Multiple MCL models of PRMT5 inhibitor drug resistance were generated to identify pathways dysregulated with disease progression.
Single-cell RNA sequencing highlighted distinct transcriptional states with PRMT5 inhibitor resistance and revealed mTOR signaling as a relevant pathway.
Visual Abstract
Mantle cell lymphoma (MCL) is an incurable B-cell non-Hodgkin lymphoma, and patients who relapse on targeted therapies have poor prognosis. Protein arginine methyltransferase 5 (PRMT5), an enzyme essential for B-cell transformation, drives multiple oncogenic pathways and is overexpressed in MCL. Despite the antitumor activity of PRMT5 inhibition (PRT-382/PRT-808), drug resistance was observed in a patient-derived xenograft (PDX) MCL model. Decreased survival of mice engrafted with these PRMT5 inhibitor–resistant cells vs treatment-naive cells was observed (P = .005). MCL cell lines showed variable sensitivity to PRMT5 inhibition. Using PRT-382, cell lines were classified as sensitive (n = 4; 50% inhibitory concentration [IC50], 20-140 nM) or primary resistant (n = 4; 340-1650 nM). Prolonged culture of sensitive MCL lines with drug escalation produced PRMT5 inhibitor–resistant cell lines (n = 4; 200-500 nM). This resistant phenotype persisted after prolonged culture in the absence of drug and was observed with PRT-808. In the resistant PDX and cell line models, symmetric dimethylarginine reduction was achieved at the original PRMT5 inhibitor IC50, suggesting activation of alternative resistance pathways. Bulk RNA sequencing of resistant cell lines and PDX relative to sensitive or short-term–treated cells, respectively, highlighted shared upregulation of multiple pathways including mechanistic target of rapamycin kinase [mTOR] signaling (P < 10-5 and z score > 0.3 or < 0.3). Single-cell RNA sequencing analysis demonstrated a strong shift in global gene expression, with upregulation of mTOR signaling in resistant PDX MCL samples. Targeted blockade of mTORC1 with temsirolimus overcame the PRMT5 inhibitor–resistant phenotype, displayed therapeutic synergy in resistant MCL cell lines, and improved survival of a resistant PDX.
Introduction
Mantle cell lymphoma (MCL) is an incurable B-cell non-Hodgkin lymphoma characterized by an expansion of CD5+ mature B cells with a high degree of genomic instability.1,2 The pathognomonic chromosomal translocation t(11;14) drives overexpression of cyclin D1 (CCND1), leading to cell cycle dysregulation.3,4 Multiple mutations that promote oncogenesis have been described, including those resulting in inactivation of the DNA damage response proteins, p53, and ATM.5,6 The disease commonly manifests in males aged >60 years and imparts a median survival time of 80 months.7 For patients ineligible for aggressive treatment using autologous stem cell transplantation, the disease is treated with immunochemotherapy or targeted therapies.8 Relapsed disease is frequently characterized by an aggressive clinical course accompanied by resistance to salvage therapy and poor outcome.9
In addition to genomic instability and numerous mutations, epigenetic dysregulation, including aberrant histone and DNA methylation, has been identified in MCL.10 Protein arginine methyltransferase 5 (PRMT5) is a driver of this epigenetic dysregulation through symmetric dimethylation of histone arginine residues.11 PRMT5 is upregulated in both solid organ and blood cancers and is required for the driver activity of multiple oncogenes.12,13 Our group was the first, to our knowledge, to describe PRMT5 dysregulation as an oncogenic driver in MCL.14 PRMT5 plays an essential role in B-cell transformation by promoting sustained activation of nuclear factor κB (NF-κB), cell cycle, and B-cell receptor signaling.15,16 Thus, PRMT5 has emerged as an attractive therapeutic target to treat malignant B-cell lymphomas.17 We and others have worked to develop several selective, small molecule inhibitors of PRMT5 that have shown promising activity in several preclinical models and clinical trials treating patients with solid organ and blood cancers.17-22
Decreased symmetric dimethylarginine (SDMA) expression is a biomarker for PRMT5 inhibition.21 In malignant cells, this inhibition leads to the restoration of multiple cellular regulatory mechanisms that drive cell cycle arrest and enhanced expression of proapoptotic genes and tumor suppressors that regulate B-cell receptor, phosphatidylinositol 3-kinase (PI3K)/AKT, and WNT/β-catenin networks in MCL.23-27 Several other pathways have become relevant to the efficacy of PRMT5 inhibition including p53, E2F transcription factor, mechanistic target of rapamycin kinase (mTOR), proto-oncogene MYC, DNA replication, and cell cycle regulation.21,26,28
Currently reported biomarkers of sensitivity to PRMT5 inhibition in MCL include MTAP deletion and p53 wild-type status.21p53 mutations and MUSASHI-2, an RNA binding protein that controls protein translation, were associated with resistance to PRMT5 inhibitor therapy in B-cell lymphomas.29 Emergence of PRMT5 inhibitor resistance in lung adenocarcinoma was dependent on the presence of stathmin 2, a microtubule regulator.30 However, the transcriptomic and genomic changes that occur with inherent or acquired resistance to PRMT5 targeted therapy remain an unexplored research field.
In this study, we focused on characterizing the mechanisms of resistance to PRMT5 inhibition in MCL and on developing new strategies to overcome it. To achieve this, we generated and used several in vitro and in vivo models of PRMT5 inhibitor–resistant MCL. Our analysis identified several prominent transcriptomic changes arising with resistance including mTOR signaling. In vivo studies showed a significant survival advantage in patient-derived xenograft (PDX) with PRMT5 inhibitor–resistant MCL with dual inhibition of PRMT5 and mTOR complex 1 (mTORC1) function. These findings highlight how transcriptomic remodeling results in resistance to PRMT5 inhibition in MCL. Based on the altered transcriptomic state of PRMT5 inhibitor–resistant cells in vivo, we have identified mTOR inhibition as a potential strategy to circumvent PRMT5 inhibitor drug resistance in MCL. This work has the potential to extend to other patient populations, given that numerous clinical trials using PRMT5 inhibitors for both solid and blood cancers are underway.19
Materials and methods
In vivo studies
The PDX-AA studies were performed at The Ohio State University under protocol 2009A0094-R4 and Insitutional Animal Care and Use Committee approval as previously described.31 Further details are provided in supplemental Methods.
Cell culture, development of PRMT5 inhibitor resistance in vitro, drugging, and synergy
The cell lines used in this work were validated by short tandem repeat typing. Resistance to PRMT5 inhibition was developed using drug escalation protocols on sensitive MCL cell lines in continuous culture. Resistance was defined as a 50% reduction in the percentage of live cells (IC50), 2 to 5 times that of the original cell line. To verify sustained resistance, resistant cell lines were cultured for 1 month in the absence of drug and rechallenged at their new IC50.32 IC50s (Table 1) were measured on day 9 for PRT-382/PRT-808 with annexin V/PI staining and flow cytometry and on day 3 for temsirolimus via MTS assay. Synergy was measured via MTS assay on day 9, with 6 days of PRT-382 treatment and 3 days of combination treatment. Scores and plots were calculated with the Loewe model via Combenefit.33 Further details including cell culture are provided in supplemental Methods.
IC50s for PRT-382 in 8 MCL cell lines at 9 days evaluated via annexin V/PI
| MCL line . | IC50 (nM) . | |
|---|---|---|
| Original . | Acquired resistance . | |
| Maver | 1650 | — |
| Mino | 610 | — |
| UPN1 | 440 | — |
| Jeko-1 | 340 | — |
| Z-138 | 140 | 400 |
| REC-1 | 70 | 230 |
| SP53 | 40 | 200 |
| CCMCL1 | 20 | 500 |
| MCL line . | IC50 (nM) . | |
|---|---|---|
| Original . | Acquired resistance . | |
| Maver | 1650 | — |
| Mino | 610 | — |
| UPN1 | 440 | — |
| Jeko-1 | 340 | — |
| Z-138 | 140 | 400 |
| REC-1 | 70 | 230 |
| SP53 | 40 | 200 |
| CCMCL1 | 20 | 500 |
Whole exome sequencing and RNA-seq
Whole exome sequencing of DNA from PDX and cell lines of MCL was analyzed to determine mutational variants and copy number variations. Details regarding sample preparation for bulk and single-cell RNA sequencing (scRNA-seq) are provided in the supplemental Methods. For the RNA-seq, TrueSeq stranded messenger RNA libraries were prepared with Poly-A selection Ribodepletion library prep and sequenced using Illumina NovaSeq 6000 on a S1 flow cell (paired end reads with 2 × 100 bp). Differentially expressed genes (DEGs) were assessed using DESeq2 with pairwise comparisons between treatment and control (q < 0.05; |log2FC| > .3). Ingenuity pathway analysis was used to prioritize pathways and candidate genes.34
After preparation of the PDX MCL samples for scRNA-seq, barcoded complementary DNA was generated by reverse transcription and then amplified in bulk according to the manufacturer's instructions. Barcoded RNA enrichment was performed by polymerase chain reaction amplification of barcoded complementary DNA using gene-specific primers. Multiplexed sequencing libraries were generated and sequenced on a 100-cycle NovaSeq SP flow cell. Bioinformatic and statistical analysis are detailed in supplemental Methods.
Western blotting
Immunoblotting was performed according to standard methods. Further details are provided in the supplemental Methods.
Statistics
Continuous data were analyzed with unpaired t tests and 2-way analysis of variance when applicable. Survival data were analyzed with the Kaplan-Meier method and tested by log rank (Mantel-Cox) test. Dose response curves were used to generate IC50 values using least square regression with bottom constraint set to zero. For this exploratory preclinical study, P values were not adjusted for potential multiple comparisons, except for the analysis of DEGs, in which the P values were adjusted, with q value < 0.05 as the significance cutoff. Error bars show standard error of the mean (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001).
Results
Development of a PDX MCL model resistant to PRMT5 inhibition
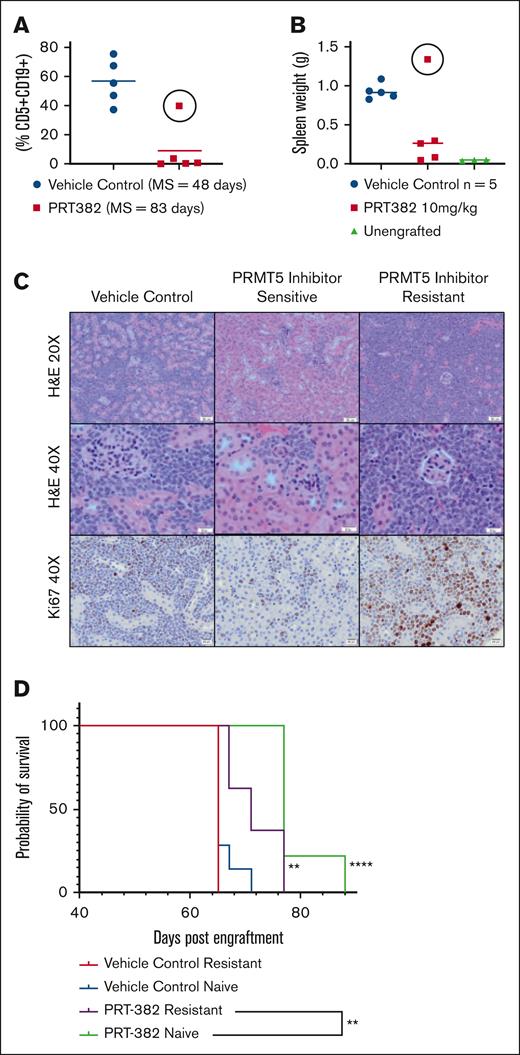
The PDX-AA MCL mouse model was derived from a patient with ibrutinib-resistant MCL. We initially characterized the efficacy of the selective PRMT5 inhibitor PRT-382 in this aggressive MCL model. Treatment was initiated via oral gavage with either PRT-382 (10 mg/kg) or vehicle at 25 days post engraftment (DPE) when peripheral disease burden was <5% (huCD5+/CD19+ lymphocytes). Samples were collected as outlined (supplemental Figure 1A). PRT-382 prolonged the median overall survival (MS) at 83 days (75-91 DPE) vs 48 days (47-56 DPE) for vehicle control (VC).21 However, 1 mouse in the PRMT5 inhibitor–treated group showed evidence of rapid disease progression despite continued treatment with PRT-382 based on peripheral disease burden and spleen weight at necropsy (Figure 1A-B, circle). This mouse also had widely disseminated neoplastic infiltration of multiple organs including the kidney compared with that of both VC and other PRMT5 inhibitor–treated mice (Figure 1C). Based on these data, MCL tumor cells were determined to be PRMT5 inhibitor resistant.
Development of PDX MCL model of PRMT5 inhibitor resistance. (A) Peripheral disease burden based on circulating neoplastic cells (CD5+/CD19+) cells in the PRT-382 (red) and VC (blue) treated cohort in the PDX MCL model. One PRT-382–treated mouse became resistant to therapy as evidenced by its increased peripheral disease burden (circle), (B) increased spleen weight in resistant mouse (circle), and (C) increased neoplastic infiltration and ki67 staining of the kidney via histopathology of the PRMT5 inhibitor–resistant mouse compared with those of both VC and other PRMT5 inhibitor–sensitive mice in its cohort; H&E, original magnification ×20 and ×40 and ki67, original magnification ×40×. (D) Kaplan-Meier plot of the in vivo experiment conducted to validate the PRMT5 inhibitor–resistant mouse. There is decreased survival (P = .005) despite PRT-382 treatment in mice engrafted with PRMT5 inhibitor–resistant (purple) vs PRMT5 inhibitor–naive (green) cells. Mice were engrafted on day 0 and treatment initiated on day 43 when PRT-382 10 mg/kg or vehicle was administered by mouth 4 days on and 3 days off. MS for PRMT5 inhibitor–resistant and –naive engrafted vehicle, 65 days; for PRMT5 inhibitor–resistant engrafted PRT-382, 71 days; and for PRMT5 inhibitor–naive engrafted PRT-382, 77 days. Unless otherwise indicated, significance is relative to the PRMT5 inhibitor–naive engrafted VC cohort; ∗∗P < .01. H&E, hematoxylin and eosin.
Development of PDX MCL model of PRMT5 inhibitor resistance. (A) Peripheral disease burden based on circulating neoplastic cells (CD5+/CD19+) cells in the PRT-382 (red) and VC (blue) treated cohort in the PDX MCL model. One PRT-382–treated mouse became resistant to therapy as evidenced by its increased peripheral disease burden (circle), (B) increased spleen weight in resistant mouse (circle), and (C) increased neoplastic infiltration and ki67 staining of the kidney via histopathology of the PRMT5 inhibitor–resistant mouse compared with those of both VC and other PRMT5 inhibitor–sensitive mice in its cohort; H&E, original magnification ×20 and ×40 and ki67, original magnification ×40×. (D) Kaplan-Meier plot of the in vivo experiment conducted to validate the PRMT5 inhibitor–resistant mouse. There is decreased survival (P = .005) despite PRT-382 treatment in mice engrafted with PRMT5 inhibitor–resistant (purple) vs PRMT5 inhibitor–naive (green) cells. Mice were engrafted on day 0 and treatment initiated on day 43 when PRT-382 10 mg/kg or vehicle was administered by mouth 4 days on and 3 days off. MS for PRMT5 inhibitor–resistant and –naive engrafted vehicle, 65 days; for PRMT5 inhibitor–resistant engrafted PRT-382, 71 days; and for PRMT5 inhibitor–naive engrafted PRT-382, 77 days. Unless otherwise indicated, significance is relative to the PRMT5 inhibitor–naive engrafted VC cohort; ∗∗P < .01. H&E, hematoxylin and eosin.
Cells from the resistant mouse were re-engrafted into a subsequent cohort of mice to validate the PRMT5 inhibitor–resistant phenotype. One from the VC and PRT-382 (10 mg/kg)-treated cohort each were engrafted IV with either these resistant splenic lymphocytes or PRMT5 inhibitor–naive splenic lymphocytes (supplemental Figure 1B). Mice were treated at 43 DPE (peripheral disease burden, <5% huCD5+/CD19+ lymphocytes) via oral gavage. Despite PRMT5 inhibitor treatment, mice engrafted with PRMT5 inhibitor–resistant MCL cells (purple) vs PRMT5 inhibitor–naive cells (green) showed decreased survival (P = .005) (median, 71; range, 67-77 days vs median, 77; range, 77-88 days; Figure 1D), suggesting reduced sensitivity to PRMT5 inhibition. These findings highlight resistance development under PRMT5 inhibitor monotherapy in a mouse model of MCL.
Development of MCL cell line models of PRMT5 inhibitor resistance and shared protein expression signatures across resistant models
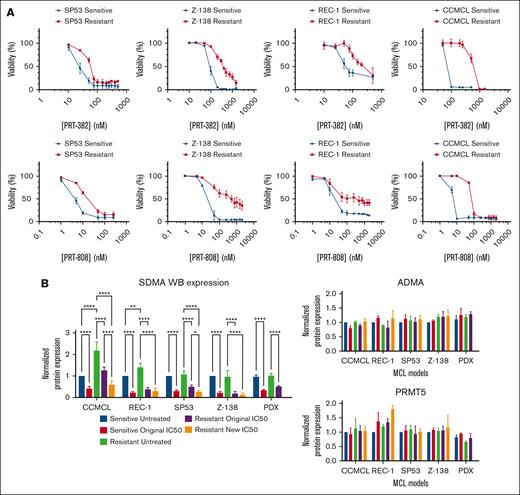
We then sought to generate in vitro models that resembled the observed resistance to PRMT5 inhibitor in vivo. PRMT5 inhibitor–sensitive MCL cell lines (n = 4) were defined by their threefold to 10-fold lower PRT-382 IC50s at 9 days (range, 20-140 nM) than that in 4 primary resistant MCL cell lines (range, 340-1650 nM; Table 1). To generate acquired PRMT5 inhibitor resistance, sensitive MCL cell lines were serially cultured following a prolonged PRT-382 dose escalation protocol (supplemental Figure 1C). This resulted in a twofold to fivefold increase in their original IC50 for PRT-382 (range, 200-500 nM) as well as for PRT-808, the active metabolite of PRT-382 (sensitive, 4-20 nM vs resistant, 12-90 nM; Figure 2A).32 The PRT-382/PRT-808–resistant phenotype persisted even after prolonged culture in media free of drug (>30 days), suggesting that the acquired phenotype was permanent (supplemental Figure 2A).
Development of MCL cell line models of PRMT5 inhibitor resistance and shared protein expression signatures across resistant models. (A) Increased IC50s for PRT-382 and PRT-808 of PRMT5 inhibitor resistance resistant (red) vs original sensitive (blue) MCL cell lines in SP53, Z-138, REC-1, and CCMCL. (B) Resistant PDX and cell lines of MCL achieve similar reduction in SDMA at their original and resistant day 9 IC50s and no substantial change in ADMA or PRMT5 after 6 days of drug exposure. Protein levels were normalized to the sensitive untreated condition in each model. ∗ P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Development of MCL cell line models of PRMT5 inhibitor resistance and shared protein expression signatures across resistant models. (A) Increased IC50s for PRT-382 and PRT-808 of PRMT5 inhibitor resistance resistant (red) vs original sensitive (blue) MCL cell lines in SP53, Z-138, REC-1, and CCMCL. (B) Resistant PDX and cell lines of MCL achieve similar reduction in SDMA at their original and resistant day 9 IC50s and no substantial change in ADMA or PRMT5 after 6 days of drug exposure. Protein levels were normalized to the sensitive untreated condition in each model. ∗ P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Despite resistance to cell death, the resistant PDX MCL cells and cell lines achieved similar reduction in SDMA protein expression at their original IC50s and new IC50s despite elevated SDMA at baseline in resistant CCMCL and REC-1. There was no substantial change in asymmetric dimethylarginine (ADMA) or PRMT5 protein expression (Figure 2B; supplemental Figure 2B-C). In summary, we established models of PRMT5 inhibitor resistance in vivo and in vitro, enabling us to investigate the molecular mechanisms associated with treatment failure.
Bulk RNA-seq reveals shared differential gene expression of MCL cell lines and PDX model with PRMT5 inhibitor resistance
Whole exome sequencing was performed to evaluate variants and mutations between the sensitive cell lines and short-term–treated PDX and their resistant and treatment-naive counterparts. Although several variants and mutations were identified with the acquisition of resistance, no shared variants or mutations were identified across all 5 models. Bulk RNA-seq was also conducted to compare drug-sensitive and -resistant MCL cell lines and PDX with PRMT5 inhibitor treatment. Dimethyl sulfoxide and PRMT5 inhibitor–treated resistant and sensitive SP53, Z-138, REC-1, and CCMCL (n = 3 each) were collected after 6 days of vehicle or PRMT5 inhibitor treatment at their respective IC50s on day 9. Six days was selected for collection to ensure cells maintained sufficient viability for RNA extraction. The PDX MCL samples included splenic lymphocytes from VC (n = 3), PRMT5 inhibitor short-term–treated (treated 2 weeks before necropsy; n = 3), and PRMT5 inhibitor–resistant (treated until ERC; n = 2) mice (supplemental Figure 1A).
To identify similar transcriptomic changes, we compared upregulated (fourth quartile) and downregulated (first quartile) transcripts after PRMT5 inhibitor treatment in the resistant and short-term–treated PDX and sensitive cell lines. Comparable results were obtained with differentially expressed transcripts of the resistant (R) and short-term–treated PDX or sensitive cell lines (S) using principal component analysis (Figure 3A). This illustrated that the 4 MCL cell lines and the PDX have similar differential expression patterns. This is further characterized by the bar chart in which no cell line model shows significantly greater shared differential transcript expression with that of the PDX (Figure 3B). Although unique gene expression landscapes can be seen in each of the investigated models, we concluded that transcriptional changes in response to PRMT5 inhibition were similar among both resistant and sensitive MCL models.
Bulk RNA-seq reveals shared differential gene expression of MCL cell lines and PDX model with PRMT5 inhibitor resistance. Bulk RNA-seq was conducted on the following PDX MCL splenic lymphocyte samples: 3 VC-treated, 3 PRMT5 inhibitor short-term–treated (treated 2 weeks before necropsy), and 2 PRMT5 inhibitor–resistant (treated until ERC) mice and MCL cell lines: dimethyl sulfoxide and PRMT5 inhibitor–treated resistant and sensitive SP53, Z-138, REC-1, and CCMCL in triplicate. (A) Principal component analysis displaying the relationship between pairs of sensitive (S) and resistant (R) MCL models in which network lines highlight the 2 nearest neighbors for each sample. (B) Bar chart depicting the overlap of differential transcript expression in R and S cell lines relative to R and S of the PDX. Fold enrichment of overlapping gene set vs expected overlap and P value (Fisher exact test) are indicated. (C) Ingenuity pathway analysis identification of dysregulated pathways based on DEGs of PRMT5 inhibitor–treated resistant vs short-term–treated PDX (P < 10-5; z score < −0.3 or > 0.3). (D) This list of pathways was used to filter for shared pathways dysregulated based on DEGs of PRMT5 inhibitor–resistant vs sensitive treated MCL cell lines (P < 10-5; z score < −0.3 or > 0.3).
Bulk RNA-seq reveals shared differential gene expression of MCL cell lines and PDX model with PRMT5 inhibitor resistance. Bulk RNA-seq was conducted on the following PDX MCL splenic lymphocyte samples: 3 VC-treated, 3 PRMT5 inhibitor short-term–treated (treated 2 weeks before necropsy), and 2 PRMT5 inhibitor–resistant (treated until ERC) mice and MCL cell lines: dimethyl sulfoxide and PRMT5 inhibitor–treated resistant and sensitive SP53, Z-138, REC-1, and CCMCL in triplicate. (A) Principal component analysis displaying the relationship between pairs of sensitive (S) and resistant (R) MCL models in which network lines highlight the 2 nearest neighbors for each sample. (B) Bar chart depicting the overlap of differential transcript expression in R and S cell lines relative to R and S of the PDX. Fold enrichment of overlapping gene set vs expected overlap and P value (Fisher exact test) are indicated. (C) Ingenuity pathway analysis identification of dysregulated pathways based on DEGs of PRMT5 inhibitor–treated resistant vs short-term–treated PDX (P < 10-5; z score < −0.3 or > 0.3). (D) This list of pathways was used to filter for shared pathways dysregulated based on DEGs of PRMT5 inhibitor–resistant vs sensitive treated MCL cell lines (P < 10-5; z score < −0.3 or > 0.3).
Because this analysis indicated common transcriptional pathways in the resistant and sensitive phenotypes in all 5 MCL models with PRMT5 inhibitor treatment, we used pathway analysis to reveal potential molecular drivers of PRMT5 inhibitor resistance across these models. Ingenuity pathway analysis identified several key pathways (P < 10-5; z score < −0.3 or > 0.3) affected with PRMT5 inhibitor resistance in the PDX compared with the short-term–treated counterparts (Figure 3C). Changes in these pathways were then evaluated in each of the 4 resistant vs sensitive MCL cell lines with PRMT5 inhibition. Several shared pathways (P < 10-5; z score < −0.3 or > 0.3) were identified, suggesting common mechanisms of PRMT5 inhibitor resistance (Figure 3D). These included lipid and protein biosynthesis as well as signaling pathways known to control these features. Most notably, a significant enrichment across all models in mTOR, PI3K, and insulin-like growth factor signaling pathway expression signatures and downmodulation of p53 signaling was observed (P < .01). The degree of similarity between our models suggests that resistance to PRMT5 inhibition converges on common transcriptional cell growth–promotion and survival pathways. A complementary analysis was conducted on the resistant models in comparison with their sensitive (cell line) or treatment-naive (PDX) counterparts without PRMT5 inhibition (supplemental Figure 3). However, in our subsequent analyses comparisons based on the differential gene expression with PRMT5 inhibition were used to provide the most significant effects arising from selective pressure of the inhibitor.
scRNA-seq demonstrates transcriptomic changes with PRMT5 inhibitor resistance in PDX MCL model
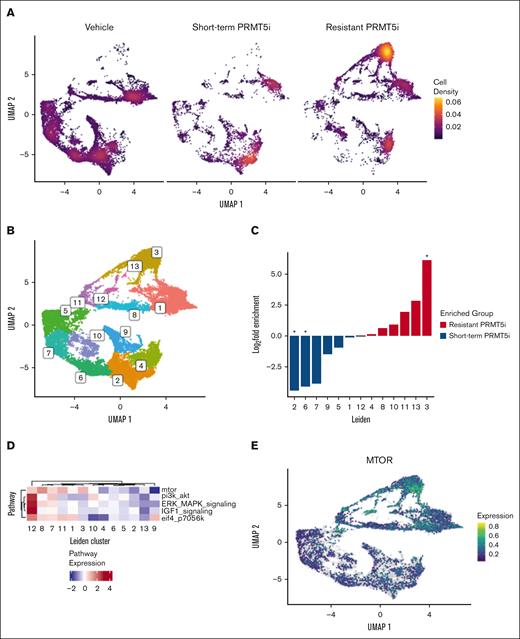
To understand the heterogeneity of transcriptional states within the MCL population during the development of PRMT5 inhibitor resistance, scRNA-seq was performed on 6 representative PDX samples. Together, these represent 3 different treatment conditions: (1) VC including parental, short-term, and long-term vehicle treated (n = 3); (2) short-term PRMT5 inhibitor treated (treated 2 weeks before necropsy; n = 1); and (3) PRMT5 inhibitor–treated resistant (treated until ERC; n = 2). Individual Uniform Manifold Approximation and Project (UMAP) plots are provided, illustrating how the transcriptional states change with length of treatment and treatment conditions (supplemental Figure 4A). Together, the UMAP plots demonstrate a strong shift in global gene expression from the untreated to PRMT5 inhibitor short-term–treated and PRMT5 inhibitor–resistant–treated samples (Figure 4A).
scRNA-seq demonstrates distinct transcriptomic changes with PRMT5 inhibitor resistance in the PDX MCL model. scRNA-seq was performed on PDX MCL splenic lymphocyte samples representing 3 different treatment conditions: (1) VC including parental, short-term, and long-term vehicle treated (n = 3); (2) short-term PRMT5 inhibitor–treated (treated 2 weeks before necropsy; n = 1); and (3) PRMT5-inhibitor–treated resistant (treated until ERC; n = 2). (A) Density UMAP plots demonstrating the transcriptional state change based on the treatment condition (VC, short-term treated, and resistant treated). (B) The Leiden method defines clusters of cells with shared transcriptional profiles independent of sample identity. (C) Differential abundance across Leiden clusters according to treatment status (resistant vs short-term treated). (D) Aggregated expression of indicated pathways according to Leiden cluster. (E) UMAP representation of mTOR differential expression in single cells.
scRNA-seq demonstrates distinct transcriptomic changes with PRMT5 inhibitor resistance in the PDX MCL model. scRNA-seq was performed on PDX MCL splenic lymphocyte samples representing 3 different treatment conditions: (1) VC including parental, short-term, and long-term vehicle treated (n = 3); (2) short-term PRMT5 inhibitor–treated (treated 2 weeks before necropsy; n = 1); and (3) PRMT5-inhibitor–treated resistant (treated until ERC; n = 2). (A) Density UMAP plots demonstrating the transcriptional state change based on the treatment condition (VC, short-term treated, and resistant treated). (B) The Leiden method defines clusters of cells with shared transcriptional profiles independent of sample identity. (C) Differential abundance across Leiden clusters according to treatment status (resistant vs short-term treated). (D) Aggregated expression of indicated pathways according to Leiden cluster. (E) UMAP representation of mTOR differential expression in single cells.
The Leiden clustering method was used to objectively define clusters of cells with distinct transcriptomes across all samples (Figure 4B). In contrast to the treated samples, untreated UMAP plots do not change significantly, illustrating transcriptional stability of the PDX model over time (supplemental Figure 4B). In the PRMT5 inhibitor–resistant state, there are loss of cells in clusters 2 and 6 and gain of cells in cluster 3 compared with short-term–treated animals (Figure 4B-C). We next evaluated the top 5 pathways found upregulated across resistant MCL cell lines and PDX. Enrichment analysis in cluster 3 transcriptomes highlighted mTOR signaling as the most upregulated in the PRMT5 inhibitor–resistant cells (Figure 4D-E).
In conclusion, both global transcriptome and scRNA-seq have revealed a defined set of consistently enriched transcriptional patterns that evolve with PRMT5 inhibitor resistance. Among these pathways, the mTOR pathway is known as the central signaling hub of metabolism, cell growth, and protein synthesis.35 We then went on to assess the effect of mTOR inhibition on PRMT5 inhibitor resistance in our MCL models.
Cotargeting mTOR and PRMT5 results in synergistic reduction in cell proliferation in PRMT5 inhibitor–resistant MCL
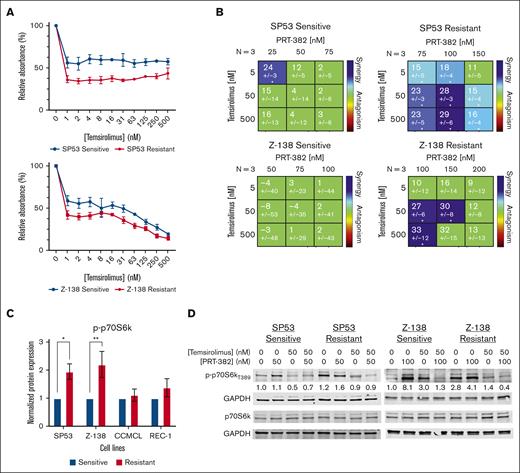
To target mTOR signaling, the mTORC1 inhibitor temsirolimus was used. We first assessed cell growth inhibition by temsirolimus in our 4 sensitive and PRMT5 inhibitor–resistant cell lines. PRMT5 inhibitor–resistant SP53 (P = .0024), Z-138 (P = .0115), and CCMCL (P = .0009) but not REC-1 were more sensitive to single-agent temsirolimus than their sensitive counterparts (Figure 5A; supplemental Figure 5A). Next, we assessed drug synergy when combining temsirolimus with PRT-382 against sensitive and resistant cell lines. The resistant SP53 and Z-138 lines showed a synergistic effect in comparison with their sensitive counterparts based on the Lowe model of synergy (Figure 5B). However, REC-1 showed only an additive effect and resistant CCMCL showed an antagonistic effect in comparison with their sensitive counterparts (supplemental Figure 5B).
Cotargeting mTOR and PRMT5 results in synergistic reduction in cell proliferation in PRMT5 inhibitor–resistant MCL cell line models. (A) IC50 curves measured via MTS after 72 hours of temsirolimus treatment in resistant and sensitive MCL cell lines (SP53 and Z-138). (B) Synergy matrixes for the PRMT5 inhibitor (PRT-382) and mTORC1 Inhibitor (temsirolimus) were calculated with Combenefit via the Lowe model of synergy (x < −10 = antagonistic; −10 < x < 10 = additive; x > 10 = synergistic); ∗P < .05; ∗∗P < .01; ∗∗∗ P < .001. Drugging was conducted with 6 days of PRT-382, followed by 3 days of PRT-382 with and without temsirolimus. (C) p-p70S6k protein levels at baseline in PRMT5 inhibitor–sensitive and -resistant MCL cell lines. (D) Total and p-p70S6k protein levels by treatment condition in SP53 and Z-138 resistant and sensitive cell lines. Drugging was conducted for 3 days with or without PRT-382, followed by 3 days with or without PRT-382 and/or temsirolimus. Protein levels were normalized to the untreated sensitive cell condition.
Cotargeting mTOR and PRMT5 results in synergistic reduction in cell proliferation in PRMT5 inhibitor–resistant MCL cell line models. (A) IC50 curves measured via MTS after 72 hours of temsirolimus treatment in resistant and sensitive MCL cell lines (SP53 and Z-138). (B) Synergy matrixes for the PRMT5 inhibitor (PRT-382) and mTORC1 Inhibitor (temsirolimus) were calculated with Combenefit via the Lowe model of synergy (x < −10 = antagonistic; −10 < x < 10 = additive; x > 10 = synergistic); ∗P < .05; ∗∗P < .01; ∗∗∗ P < .001. Drugging was conducted with 6 days of PRT-382, followed by 3 days of PRT-382 with and without temsirolimus. (C) p-p70S6k protein levels at baseline in PRMT5 inhibitor–sensitive and -resistant MCL cell lines. (D) Total and p-p70S6k protein levels by treatment condition in SP53 and Z-138 resistant and sensitive cell lines. Drugging was conducted for 3 days with or without PRT-382, followed by 3 days with or without PRT-382 and/or temsirolimus. Protein levels were normalized to the untreated sensitive cell condition.
mTOR regulates the translation machinery with activation of numerous downstream targets including p70-S6 kinase 1 (p70S6k) via phosphorylation at T389, which is a posttranslational modification affected by temsirolimus.36,37 SP53- and Z-138–resistant cell lines had higher baseline protein expression of phospho-p70S6k (p-p70S6k) than their sensitive counterparts (Figure 5C). Additionally, treating these resistant lines with temsirolimus alone or in combination with PRT-382 decreased p-p70S6k levels confirming reduced mTOR signaling (Figure 5D). Although activation of p-p70S6k was present in sensitive and resistant cells with PRT-382 treatment, we conducted a time course experiment and showed that resistant MCL cell lines had a more pronounced activation of mTOR signaling as reflected by p-p70S6k protein expression over 9 days of PRT-382 treatment (supplemental Figure 5C). Thus, we were able to confirm upregulated mTOR signaling with PRMT5 inhibitor resistance and enhanced cell growth suppression with combined inhibition of PRMT5 and mTOR in vitro.
Cotargeting mTOR and PRMT5 confers a survival advantage in a PRMT5 inhibitor–resistant PDX MCL model
To confirm the efficacy of targeting mTOR and PRMT5 in the setting of PRMT5 inhibitor resistance in vivo, the resistant PDX MCL model was used. Splenic lymphocytes from PRMT5 inhibitor–resistant mice were engrafted, and the mice were randomized to receive either VC, temsirolimus (12.5 mg/kg; intraperitoneal), PRT-808 (10 mg/kg; chow), or combination at 10 DPE (supplemental Figure 6A). PRT-808 was used because of its favorable bioavailability as a chow formulation that provided less stress on the animals and greater ease of administration as opposed to PRT-382, which must be administered via oral gavage.
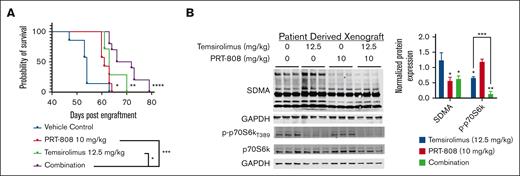
The Kaplan-Meier plot shows prolonged survival with combination (purple) with an MS of 69 days (range, 63-79 DPE) vs VC (blue) with an MS of 54 days (range, 47-63 DPE; P < .0001), single-agent PRT-808 (red) with an MS of 61 days (range, 61-64 DPE; P < .001), or temsirolimus (green) with an MS of 63 days (range, 61-70 DPE; P < .05; Figure 6A). Protein evaluation of SDMA and p-p70S6k reflects reduction of these targets with PRT-808 and temsirolimus treatment, respectively, in which p-p70S6k was significantly reduced (P < .001) as a combination compared with single-agent temsirolimus (Figure 6B). In summary, these findings highlight therapeutic benefits when combining PRMT5 inhibitor with temsirolimus leading to increased MCL growth suppression and survival advantages in a PRMT5 inhibitor resistance setting.
Cotargeting mTOR and PRMT5 confers a survival advantage in PRMT5 inhibitor PDX resistant MCL model. (A) Kaplan-Meier plot for the in vivo experiment conducted to validate combination therapy in the PRMT5 inhibitor–resistant PDX mouse model. Mice were engrafted with splenic lymphocytes from the PRMT5 inhibitor–resistant MCL PDX on day 0 and treatment initiated on day 10 with temsirolimus (12.5 mg/kg) or vehicle administered intraperitoneally weekly and PRT-808 (10 mg/kg) or vehicle chow administered in intervals (5 days on/2 days off). MS was as follows: for VC (n = 7), 54 days; for PRT 808 (n = 7), 61 days; temsirolimus (n = 8), 63 days; and combination (n = 10), 69 days. ∗ P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗ P < .0001. (B) Protein evaluation of SDMA and p-P70S6k across the 4 treatment conditions. Protein levels were normalized to VC group and indicated significance is relative to the VC. ∗ P < .05; ∗∗P < .01; ∗∗∗P < .001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Cotargeting mTOR and PRMT5 confers a survival advantage in PRMT5 inhibitor PDX resistant MCL model. (A) Kaplan-Meier plot for the in vivo experiment conducted to validate combination therapy in the PRMT5 inhibitor–resistant PDX mouse model. Mice were engrafted with splenic lymphocytes from the PRMT5 inhibitor–resistant MCL PDX on day 0 and treatment initiated on day 10 with temsirolimus (12.5 mg/kg) or vehicle administered intraperitoneally weekly and PRT-808 (10 mg/kg) or vehicle chow administered in intervals (5 days on/2 days off). MS was as follows: for VC (n = 7), 54 days; for PRT 808 (n = 7), 61 days; temsirolimus (n = 8), 63 days; and combination (n = 10), 69 days. ∗ P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗ P < .0001. (B) Protein evaluation of SDMA and p-P70S6k across the 4 treatment conditions. Protein levels were normalized to VC group and indicated significance is relative to the VC. ∗ P < .05; ∗∗P < .01; ∗∗∗P < .001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
Despite advances in therapeutic options for patients with MCL, virtually all patients eventually develop relapsed/refractory disease.38,39 In addition, the overall heterogeneity of MCL both clinically and genetically presents challenges in the treatment of this disease.40,41 For this reason, several novel targeted strategies such as BTK and BCL2 inhibitors and chimeric antigen receptor T cells have been developed.42-44 However, the use of single-agent monotherapy is unlikely to provide a durable response leading to resistance.43,45,46 We and others have developed selective agents that target PRMT5 and have shown impressive preclinical activity in MCL and other aggressive lymphomas.17-22
Development of PRMT5 inhibitor resistance models in vitro and in vivo (Figures 1 and 2) provided a unique opportunity to assess transcriptomic changes that evolve with the acquisition of resistance to this therapy. Reduction of SDMA was still achieved at the original dose of the inhibitor indicating that resistance was not due to the loss of interaction between the inhibitor and PRMT5 but rather compensatory mechanisms were at play (Figure 2B). The fact that PRMT5 protein expression remained unchanged ruled out the possibility that upregulation of PRMT5 itself was contributing as a resistance mechanism. ADMA expression was also unchanged with treatment, arguing against compensatory ADMA methylation with PRMT5 inhibition that has been reported.47 No site specific mutations were found with resistance across the PDX and cell line models, as has been reported for other BCL-2 and BTK inhibitors in MCL.48-51 Interestingly, SAM competitive inhibitors such as PRT-808 and -382 have a lower tendency to develop compound-specific resistance mutations in the PRMT5 enzyme.52
The overall genomic heterogeneity of these 5 models also recapitulates the heterogeneity of MCL’s presentation in the patient population.53-56 Despite this heterogeneity, differentially expressed transcripts with PRMT5 inhibition in the resistant models demonstrated strong overlap with each other and with their sensitive counterparts (Figure 3A-B). Specifically, pathway analysis of the DEGs of the resistant models highlighted shared pathways and potential resistance mechanisms (Figure 3D). In other lymphoid diseases, PRMT5 has been shown to activate PI3K-AKT-mTOR signaling, possibly providing a molecular link of PRMT5 to the mTOR pathway.26 Our data expand on these findings, providing a link with this pathway in a setting in which PRMT5 pathways have been challenged by prolonged pharmacological inhibition to the point of resistance development.
When assessing transcriptional profiles in the context of drug resistance, it is important to consider how resistance can initially be driven by a relatively small subset of cells with favorable adaptation. Under constant selective pressure, such a cell population may eventually outgrow susceptible cells and evolve as treatment-resistant cancer.39 We deployed scRNA-seq to evaluate the cellular heterogeneity of our PDX MCL model with the development of treatment resistance, as has been appreciated in other therapies.57 Distinct gene expression profiles were found among the vehicle, short-term–treated, and resistant treated samples. Thus, this analysis provided a unique opportunity to evaluate the transcriptomic changes at a single-cell level that developed over time with resistance. Resistance-associated expression profiles were distinct from initial PRMT5 inhibitor treatment and prominently featured upregulation of mTOR signaling in a cluster enriched for the resistant phenotype (Figure 4).
After identification of mTOR pathway enrichment, the mTORC1 inhibitor, temsirolimus was used. Temsirolimus has demonstrated single-agent efficacy in the setting of refractory MCL but was inferior to ibrutinib.58 However, this study suggests that mTOR kinase inhibitors, which have been approved clinically, may be repurposed to fight resistance to PRMT5 inhibitors in MCL.59 In MCL cell lines, temsirolimus has been reported to induce autophagy and G0/G1 cell cycle arrest.60 In 1 study, synergy between temsirolimus and the histone deacetylase inhibitor vorinostat was ascribed to the targeting of 2 distinct pathways, autophagy and apoptosis, respectively.55 A similar mechanism could be hypothesized for combination with PRMT5 inhibitors given the induction of apoptosis characterized by PRMT5 inhibition.61
Temsirolimus resistance has been observed over time and attributed to compensatory increased phospho-AKT levels by the uninhibited mTORC2 kinase complex.62 It is possible that PRMT5 inhibition could be reducing compensatory upregulation of this pathway as previously described.23,24 Interestingly, a study in glioblastoma found that PRMT5 inhibition enhanced sensitivity to mTOR inhibition and resulted in decreased CCND1 and c-MYC using a dual mTORC1 and 2 inhibitor (PP242).63 In addition, it was previously discovered that the metabolic enzyme, methionine adenosyltransferase 2 alpha, generates the PRMT5 substrate S-adenosylmethionine.64 mTORC1 mediated upregulation of S-adenosylmethionine via methionine adenosyltransferase 2 alpha could be considered as a potential mechanism of synergy observed in cotargeting mTORC1 and PRMT5.65 The lack of synergy observed in PRMT5 inhibitor–resistant CCMCL could arise from its upregulated EIF2 signaling resulting in greater induction of autophagy after mTORC1 inhibition.66 Of note, we found distinct p70S6k activation kinetics when comparing PRMT5 inhibitor–resistant and sensitive MCL cells (supplemental Figure 5C). Although some activation of the mTOR pathway is also evident in sensitive cell lines undergoing PRMT5 inhibition, this is insufficient to provide them with resistance. This indicates context-specific alterations in the mTOR pathway, potentially involving specific upstream and downstream mediators that shape resistance-associated mTOR singaling.
We have previously reported downregulation of mTOR signaling with PRMT5 inhibition in PRMT5 inhibitor–sensitive MCL.21 Although mTOR signaling was prioritized given its upregulation in the resistant Leiden cluster analysis, many other potentially relevant pathways were found to be dysregulated across our models of PRMT5 inhibitor resistance. It is possible that dysregulation of other pathways or upstream targets could be contributing to resistance. For example, PRMT5 inhibition results in stabilization of the tumor suppressor p53, one of the most powerful mTORC1 antagonists.15,67 p53 can also inhibit the mTORC1 pathway in response to cellular stress, including DNA damage.68 However, downregulation of p53 signaling was observed across our PRMT5 inhibitor–resistant models. Thus, these findings could support cotargeting other pathways such as DNA damage response, because increased genotoxic stress leads to p53-mediated restraint of mTORC1.69 Similarly, upregulated mRNA expression of the p53 inhibitor MDM2 has been reported as a mechanism of p53 inactivation in blastoid MCL.70MDM2 gene expression was increased with PRMT5 inhibitor treatment in several of our resistant models. Another study found that given PRMT5 inhibition resulted in DNA damage response and subsequent p53 activation and G1 cell cycle arrest, there could be rationale for targeting these pathways in combination with PRMT5.61
Taken together, our findings provide a promising lead for the identification of patients with MCL who may benefit from mTOR inhibitors. Although initially effective, most monotherapies eventually result in treatment resistance. Thus, the purpose of these studies was to determine pathways dysregulated with PRMT5 inhibitor resistance that could be targeted to serve as rationale for future combinatorial therapies. Incorporating multiple MCL models and investigating the sequential development of resistance to therapy via scRNA-seq identified many potential pathways of resistance that warrant further investigation. We propose that future studies will identify additional synergistic combinations with PRMT5 inhibitors that will enhance therapeutic response. These findings have the potential to extend to patient populations including other hematologic malignancies and solid tumors.19
Acknowledgments
The authors thank Reena Shakya and University Lab Animal Resources staff for help with the animal studies conducted at The Ohio State University. All mouse studies were approved by The Ohio State University Institutional Animal Care and Use Committee and the animals were maintained under compliance with institutional guidelines. The authors are grateful for the patients who provided tissue samples to The Ohio State University Comprehensive Cancer Center Leukemia Tissue Bank Shared Resource.
M.E.L. was supported by The Ohio State University Comparative Biomedical Sciences Graduate Program, the Genentech Veterinary Pathology Fellowship, and the F-32: Ruth L. Kirschstein Postdoctoral Individual NRSA (1F32CA265099-01) during this work. Histology, immunohistochemistry, and pathology support services were provided by the Comparative Pathology & Digital Imaging Shared Resource, Department of Veterinary Biosciences, and the Comprehensive Cancer Center, The Ohio State University, Columbus, OH, and supported in part by grant P30 CA16058, National Institutes of Health, National Cancer Institute, Bethesda, MD. This work was also supported by The Ohio State University Genomics Shared Resource and Comprehensive Cancer Center, Weill Cornell Medicine Genomics Core Facility, the National Cancer Institute Cancer Center Support Grant (5P01CA214274-03) (R. B.), the Leukemia and Lymphoma Society (longitudinal functional genomics in mantle cell lymphoma therapy and drug resistance) (R. B.), and The James Comprehensive Cancer Center at The Ohio State University (NCIP30 CA016058).
Authorship
Contribution: M.E.L., L.A., B.W.B., and R.B. conceptualized and designed the study; M.E.L., S.S., F.B.-B., B.W.B, R.B and L.A. developed the methodology; M.E.L., S.K., S.S., A.S., I.H., M.H., J.H.-M., L.V., and S.T. participated in the acquisition of data; M.E.L., S.S, F.B.-B., S.K., C.W., K.C., C. Mason, J.F., D.B., C. Meydan, Z.C., and B.W.B. analyzed and interpreted the data; M.E.L, S.S., C.W., L.A., B.W.B., and R.B. wrote the manuscript; M.E.L., P.S., K.V., C.W., S.C.-K., M.D.L., L.A., B.W.B., and R.B. provided administrative, technical, or material support; and L.A., B.W.B., and R.B. supervised the study.
Conflict-of-interest disclosure: P.S. and K.V. report employment with Prelude Therapeutics. K.V. and P.S. report stock holdings in Prelude Therapeutics. R.B. reports research support from Prelude Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Robert Baiocchi, Division of Hematology, Internal Medicine, The Ohio State University, 400 W 12th Ave, Room 481A, 481B Wiseman CCC, Columbus, OH 43210; email: robert.baiocch@osumc.edu.
References
Author notes
∗L.A., B.W.B., and R.B. contributed equally to this work.
Whole exome sequencing and RNA-seq data are deposited in the Gene Expression Omnibus database (accession number GSE240726).
Processed experimental data are available at figshare (private link for reviewer access https://figshare.com/s/7a755a5ad0015e643c93), and the code is available at https://github.com/blaserlab/baiocchi_long.
The full-text version of this article contains a data supplement.