Key Points
CART-ddBCMAs are safe for use in patients with relapsed or refractory multiple myeloma.
CART-ddBCMAs produce deep and durable responses in patients with poor prognostic factors.
Abstract
Relapsed and refractory multiple myeloma (RRMM) is a plasma cell neoplasm defined by progressively refractory disease necessitating chronic and increasingly intensive therapy. Despite recent advances, limited treatment options exist for RRMM. This single-arm, open label phase 1 study aimed to evaluate the safety of novel B-cell maturation antigen (BCMA)-targeting chimeric antigen receptor (CAR) T construct that leverages a completely synthetic antigen-binding domain (CART-ddBCMA), which was specifically engineered to reduce immunogenicity and improve CAR cell surface stability. Thirteen patients ≥18 years with RRMM who received at least 3 prior regimens of systemic therapy were enrolled in the study. Patients received a single dose of 100 × 106 CART-ddBCMA (DL1) or 300 × 106 CART-ddBCMA (DL2) following standard lymphodepleting chemotherapy. The primary endpoints of the study were to evaluate the incidence of treatment emergent adverse events, including dose-limiting toxicities, and establish a recommended phase 2 dose. Results showed that CART-ddBCMA was well tolerated and demonstrated a favorable toxicity profile. Only 1 case of grade ≥3 cytokine release syndrome and 1 case of immune effector cell–associated neurotoxicity were reported; both were at DL2 and were manageable with standard treatment. No atypical neurological toxicities and Parkinson disease-like movement disorders were observed. The maximum tolerated dose was not reached. All infused patients responded to CART-ddBCMA, and 9/12 (75%) patients achieved complete response/stringent complete response. Responses deepened over time, and at the time of last data-cut (median follow-up 56 weeks), 8/9 (89%) evaluable patients achieved minimal residual disease negativity. In conclusion, the findings demonstrate the safety of CART-ddBCMA cells and document durable responses to CART-ddBCMA in patients with RRMM. This trial was registered at www.clinicaltrials.gov as #NCT04155749.
Introduction
Multiple myeloma (MM) is a plasma cell neoplasm with treatment aimed at disease control rather than cure. Despite new therapeutic options, which include immunomodulatory agents (IMiDs), proteasome inhibitors, and anti-CD38 monoclonal antibodies (mAbs), the natural disease course is characterized by relapse with progressively refractory disease, and patients accumulate disease- and treatment-related toxicities.1 Historically, patients with triple- (proteasome inhibitors, IMiD, and anti-CD38 mAbs) and penta-refractory (2 IMiDs, 2 proteasome inhibitors, and anti-CD38 mAbs) disease have demonstrated median progression-free survival (PFS) of 3.5 and 2.3 months and median overall survival (OS) of 14.7 and 6.6 months, respectively.2-4 Due to the poor survival in this highly refractory patient population, novel treatment strategies are critically needed to improve outcomes in patients with MM.
Recently, MM therapies targeting the B-cell maturation antigen (BCMA) have emerged as promising options for highly refractory patients.5,6 BCMA is highly expressed on MM cells, with limited expression outside of terminally differentiated B cells and normal plasma cells, and is involved in promoting MM cell survival and proliferation.7-10 Approaches to target BCMA using bispecific T-cell engagers and antibody-drug conjugates (ADCs) have shown overall response rate (ORR) of 60% to 75% and complete response/stringent complete response rate (CR/sCR) of 30% to 40% but require frequent repeated infusions.11-15 In contrast, autologous chimeric antigen receptor (CAR) T cells engineered to target surface antigens in hematologic malignancies are typically given once and mediate prolonged remissions.16-21 The KarMMA trial of idecabtagene vicleucel (ide-cel) demonstrated an ORR of 73% and led to US Food and Drug Administration (FDA) approval of the first CAR T-cell targeting BCMA in March 2021.22,23 In the CARTITUDE-1 trial, ciltacabtagene autoleucel (cilta-cel) showed an ORR of 97% at the time of reporting,24 and the respective Biological License Application was approved in February 2022. Additional therapeutics products targeting BCMA are currently in development.25-31
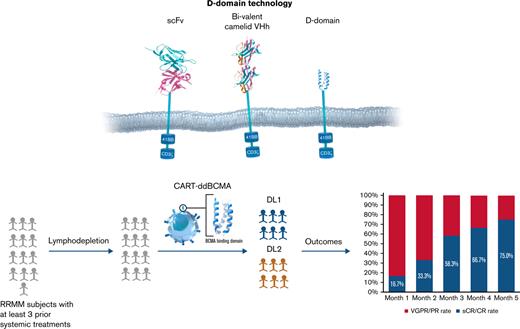
Although effective, the available CAR T-cell therapies are limited by durability of response, disease resistance, and safety and production issues.32-34 The typical antigen-binding domains of CARs are derived from animal antibodies and use combinations of either single-chain variable fragments (scFv) that link variable light and heavy chains or single-domain heavy-chain (VHH) antibodies. Despite specific antigen binding, these molecules can have promiscuous oligomerization of the scFv fragments, leading to ineffective or tonic downstream signaling, which can be detrimental to CAR T-cell effector function and persistence.35-37 These nonhuman-derived proteins can also induce development of anti-CAR antibodies and prime T-cell responses that may to lead rejection and decreased persistence of otherwise autologous products.38 To overcome these limitations, alternatives to scFVs such as ankyrin repeats,39 adnectins,40 thermo-stable DNA-binding proteins,41 affibodies,42 and d-domain proteins43 have been proposed. d-domain proteins are synthetic proteins with unique advantages, including small size (∼8 kDa), lack of disulfide bonds, and N-linked glycosylation, which allow for rapid protein folding, absence of tonic signaling, and high cell surface stability.43,44
We developed anti-BCMA CAR T cells with a CAR composed of a d-domain-based antigen binder fused to the CD8 hinge and transmembrane domain in tandem with the intracellular signaling domains of 4-1BB and CD3ζ and introduced into human T cells via lentiviral vector (CART-ddBCMA). Based on the encouraging efficacy seen in preclinical studies, we initiated a phase 1 clinical study of CART-ddBCMA patients with relapsed/refractory MM (#NCT04155749).
Methods
CART-ddBCMA
CART-ddBCMA drug product consists of autologous T cells genetically modified ex vivo to express a binding domain that specifically recognizes BCMA. The binding domain was identified in a library of randomized α3D sequences using standard phage-display technologies, and site-directed mutagenesis was used to enhance target affinity and minimize immunogenicity.43 The resulting sequence encoding a 73 amino acid d-domain with nanomolar affinity for human BCMA was cloned into a lentiviral vector along with CD8 hinge and transmembrane region, 4-1-BB and CD3ζ intracellular signaling domains. Preclinical studies of CAR T cells using d-domains showed the absence of tonic signaling, consistently high levels of cell surface expression, and low immunogenicity based on in silico modeling.45 CART-ddBCMA displayed reproducible BCMA-dependent NFAT signaling, cytokine (interleukin-2, interferon-γ) secretion, and induced BCMA-specific cytotoxicity in tumor cell lines. In the mouse-human xenograft models, CART-ddBCMA eradicated BCMA-expressing tumors within 2 weeks of single administration. Body weights of the mice were not impacted by CART-ddBCMA treatment, and no histopathological findings in the in vivo studies were attributable to ddBCMA exposure.46-48
Study design
This open-label, multicenter phase 1 trial enrolled adults with RRMM to evaluate the safety of intravenous administration of CART-ddBCMA (supplemental Figure 1). The protocol was approved by the Institutional Review Board at each center, and the trial was performed in accordance with the principles of the Declaration of Helsinki. Eligible patients required treatment with at least 3 prior lines of systemic therapy, including a proteasome inhibitor, an IMiD, and an anti-CD38 antibody. Alternatively, patients were eligible if deemed to have “triple-refractory” disease following treatment with proteasome inhibitor, IMiD, and anti-CD38 antibody as part of the same or different regimens. Eligibility criteria also included adequate organ function (creatine clearance ≥50 mL/min and left ventricular ejection fraction ≥45%) and an Eastern Cooperative Group Performance Status of 0-1. Patients with plasma cell leukemia or active central nervous system involvement were excluded but ongoing anticoagulation was allowed. Patients with prior BCMA-targeted therapy were eligible after medical monitor discussion.
After providing written informed consent, patients were enrolled and underwent leukapheresis. Bridging therapy was allowed, but a 2-week washout was required prior to lymphodepleting chemotherapy and cell infusion. Repeat baseline assessments were required prior to initiation of lymphodepleting chemotherapy. For lymphodepleting chemotherapy, patients received a regimen of fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) daily on days −5, −4, and −3 prior to cell infusion. Cells were manufactured by the Connell and O’Reilly Families Cell Manipulation Core Facility of the Dana-Farber/Harvard Cancer Center. Patients received a dose of 100 × 106 CART-ddBCMA (dose level 1, DL1) or 300 × 106 CART-ddBCMA (dose level 2, DL2) on day 0. Blood was collected at days 0, 1 to 4, 7, 9, 11, 14, 21, and 28 after CART-ddBCMA infusion and then months 2 to 6, 9, 12, 15, 18, 21, and 24 to monitor CAR T-cell expansion and persistence. Additional blood was drawn to evaluate correlative pharmacodynamic effects. Patients were also monitored for disease progression up to 24 months. At the time of disease progression, or at 24 months if progression did not occur, patients were transferred to long-term follow-up phase of the study. Retreatment of patients was possible with FDA and institutional review board approval, and patients were dosed from material remaining from the initial manufacturing run.
End points
The primary end points of the study were to evaluate the incidence of treatment-emergent adverse events (AEs), including dose-limiting toxicities (DLTs), and establish the recommended phase 2 dose. DLTs were defined as any investigational study drug–related grade 3+ toxicity occurring within the first 28 days as well as any grade 4 life-threatening toxicity, grade ≥3 cytokine release syndrome (CRS) that did not improve to ≤grade 2 within 72 hours, any grade ≥3 neurotoxicity including any seizures, any grade ≥3 toxicity involving vital organs (eg, cardiac, pulmonary) that resulted in significant and irreversible organ damage, any grade ≥3 nonhematologic toxicity that did not improve to ≤grade 2 within 72 hours, and any death not attributed to underlying malignancy. Toxicity grading was performed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. CRS and immune effector cell-associated neurotoxicity (ICANS) were graded according to the American Society for Transplant and Cellular Therapy consensus criteria.49 Secondary endpoints included, but were not limited to, duration of response, PFS, OS, and correlative and exploratory studies. Response assessments were performed per the International Myeloma Working Group (IMWG) consensus criteria.50
Statistical analysis
Sample size was based on a 3 + 3 dose escalation design.51 A total of 6 evaluable subjects were enrolled in each dose level to ensure adequate evaluation for potential DLT incidence in selecting a recommended phase 2 dose. Data are presented as the median and range for continuous variables and frequency for categorical variables. Time-to-event analyses and the associated 95% confidence intervals were estimated using the Kaplan-Meier method. Subjects were censored at the date of last follow-up. All patients who received CART-ddBCMA infusion were included in this analysis as was planned per protocol.
Results
Patient disposition and characteristics
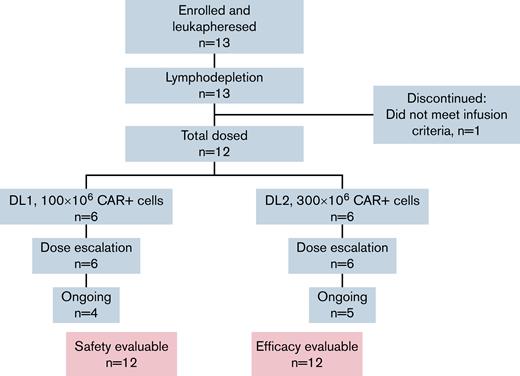
Between 19 November 2019 and 14 April 2021, 13 subjects were enrolled, and 12 were infused with CART-ddBCMA, 6 at DL1 and 6 at DL2 (Figure 1). One subject discontinued prior to cell infusion due to disease-related complications unrelated to the investigational product. As of the data cut for this analysis, on 4 November 2021, the median follow-up was 56 weeks (range, 33-90). Drug products were successfully manufactured for all 13 patients with a median vein-to-vein time of 35 days (range, 33-42 days) for infused patients. CAR expression in the final product was consistent for all patients, and the variability in % CAR+ cells in the final product was low between patients. The median CAR+ cells in total CD3+ cells was 74.5% (range, 61% to 87%). The median patient age was 69 years (range, 44-76) for patients treated with 100 × 106 CART-ddBCMA and 60 years (range, 52-65) for those treated with 300 × 106 CART-ddBCMA (Table 1). The median time since diagnosis was 6.5 years (range, 1.8-11.8 years), and patients had received a median of 5 (range, 5-7), 4 (range, 3-16), and 5 (range, 3-16) prior lines of therapy in DL1, DL2, and overall, respectively. Nine of the 10 subjects with evaluable cytogenetics (90%) had high-risk features per IMWG, 7/12 subjects (58%) had extramedullary disease at time of treatment, and 10/12 subjects (83%) were considered penta-refractory at time of enrollment. One patient had progressed following treatment with a BCMA ADC. All enrolled patients received bridging therapy following leukapheresis for progressive and/or symptomatic disease.
Patient demographics
| Characteristics . | Dose level 1 100 million CAR-T (n = 6) . | Dose level 2 300 million CAR-T (n = 6) . |
|---|---|---|
| Age, y, median (min-max) | 73 (66-75) | 60 (53-65) |
| Sex | 3 male | 5 male |
| 3 female | 1 female | |
| BMPC >50% | 3/6 | 4/6 |
| Extramedullary disease | 4/6 | 3/6 |
| High-risk cytogenetics per IMWG | 5/5∗ | 4/5∗ |
| Prior lines of therapy, median (min-max) | 5 (5-7) | 4 (3-16) |
| Prior HSCT | 3/6 | 4/6 |
| Penta-refractory† | 6/6 | 4/6 |
| IgG myeloma | 1 | 5 |
| IgA myeloma | 3 | 0 |
| Light chain only | 2 | 1 |
| Characteristics . | Dose level 1 100 million CAR-T (n = 6) . | Dose level 2 300 million CAR-T (n = 6) . |
|---|---|---|
| Age, y, median (min-max) | 73 (66-75) | 60 (53-65) |
| Sex | 3 male | 5 male |
| 3 female | 1 female | |
| BMPC >50% | 3/6 | 4/6 |
| Extramedullary disease | 4/6 | 3/6 |
| High-risk cytogenetics per IMWG | 5/5∗ | 4/5∗ |
| Prior lines of therapy, median (min-max) | 5 (5-7) | 4 (3-16) |
| Prior HSCT | 3/6 | 4/6 |
| Penta-refractory† | 6/6 | 4/6 |
| IgG myeloma | 1 | 5 |
| IgA myeloma | 3 | 0 |
| Light chain only | 2 | 1 |
BMPC, bone marrow plasma cell; HSCT, hematopoietic stem cell transplant.
Some subjects were not evaluable or data were not available at time of data cutoff.
Penta-refractory patients are refractory to bortezomib, carfilzomib, daratumumab, lenalidomide, and pomalidomide.
Safety
All patients experienced grade 3 or 4 treatment-emergent AEs following CART-ddBCMA infusion, as shown in Table 2. The most common AEs were hematologic, including neutropenia (92%), anemia (83%), lymphocytopenia (67%), and decreased hemoglobin (75%). Most patients had cytopenias resolved to baseline or ≤grade 2 by 28 days. Of those patients who did not, all but 1 patient resolved to baseline or ≤grade 2 with standard interventions by month 5. Lymphocytopenia in 1 patient was resolved to baseline levels by month 12. Investigator attribution of these events was related to lymphodepletion chemotherapy plus underlying bone marrow function. In all cases of CR or sCR, improvement in cytopenias occurred compared with a screening of baseline value. The most common nonhematologic grade 3 or 4 AEs were hypertension (25%) and electrolyte imbalances (17%). There were no treatment-emergent grade 3 or 4 infections.
Adverse events following CART-ddBCMA infusion
Event . | Cohort . | ||
|---|---|---|---|
| 100 × 106 (N = 6) n (%) . | 300 × 106 (N = 6) n (%) . | Total (N = 12) n (%) . | |
| Subjects with at least 1 ≥grade 3 AE, % | 6 (100) | 6 (100) | 12 (100) |
| Neutropenia, % | 6 (100) | 5 (83.3) | 11 (91.7) |
| Anemia, % | 5 (83.3) | 5 (83.3) | 10 (83.3) |
| Lymphocytopenia, % | 5 (83.3) | 3 (50.0) | 8 (66.7) |
| Thrombocytopenia, % | 2 (33.3) | 4 (66.7) | 6 (50.0) |
| Leukopenia, % | 3 (50.0) | 2 (33.3) | 5 (41.7) |
| Hyponatremia, % | 2 (33.3) | 0 | 2 (16.7) |
| Febrile neutropenia, % | 3 (50.0) | 1 (16.7) | 4 (33.3) |
| Hypertension, % | 2 (33.3) | 1 (16.7) | 3 (25.0) |
Event . | Cohort . | ||
|---|---|---|---|
| 100 × 106 (N = 6) n (%) . | 300 × 106 (N = 6) n (%) . | Total (N = 12) n (%) . | |
| Subjects with at least 1 ≥grade 3 AE, % | 6 (100) | 6 (100) | 12 (100) |
| Neutropenia, % | 6 (100) | 5 (83.3) | 11 (91.7) |
| Anemia, % | 5 (83.3) | 5 (83.3) | 10 (83.3) |
| Lymphocytopenia, % | 5 (83.3) | 3 (50.0) | 8 (66.7) |
| Thrombocytopenia, % | 2 (33.3) | 4 (66.7) | 6 (50.0) |
| Leukopenia, % | 3 (50.0) | 2 (33.3) | 5 (41.7) |
| Hyponatremia, % | 2 (33.3) | 0 | 2 (16.7) |
| Febrile neutropenia, % | 3 (50.0) | 1 (16.7) | 4 (33.3) |
| Hypertension, % | 2 (33.3) | 1 (16.7) | 3 (25.0) |
CAR T-cell–associated toxicities occurred in all subjects, but most were low grade and manageable. CRS occurred in all patients, with a median onset of 2.5 days (range, 0-6 days) and duration of 7 days (range, 3-8 days) in DL1 and 1 day (range, 0-3 days) and 4.5 days (range, 3-6 days), respectively, in DL2. No patient in DL1 experienced grade 3+ CRS, but 1 patient experienced grade 3 CRS in DL2 that was partly attributed to a delay in defined tocilizumab administration (Table 3). Four subjects in DL1 and 5 subjects in DL2 (9/12 overall) required tocilizumab for the management of CRS (median 1 dose; range, 1-2 doses). Two subjects in DL1 and 3 subjects in DL2 received 1 dose of dexamethasone for CRS management. ICANS occurred in 2 subjects: 1 in DL1 and 1 in DL2. The subject in DL1 experienced grade 2 ICANS that began on D+2 and resolved by D+5 with administration of steroids. The subject in DL2 experienced ICANS on D+6 as decreased mental status and decreasing immune effector cell-associated encephalopathy score of 7 consistent with grade 1 characteristics. Severe CRS was not seen in this subject, and the patient did not require tocilizumab. The severity of ICANS was grade 3 on D+9 based on clinical presentation and immune effector cell-associated encephalopathy score of 2, which was solely driven by global aphasia rather than decreased level of consciousness because the patient remained able to follow commands and intermittently respond. Following treatment with anakinra and steroids, the subject improved to grade 2 ICANS on D+19 and to grade 1 on D+20, and the AE was completely resolved on D+22. No long-term deficits or sequela have been identified in both subjects. Also, at the time of last data cut there were no cases of delayed-onset progressive movement disorders with features of Parkinson disease, as described in other investigational and commercially available BCMA-targeted CAR T-cell products.52,53 Given the low incidence of high-grade CAR T-cell–related AEs and only 1 observed DLT, a maximum tolerated dose of CART-ddBCMA was not reached.
CAR-T–associated adverse events
| CAR-T-associated AEs Per ASTCT criteria CRS . | 100 million (N = 6) . | 300 million (N = 6) . | ||
|---|---|---|---|---|
| Grade 1/2 . | Grade 3 . | Grade 1/2 . | Grade 3 . | |
| 6 | 0 | 5 | 1 | |
| Median onset (min-max) | 2.5 d (0-4 d) | <24 h (0-1 d) | ||
| Median duration (min-max) | 5 d (2-7 d) | 3 d (1-9 d) | ||
| CAR-T-associated AEs Per ASTCT criteria CRS . | 100 million (N = 6) . | 300 million (N = 6) . | ||
|---|---|---|---|---|
| Grade 1/2 . | Grade 3 . | Grade 1/2 . | Grade 3 . | |
| 6 | 0 | 5 | 1 | |
| Median onset (min-max) | 2.5 d (0-4 d) | <24 h (0-1 d) | ||
| Median duration (min-max) | 5 d (2-7 d) | 3 d (1-9 d) | ||
| Neurotoxicity (ICANs) . | Grade 1/2 . | Grade 3 . | Grade 1/2 . | Grade 3 . |
|---|---|---|---|---|
| 1 | 0 | 0 | 1 | |
| Onset | 2 d | 6 d | ||
| Duration | 2 d | 14 d | ||
| Toxicity management | ||||
| Tocilizumab | 4 | 5 | ||
| Dexamethasone | 3 | 2 | ||
| Anakinra | 0 | 1 | ||
| Neurotoxicity (ICANs) . | Grade 1/2 . | Grade 3 . | Grade 1/2 . | Grade 3 . |
|---|---|---|---|---|
| 1 | 0 | 0 | 1 | |
| Onset | 2 d | 6 d | ||
| Duration | 2 d | 14 d | ||
| Toxicity management | ||||
| Tocilizumab | 4 | 5 | ||
| Dexamethasone | 3 | 2 | ||
| Anakinra | 0 | 1 | ||
ASTCT, American Society for Transplantation and Cellular Therapy; ICANS, immune effector cell-associated neurotoxicity.
Efficacy
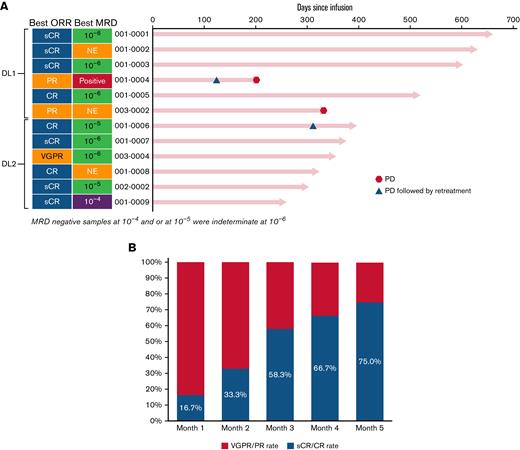
At the time of data-cut, all subjects in the study had >200 days of follow-up. The median duration of follow-up was 12.6 months in all patients (15.6 months in DL1 and 8.3 months in DL2). The objective response rate was 100% across both dose levels of CART-ddBCMA, with 9 patients (75.0%) having CR/sCR, 1 (8.3%) having a very good partial response (PR), and 2 (16.7%) having a PR (Figure 2). The median time to response for all subjects was 28 days (range, 28-87) with deepening of responses over time. Median time to response was 28.5 days (range, 28-57) in DL1 and 28 days (range, 28-87) in DL2. Median duration of response, PFS, and OS was not reached at both DLs. Because the ORR was comparable between DL1 and DL2 and the toxicities were lower at DL1, additional subjects were enrolled in DL1.
Objective responses in patients treated with CART-ddBCMA. Responses were assessed according to the IMWG consensus criteria. MRD status is also indicated along with extent of MRD, presented as the number of multiple myeloma cells detected in the bone marrow per 1 × 104, 1 × 105, or 1 × 106 total nucleated cells. An MRD of ≤1 × 10−4 is considered MRD-negative. (A) The best responses for each patient are shown, grouped by dose cohorts. (B) OR and sCR/CR rate over time.
Objective responses in patients treated with CART-ddBCMA. Responses were assessed according to the IMWG consensus criteria. MRD status is also indicated along with extent of MRD, presented as the number of multiple myeloma cells detected in the bone marrow per 1 × 104, 1 × 105, or 1 × 106 total nucleated cells. An MRD of ≤1 × 10−4 is considered MRD-negative. (A) The best responses for each patient are shown, grouped by dose cohorts. (B) OR and sCR/CR rate over time.
Minimal residual disease (MRD) was evaluated by next-generation sequencing at day +28 in 9/12 patients. One month after CART-ddBCMA infusion, 5/9 patients were MRD negative (10−5, n = 3; 10−6, n = 2) with further deepening of responses over time (Figure 2). At the time of last data cut, 5 subjects were MRD negative at 10−6 and 2 were MRD negative at 10−5. Of those who achieved MRD negativity at 10−6 (n = 5), none have had progressive disease.
Disease progression was observed in 3 subjects. One subject treated on DL1 had progression at day +115 after a best response of PR at day +28. The subject was retreated with CART-ddBCMA at DL2 on day +136 and had further progression at day +205 from initial CAR T infusion. The second subject with disease progression was treated on DL2, reached PR at day 28, very good PR at month 4, and sCR at month 9 (concurrently MRD negative at 10−5) but had progressive disease at day +320 with new lymphadenopathy and rising M-protein (Figure 2). This subject had received a BCMA ADC prior to enrollment in the study. After progression, the subject was retreated at DL1. The subject had an ongoing PR at the time of reporting following reinfusion. The third subject with disease progression was treated on DL1 and had a PR, which was maintained for almost 1 year but had disease progression by day +336. The subject did not receive any retreatment at the time of reporting.
CAR T-cell expansion and persistence
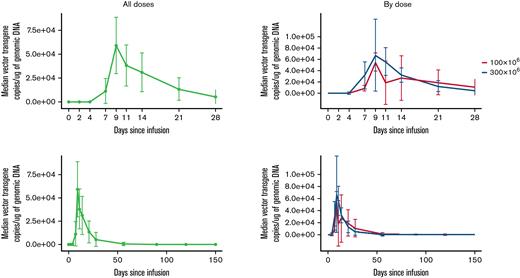
CART-ddBCMA expansion was measured by transgene vector copy number in whole blood. The median time to peak expansion of CART-ddBCMA after infusion was 14 days (range, 9-21) in DL1, 10 days (range, 7-14) in DL2, and 11 days (range, 7-21) in all subjects. The median copies of vector transgene per microgram of genomic DNA at the peak level was 71 992 (range, 10 068-204 300) in DL1, 91 829 (range, 43 785-351 000) in DL2, and 90 147 (range, 10 068-351 000) in all subjects. Median area under the curve (0-28, days × vector copy number/microgram of genomic DNA) for CART-ddBCMA was 514 374 (range, 76 916-3 026 634) in DL1, 644 965 (range, 42 7583-1 777 748) in DL2, and 644 965 (range, 76 916-3 026 634) in all subjects. Median persistence of CART-ddBCMA was 59 days (range, 21-180) in DL1, 42 days (range, 28-180) in DL2, and 42 days (range, 21-180) in all subjects. CART-ddBCMA kinetics, including peak level, time to peak expansion, and persistence, were similar for DL1 and DL2 (Figure 3).
CART-ddBCMA expansion and persistence in patients. The kinetics of CART-ddBCMA over time is shown for each patient as measured by the copies of vector transgene per microgram of genomic DNA.
CART-ddBCMA expansion and persistence in patients. The kinetics of CART-ddBCMA over time is shown for each patient as measured by the copies of vector transgene per microgram of genomic DNA.
Soluble BCMA (s-BCMA) levels in the serum, a marker for plasma cells and myeloma cells,8,10 decreased in all subjects following CART-ddBCMA treatment. The fall in s-BCMA levels in the peripheral blood continued even after CART-ddBCMA was undetectable in the peripheral blood (supplemental Figures 2-3). The recovery of s-BCMA levels was relatively slow in patients with ongoing response, and most patients had lower s-BCMA levels for >6 months, suggesting a slower recovery of plasma cells.
Discussion
This study demonstrated that the maximally tolerated dose of CART-ddBCMA was not exceeded at a flat dose of 300 × 106 CAR+ cells. Evaluation of secondary endpoints indicates an ORR of 100%, with 75% of those responses being CR or better collectively and ≥67% being CR or better in each dose level. The AEs observed in this trial were consistent with previously observed AEs in BCMA CAR T-cell trials,27 including the pivotal study that led to ide-cel approval.22 In this study, only 1 patient (8.3%) had grade 3 neurotoxicity occurring at DL2 within the first week of treatment, which was the only DLT observed in the study. Importantly, no grade ≥3 CRS or ICANS occurred in DL1, and no cases of delayed-onset Parkinson-like progressive movement disorders52,53 were observed at either dose level. At DL1, the lack of grade ≥3 CRS and ICANS occurred in the context of 100% ORR (6/6) and 66.7% (4/6) sCR. No patients experienced atypical neurotoxicity despite a median follow-up of 12.6 months. Ten of the 12 subjects dosed with CART-ddBCMA (83.3%) remained in ongoing response at time of data cut (median follow-up 395 days). Additionally, of the patients who were evaluable, 88.9% were MRD-negative within 1 month of treatment, and many (5/6 patients who were tested multiple times) maintained or developed a deeper response to treatment over time based on their MRD status.
These responses occurred in patients with relatively poor prognostic indicators, including 7/12 (58.3%) with high tumor burden (bone marrow plasma cell >50%), 7/12 (58.3%) with extramedullary disease, and 9/10 evaluable (90%) with high-risk cytogenetics. They were also heavily pretreated, with 7/12 (58.3%) patients having prior hematopoietic stem cell transplant and 10/12 (83.3%) having penta-refractory disease. Given the comparable ORRs between DL1 and DL2 and the comparatively lower CAR T-related toxicities in patients treated with 100 × 106 CART-ddBCMA, we have continued enrollment of the expansion cohort at DL1. If the response rate observed in this study continues in a larger cohort, this dose level will be used in a pivotal trial that is currently in development.
CART-ddBCMA kinetics, including median time to reach peak expansion (10 days) and median time to onset of response (28 days), were similar and consistent with kinetics of CAR T-cell therapies, including ide-cel22 and cilta-cel.24 The ORR and CR observed with CART-ddBCMA was comparable to ORR observed with ide-cel and cilta-cel.22,25,26,54 These results are promising when compared with ide-cel and cilta-cel given the higher rates of high-risk cytogenetics (90% vs 35% and 24%, respectively), extra-medullary involvement (58% vs 39% and 13%, respectively) and penta-refractory disease (83% vs 26% and 42%, respectively).22,25,26,54 After a median follow-up of 12.3 months, median duration of response, PFS, and OS have not been reached at either dose level. More importantly, CART-ddBCMA responses deepened over time, and 6 subjects (of 8 evaluable) remained relapse-free beyond 12-month evaluation, including 3 subjects remaining relapse-free beyond 20 months (Figure 2A), indicating the durability of the efficacy.
The persistence of CART-ddBCMA cells in the peripheral blood was also similar to most BCMA targeting CAR T-cell therapies, which noted a drop in peripheral CAR+ cells within 60 days and lack of detectable CAR+ cells in peripheral blood within 120 days in most subjects.25,55,56 We believe the drop in CART-ddBCMA levels is mainly due to lack of antigen stimulation following tumor elimination. Intriguingly, durability of efficacy was not found to correlate with presence of detectable CAR T cells in multiple myeloma in previous studies.25,56 In our study, although CART-ddBCMA cells were not detectable after 120 days, responses were durable for >12 months in 6/8 evaluable subjects. Furthermore, s-BCMA levels remained low in all subjects with ongoing response, and the recovery rate was slow, suggesting a slower recovery of BCMA-expressing plasma cells in the peripheral blood. Further studies and additional data are needed to verify if slower recovery of BCMA-expressing plasma cells is due to ongoing immunosurveillance against BCMA expressing plasma cells by CART-ddBCMA cells.
This trial is the first to demonstrate the use of d-domain antigen-binding domain-based CAR T cells. d-domains have distinct advantages, such as triple α-helical bundle stabilized by a hydrophobic core with no disulfide bonds or N-linked glycosylation sites,43 and are easily manipulatable, allowing for removal of immunogenic epitopes and modulation of the target binding specificities. Therefore, the production of d-domain based CAR T cells is expected to provide consistent manufacturing with lower interpatient variability and decreased tonic signaling, which may improve the durability of BCMA CART responses. The current study provides the first evidence on clinical application of d-domains. The durable responses, consistent CAR+ expression rate per cell (median vector copy number of 2.33; range, 1.33-3.55), and low interpatient variability (median CAR+CD3+ cells in the product of 74%; range, 61% to 87%) noted in the current study are encouraging and support the development of binding domains for other targets.
This study is limited by a small sample size and is mainly designed to evaluate the initial safety of CART-ddBCMA administration. This limitation of the study should be considered during the interpretation of the findings on safety and efficacy. Further studies in a larger cohort are needed to confirm the safety and efficacy of CART-ddBCMA cells for treatment of RRMM.
In conclusion, we characterized the safety of CART-ddBCMA at doses of 100 and 300 × 106 cells per patient. We further showed that CART-ddBCMA administration can induce deep and durable responses in patients with high-risk RRMM.
Acknowledgments
The authors thank Trisha R. Berger for drafting the manuscript, Anand Rotte for editorial assistance, Sujatha Kuruvakkat and Sigal Shachar for data analyses, Jerome Ritz for CART-ddBCMA support with cell manufacturing, and Scott Currence and Travis Quigley for clinical operations support.
Authorship
Contribution: M.J.F., M.R.B., J.R., E.K.O., N.R., D.C., A.J.Y., E.L., D.E.A., A.J., K.S., H.D., and S.N. contributed to the study design, collection of data, and analyses and interpretation of data; F.G. and C.C. contributed to study design and data analyses; and A.S., C.H., and M.V.M. contributed to the study design and interpretation of data.
Conflict-of-interest disclosure: M.J.F. reported consultancy fees from Celgene, consultancy and research funding from Novartis, consultancy fees from Arcellx, and consultancy fees and research funding from Gilead/Kite. M.R.B. reported honoraria, membership on an entity’s board of directors or advisory committees, research funding, and speakers bureau fees from Kite; honoraria and speakers bureau fees from Incyte; membership on an entity’s board of directors or advisory committees from Autolus; membership on an entity’s board of directors or advisory committees and research funding from Novartis; membership on an entity’s board of directors or advisory committees and research funding from CRSPPR Therapeutics; honoraria and speakers bureau fees from Bristol Myers Squibb. E.K.O. reported consultancy fees from Celgene. J.R. reported consultancy fees from Attivare Therapeutics; research funding from Bristol Myers Squibb; consultancy fees from Parexel; consultancy fees and patents and royalties from Wolters Kluwer Health; consultancy fees from Imaging Endpoints; membership on an entity's board of directors or advisory committees from Karyopharm. N.R. reported consultancy fees from Celgene. A.J.Y. reported consultancy fees from Adaptive, Bristol Myers Squibb, GSK, Oncopeptides, Karyopharm, Amgen, Takeda, Sanofi, and Janssen. A.S. reported former employment at and current equity holder in private company Arcellx. D.E.A. reported membership on an entity's board of directors or advisory committees and research funding from Celgene; research funding from Pharmacyclics; consultancy fees and research funding from Kite Pharma; membership on an entity's board of directors or advisory committees from Juno, Partner Tx, Karyopharm, Bristol Myers Squibb, Aviv MedTech Ltd., Takeda, Legend Biotech, and Chugai, consultancy fees from Janssen, Parexcel, Takeda, and Sanofi. A.J. reported membership on an entity's board of directors or advisory committees from Bristol Myers Squibb, Celgene, AbbVie, Gracell, GSK, Janssen, Karyopharm, Amgen, and Sanofi. K.S. reported current equity holder in publicly traded company Orchard Therapeutics, Ltd. S.N. reported ad hoc advisory boards for Kite/Gilead, Novartis, Iovance, and GlaxoSmithKline. F.G. reported current employment at and current equity holder in private company Arcellx. C.C. reported current employment at and current equity holder in private company Arcellx. C.H. reported current employment at and current equity holder in private company Arcellx. M.V.M. reported consultancy fees and research funding from Arcellx, Kite, and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Matthew J. Frigault, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114; e-mail: mfrigault@partners.org.
References
Author notes
Presented in abstract form at the American Society of Clinical Oncology 2021 Virtual Meeting, 4-8 June 2021 and the 63rd annual meeting of the American Society of Hematology, Atlanta, GA; 11-14 December 2021.57
For data sharing, contact the corresponding author, Matthew J. Frigault (mfrigault@partners.org). Individual participant data will not be shared.
The full-text version of this article contains a data supplement.