic memory CD8 T-cells increased in 8 of 9 patients, whereas tumor-reactive activated CD4 memory T-cells increased in 5 of 9 patients for at least 2 time points after treatment (supplemental Figure 9B). Three weeks after treatment start, tumor-specific activation in both T-cell populations trended with subsequent clinical responses (Figure 6F; R = 0.62 for CD8s and R = 0.63 for CD4s). Thus, ISV induced tumor-reactive memory CD4 and CD8 T-cell populations that could be detected in the peripheral blood, indicating systemic antitumor immune response.
Discussion
We report on a clinical trial testing in situ cancer vaccination for patients with B-cell non-Hodgkin lymphoma. We treated 1 tumor site with local low-dose radiation and an intratumoral TLR9 agonist, followed by oral ibrutinib. Fifty percent of patients experienced an overall response, and 70% had >30% tumor reduction outside the injected site, reflecting effective treatment-induced systemic immune responses. In-depth analyses via orthogonal methods on longitudinal samples from multiple tumors allowed us to identify potential key mechanistic roles for tumor cell antigen presentation, activation of effector T and NK cells, and dampening of immunosuppressive and tumor-promoting elements of the TME. Furthermore, by analyzing TCR clonotypes and obtaining experimental evidence for the expansion of tumor-reactive T-cells, we could infer the generation of systemic tumor-specific immune responses.
Intratumoral CpG and LDRT results in a response rate of 28% in untreated low-grade lymphoma.3 Ibrutinib monotherapy in patients with FL, the predominant histology in our study, results in a response rate ranging from 21% to 35%.16,17 The overall response rate in this combination study was 50%. The value of cross-study efficacy comparisons is limited; however, our correlative analyses did suggest separate, potentially synergistic contributions of intratumoral CpG/LDRT and ibrutinib. Induction of IFN responses and antigen presentation as well as activation of T and NK cells were seen prominently on day 8, before ibrutinib therapy, suggesting these were key contributions of the local treatment. Reductions in TGF-β pathway genes in multiple cell types and cell surface PD-1 on CD8 T-cells were observed only at week 6 and seen at injected and uninjected sites, suggesting a systemic ibrutinib effect. Abrogation of TR1 cells began at week 2 and deepened at week 6, signifying an effect of the combined therapy. Thus, we were able to induce collaborative and effective antitumor immune responses by simultaneously modulating different aspects of the immune system.
Although we observed the most pronounced biological effects of treatment at the injected site, most of these effects, such as IFN responses, did not explain the heterogeneity in clinical responses. In contrast, several biomarkers changed differentially among patients with better vs worse clinical responses at the uninjected site. Patients with deeper clinical responses exhibited decreased TGF-β–associated signaling, decreased TR1 and TFH populations, decreased PD-1 on CD8 T-cells, and expanded TCR clones at their uninjected (distal) sites. We also observed increased tumor-specific T-cells in the peripheral blood after treatment, implicating the generation of systemic immunity. Thus, local therapy induced a strong immune response at the injected site, and the addition of ibrutinib facilitated changes at distant tumor sites, particularly relief of immune suppression, which enabled effective systemic immune responses to develop.
Tumor cell antigen presentation via MHCII emerged as a strong predictor of superior clinical response. MHCII expression by other antigen-presenting cells and general markers of IFN effects did not correlate with clinical response, suggesting that tumor cell antigen presentation via MHCII may be uniquely important. This is a particularly robust finding as it was also observed in our prior study of CpG and LDRT3 and concurs with prior demonstrations of lymphoma neoantigen presentation by human lymphoma B-cells18,19 and direct T-cell priming by mouse lymphoma B-cells.20 In our study, there was evidence of increased CD4 T-cell activation. These findings emphasize the potential role of CD4 T-cell activation via tumor cell MHCII in generating antitumor immune responses, with the tumor cells themselves being coopted for therapeutic gain.
Although treatment-induced changes were prominent in micronevironmental cells, baseline features predicting response were enriched in tumor cells. Higher baseline tumor cell expression of CD47 and CD37, 2 cell surface receptors against which antibody therapeutics are in clinical development,21-23 correlated with worse clinical outcomes. Both interact with integrins, which may promote interactions with protective niches and TFH cells.24,25 CD47 additionally interacts with signal regulatory protein α (SIRP-α), inhibiting macrophage-mediated phagocytosis, and has been linked to poor prognosis in hematologic malignancies.26,27 If these receptors are actively inhibiting immunotherapy response, ISV strategies incorporating CD47 or CD37 blockade may increase efficacy.
Immune checkpoint blockade (ICB) has improved outcomes in many solid malignancies and Hodgkin lymphoma but has had very limited activity in FL.28 This could be due to a lack of pre-existing antitumor T-cells for ICB to reinvigorate, the inability to overcome a suppressive immune microenvironment, or, particularly in FL, unpredictable effects on TFH cells, known to express high levels of PD-1. ISV is a wholly different approach, aimed at generating de novo immune responses in an activated tumor environment. Our study demonstrates that with the right mix of agents, a collaborative immune response can be achieved: release of tumor antigens and initiation of inflammatory cascades by LDRT, activation of innate immune cells and tumor cell antigen presentation by CpG, and dampening of critical immune suppressors by ibrutinib. Such approaches may be useful in other settings in which ICB is less effective.
This work supports the development of further ISV-based strategies and underscores the utility of serial biopsies for understanding in vivo mechanisms of immunotherapy. Application of modern high-dimensional methods to longitudinal samples allowed us to glean critical insights into mechanisms of action and features associated with clinical response. Although some of our findings are likely specific to lymphoma, such as the role of TFH cells, others, such as reductions in TGF-β signaling and TR1 suppressive T-cells, may apply broadly. Therapeutic cancer vaccination can be safe and effective, and our study suggests that future efforts could focus on combining agents that can reduce global immunosuppression while providing effective local cancer immunization.
Acknowledgments
The authors thank the patients and their families; our clinical research staff, including Alyssa Kanegai, Rachel Greenstein, Destiny Philips, and Summer Guo; and laboratory staff, particularly Etelka Gabriel, and members of the Levy and Ji laboratories for critical discussions, particularly Vishnu Shankar and Shoshana Levy. Figure 1A was created with the aid of BioRender.com.
Janssen provided financial support and ibrutinib for the clinical trial. TriSalus Life Sciences provided SD101 for the clinical trial. The study was supported by National Institutes of Health (NIH) National Cancer Institute grant R35 CA197353, Leukemia & Lymphoma Society grant TRP 6539-18, and the Hoogland Lymphoma Research Fund (R.L.); and was supported at different times by NIH National Cancer Institute grant K08CA252637, National Heart, Lung, and Blood Institute grant 5T32HL120824-04, and an American Cancer Society Postdoctoral Fellowship PF-17-239-01-LIB (T.S.); and by a Mildred Scheel postdoctoral fellowship from the Deutsche Krebshilfe (70113507) (S.H.).
The content is solely the responsibility of the authors and does not represent the official views of any funding agency listed.
Authorship
Contribution: R.L. and M.S.K. conceived and designed the clinical trial; T.S., M.S.K., M.J.F., R.L., A.A.A., R.A., and L.S.M. referred and enrolled patients on the trial; S.R.L., B.M., and M.G.O. performed fine needle aspirates and R.T.H. oversaw radiotherapy; D.K.C. processed patient samples and conducted flow cytometry assays and in vitro immune response assays; D.K.C., T.S., and S.H. analyzed, interpreted, and presented flow cytometry and immune response data; T.S., S.H., and A.S. conducted single-cell sequencing assays; S.G., A.S., and G.D. processed raw single-cell sequencing data; T.S., S.H., and G.D., analyzed, interpreted, and presented single-cell sequencing data; T.S. and E.E. collected, analyzed, interpreted, and presented clinical efficacy and safety data; R.L. and H.P.J. provided supervision and resources for all aspects of the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Levy, Division of Oncology, Department of Medicine, Center for Clinical Science Research, Stanford University, 269 Campus Dr, Center for Clinical Sciences Research, Room 1105, Stanford, CA 94305; email: levy@stanford.edu.
References
Author notes
∗T.S. and S.H. contributed equally to the study.
The scRNA-seq data are deposited in the NCBI database of Genotypes and Phenotypes (dbGaP, accession number phs002188).
Deidentified individual participant data relevant to this study are available on request from the corresponding author, Ronald Levy (levy@stanford.edu).
The full-text version of this article contains a data supplement.