Key Points
KLF2 is downregulated by augmented JAK-STAT signaling in PV and ET, leading to upregulated thrombotic gene expression.
KLF2 transcript levels in PV and ET are inversely correlated with neutrophil and platelet counts but not hemoglobin and hematocrit.
Abstract
Thromboses are major causes of morbidity and mortality in polycythemia vera (PV) and essential thrombocythemia (ET) diseases associated with JAK2V617F mutation. However, the molecular mechanism(s) of increased thrombosis in PV and ET remain unknown. Kruppel-like factor 2 (KLF2) is a transcription factor that regulates expression of genes associated with inflammation and thrombosis; the absence of KLF2 in neutrophils causes thrombosis by inducing tissue factor. We studied the role of KLF2 in regulating prothrombotic gene expression in PV and ET. Neutrophils and platelets KLF2 expression in PV and ET was lower than the controls. Furthermore, in patients with thromboses, KLF2 transcripts were lower in platelets than those without thromboses. JAK2V617F allelic burden was inversely correlated with KLF2 transcript levels, suggesting JAK-STAT pathway may downregulate KLF2 expression. Whole transcriptome analyses of neutrophils and platelets showed that a lower KLF2 expression was associated with an upregulation of KLF2-regulated thrombotic genes. In addition, low KLF2 expression in platelets positively correlated with thrombotic events. In patients with PV and ET, KLF2 expression was induced by pegylated interferon alfa (PegINF-α) but not by hydroxyurea treatments. These data suggest that KLF2 may be a regulator of PV and ET thrombosis and a novel therapeutic target to prevent thrombosis.
Introduction
Thrombosis is the most common complication of polycythemia vera (PV) and essential thrombocythemia (ET).1 The risk factors of thrombosis include high leukocyte and neutrophil counts;2 however, the molecular mechanisms of thrombosis are not fully known. We previously reported that increased hypoxia inducible factor transcriptional activity in PV and ET induces transcription of proinflammatory and prothrombotic genes.3
Kruppel-like factors (KLFs) are zinc finger transcription factors.4 KLF2 is highly expressed in endothelium and hematopoietic cells and induces myeloid quiescence by inhibiting the recruitment of coactivators of nuclear factor kappa B (NF-κB).5 The knockdown of KLF2 in endothelial cells increases prothrombotic gene expression including F3 (encoding tissue factor [TF]) and SERPINE1 (encoding plasminogen activator inhibitor 1 [PAI-1]),6 and low neutrophil KLF2 activates neutrophils and induces TF.7
We investigated possible KLF2 downregulation in PV and ET and its effect on thromboses and interaction with JAK2V617F.
Study design
In this institutional review board–approved study JAK2V617F allele burden,8KLF2, and thrombotic gene transcripts were measured as previously described.3,9 Ruxolitinib was used for JAK2 inhibition and pCMV6-entry vector containing KLF2 complementary DNA was used for KLF2 overexpression; for details, see supplemental Methods.
Results and discussion
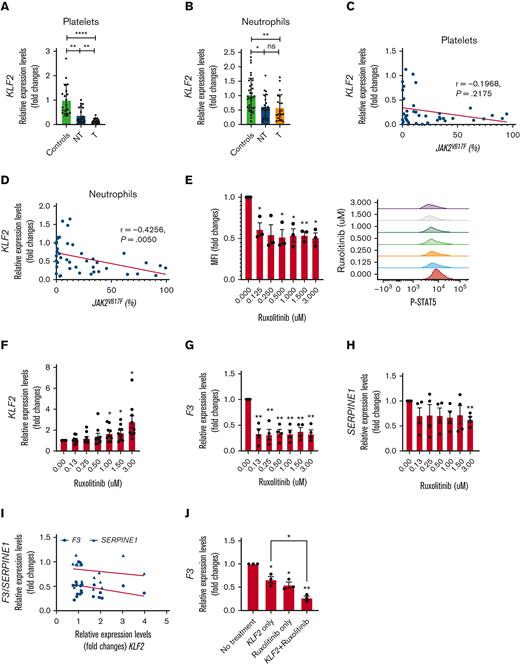
KLF2 transcript levels were downregulated in neutrophils and platelets of PV and ET (n = 45) compared with controls (n = 36, granulocytes; n = 18, platelets) (Figure 1A-B). In those with thrombosis (n = 23), platelet KLF2 transcript levels were lower than those without thrombosis (n = 22). In neutrophils, KLF2 transcript levels were comparable in both groups (Figure 1A-B). To determine if downregulation of KLF2 alters the expression of its target genes, we analyzed whole transcriptome data of neutrophils (22 PV and 10 controls) and platelets (24 PV and 4 controls)3 available from our prior work. In neutrophils, 149 KLF2-targeted genes were expressed, 30 were upregulated, and 5 were downregulated. In platelets, among 169 KLF2-regulated genes, 26 genes were upregulated, and 2 genes were downregulated (adjusted P value <.05, Log2 fold changes >1) (supplemental Figure 1A,B). The transcript levels of these dysregulated genes correlated with KLF2 transcript levels (supplemental Tables 1 and 2). We quantified transcript levels of some of the KLF2 target genes including prothrombotic genes of F3, CD36 (platelet glycoprotein 4), VWF (von Willebrand factor), SERPINE1, and THBS1 (Thrombospondin 1) and antithrombotic genes of COL4A1 (Collagen Type IV Alpha 1) and CD40LG (CD40 Ligand). Then, these transcript levels were correlated with KLF2 transcript levels (supplemental Figure 2). In general, prothrombotic genes were upregulated in PV and ET neutrophils and platelets (supplemental Figure 2A-E), whereas antithrombotic genes were downregulated (supplemental Figure 2F,G). In platelets, F3, CD36, and VWF transcript levels were higher in those with thrombosis than in those without thrombosis (supplemental Figure 2A-C). In neutrophils, CD36 levels were higher in patients with thrombosis (supplemental Figure2B). KLF2 transcript levels in platelets but not in neutrophils inversely correlated with prothrombotic gene expression (supplemental Figure 2A-E). In contrast, antithrombotic gene expression both in platelets and neutrophils positively correlated with KLF2 transcript levels (supplemental Figure 2F-G). Although the absence of KLF2 in neutrophils alone induces thrombosis in murine models,7 we found an even stronger correlation of KLF2 and its target gene expression in platelets. Thus, downregulated KLF2 in PV and ET in platelets induces prothrombotic gene expression.
KLF2 is downregulated in PV and ET platelets and neutrophils by JAK-STAT signaling.KLF2 transcript levels were measured in (A) platelets and (B) neutrophils and expressed as a fold changes. KLF2 transcript levels in (C) platelets and (D) neutrophils correlated with JAK2V617F allele burden measured in neutrophils. (E) Phosphorylated STAT5 (P-STAT5) were measured in HEL cells treated with various concentrations of ruxolitinib by fluorescence activated cell sorting analysis and mean fluorescence intensity was calculated against samples without treatment which is taken as 1 and expressed as fold changes. (F) KLF2, (G) F3, and (H) SERPINE1 transcript levels were measured in HEL cells treated with various concentration of ruxolitinib. (I) KLF2 transcript levels were not correlated with F3 and SERPINE1 transcript levels in ruxolitinib treated HEL cells. (J) F3 transcript levels were measured in HEL cells with only KLF2 overexpression, only ruxolitinib treatment, and both KLF2 overexpression and ruxolitinib treatment. Expression levels were expressed as fold changes and no treatment control was taken as 1. P value was calculated by unpaired t test or paired t test. Correlation analysis was performed by Spearman correlation using GraphPad prism. ∗∗∗∗ P < .0001, ∗∗ P < .01, ∗ P < .05. P-STAT5, Phosphorylated STAT5; NT, no history of thrombosis; T, history of thrombosis.
KLF2 is downregulated in PV and ET platelets and neutrophils by JAK-STAT signaling.KLF2 transcript levels were measured in (A) platelets and (B) neutrophils and expressed as a fold changes. KLF2 transcript levels in (C) platelets and (D) neutrophils correlated with JAK2V617F allele burden measured in neutrophils. (E) Phosphorylated STAT5 (P-STAT5) were measured in HEL cells treated with various concentrations of ruxolitinib by fluorescence activated cell sorting analysis and mean fluorescence intensity was calculated against samples without treatment which is taken as 1 and expressed as fold changes. (F) KLF2, (G) F3, and (H) SERPINE1 transcript levels were measured in HEL cells treated with various concentration of ruxolitinib. (I) KLF2 transcript levels were not correlated with F3 and SERPINE1 transcript levels in ruxolitinib treated HEL cells. (J) F3 transcript levels were measured in HEL cells with only KLF2 overexpression, only ruxolitinib treatment, and both KLF2 overexpression and ruxolitinib treatment. Expression levels were expressed as fold changes and no treatment control was taken as 1. P value was calculated by unpaired t test or paired t test. Correlation analysis was performed by Spearman correlation using GraphPad prism. ∗∗∗∗ P < .0001, ∗∗ P < .01, ∗ P < .05. P-STAT5, Phosphorylated STAT5; NT, no history of thrombosis; T, history of thrombosis.
Given the variable JAK2V617F allelic burden is a thrombosis risk in PV and ET,10,11 we measured it in PV and ET clonal neutrophils. JAK2V617F allelic burden inversely correlated with KLF2 transcript levels in neutrophils (r = −0.4160, P = .0068) only, but not in platelets (Figure1C,D), suggesting that augmented JAK2-STAT5 signaling in PV and ET12 because of JAK2V617F may downregulate neutrophil KLF2 expression. To test this hypothesis, transcript levels of KLF2 and its target genes (SERPINE1, and F3) were measured in myeloid leukemia cell lines human erythroleukemia (HEL) (JAK2V617F positive) incubated with ruxolitinib (JAK1 and JAK2 inhibitor13). We measured the JAK2 activity to confirm ruxolitinib effect by measuring phosphorylated STAT5 by flow cytometry.14 Phosphorylated STAT5 decreased with ruxolitinib treatment, whereas KLF2 transcripts increased in dose dependent manner (Figure 1E), indicating that KLF2 expression is downregulated by augmented JAK2-STAT5 activity in PV and ET (Figure 1F). We show that ruxolitinib treatment decreased F3 and SERPINE1 transcript levels (Figure 1G,H), however, it did not correlate with KLF2 transcript levels (Figure 1I).
To test if F3 transcript is regulated by KLF2 and/or JAK2-STAT5, we overexpressed KLF2 in HEL cells. KLF2 overexpression led to decreased F3 transcript levels. Ruxolitinib treatment further decreased the F3 transcript levels (Figure 1J). These data demonstrate that low KLF2 transcript levels in PV and ET are mediated by augmented JAK2-STAT5 signaling due to JAK2V617F and F3 expression. Augmented JAK2-STAT5 signaling also increases F3 expression, independently. These data are consistent with higher TF activity in PV and ET with JAK2V617F mutation than in ET with CALR mutation or controls.15
KLF2 transcript level in platelets inversely correlated with neutrophil (r = −0.3061, P = .0226) and platelet numbers (r = −0.2949, P = .032) (Figure 2A,B) but not with hemoglobin or hematocrit (Figure 2C,D). Multivariate analyses showed that only leukocytosis/neutrophilia associated with higher thrombotic risk generally require myelosuppressive therapy.2 Neutrophils make up 50% to 70% of leukocytes.16 Neutrophil counts correlated positively with leukocyte counts (r = 0.9210, P < .0001). High platelet counts increase 1.8-fold risk of arterial thrombosis in brain17 whereas lower platelet counts are associated with venous thromboses in ET,18 suggesting that high leukocyte and platelet numbers increase the risk of thrombosis because of low expression levels of KLF2 and further support that normalization of leukocyte and platelet numbers reduces the risk of thrombosis.
KLF2 levels in platelets are inversely correlated with neutrophil and platelet counts and increased with PegINF-α treatment.KLF2 transcript level in platelets was inversely correlated with (A) neutrophil counts and (B) platelet counts but not with (C) hematocrit and (D) hemoglobin concentration. KLF2 transcript levels were measured in (E) platelets and (F) neutrophils before and after PegINF-α or HU. Changes of (G) neutrophil counts and (H) platelets counts were calculated against before treatment which was taken as 100% and inversely correlated with changes of KLF2 transcript levels in platelets. Changes of (I) neutrophil counts and (J) platelets counts were calculated against before treatment which was taken as 100% and inversely correlated with changes of KLF2 transcript levels in neutrophils. P value was calculated by paired t test. Correlation analysis was performed by Spearman correlation using GraphPad prism. ∗∗ P < .01, ∗ P < .05.
KLF2 levels in platelets are inversely correlated with neutrophil and platelet counts and increased with PegINF-α treatment.KLF2 transcript level in platelets was inversely correlated with (A) neutrophil counts and (B) platelet counts but not with (C) hematocrit and (D) hemoglobin concentration. KLF2 transcript levels were measured in (E) platelets and (F) neutrophils before and after PegINF-α or HU. Changes of (G) neutrophil counts and (H) platelets counts were calculated against before treatment which was taken as 100% and inversely correlated with changes of KLF2 transcript levels in platelets. Changes of (I) neutrophil counts and (J) platelets counts were calculated against before treatment which was taken as 100% and inversely correlated with changes of KLF2 transcript levels in neutrophils. P value was calculated by paired t test. Correlation analysis was performed by Spearman correlation using GraphPad prism. ∗∗ P < .01, ∗ P < .05.
Cytoreductive treatment hydroxyurea (HU) and Pegylated interferon alfa (PegINF-α) is used for patients with high risk of thrombosis.19 We evaluated if these therapies modulate KLF2 expression in neutrophils and platelets in 13 patients treated with HU and 14 patients with PegINF-α. Because the patients have different initial KLF2 transcript levels according to their inflammation or thrombosis status, we used each pretreatment sample as a control for matching post treatment sample. Median duration of treatment with PegINF-α or HU was 165 and 175 days, respectively. PegINF-α treatment increased KLF2 transcript levels in both neutrophils and platelets, whereas HU treatment did not (Figure 2E-F). JAK2V617F allele burden decreased in 53% of patients treated with HU and 30% of the patients treated with PegINF-α. Neutrophil and platelet counts decreased from baseline after HU (83%, 85%) and PegINF-α treatment (85%, 91%), respectively. Increased KLF2 transcript levels in platelets (Figure 2G-H) but not in neutrophils (Figure 2I-J) inversely correlated with decrease of neutrophils (r = −0.5818, P = .0099) and platelet counts (r = −0.3906, P = .0591). However, these levels did not correlate with the decreased hemoglobin and hematocrit (data not shown). Tumor necrosis factor (TNF) treatment reduces KLF2 transcript and protein levels through NF-kB signaling in human umbilical vein endothelial cells.20TNF transcript levels in CD34+ cells and in vitro expanded erythroid cells decreased with PegINF-α but not with HU.21 Increased KLF2 transcripts in platelets but not in neutrophils after PegINF-α treatment correlated with decreased TNF transcript (r = −0.4682 P = .0432, figure not shown). PegINF-α decreases expression of genes associated with inflammation and immune cell function.22 Although it remains to be confirmed if PegINF-α treatment increases KLF2 transcript levels by decreasing inflammation, our observations suggest that increased KLF2 transcript levels after PegINF-α treatment may be due to PegINF-α–mediated decreased inflammatory factors such as tumor necrosis factor.
We determined that downregulated KLF2 in PV neutrophils and platelets is mediated by augmented JAK2 activity, leading to increased risk of thrombosis, which is corrected by PegINF-α treatment (supplemental Figure 3).
Acknowledgments
This research was supported by the MPN Research Foundation, the National Institutes of Health under the Ruth L. Kirschstein National Research Service Award 2T32HL007576-31 from the National Heart, Lung, and Blood Institute (J.S.), and the VA Merit Review Award (PI-Prchal, HIF-Mediated Detrimental Consequences of Chronic Intermittent Hypoxia).
Authorship
Contribution: J.G. suggested to P.T., J.T.P., and J.S. to focus on KLF2 in PV and ET; J.S. designed and performed the experiments; P.T. and J.T.P. contributed to the study design; J.S. and S.J.K. performed whole transcriptome analysis; S.J.K. collected and critically analyzed clinical data with J.T.P.; J.S. and J.T.P. wrote the manuscript; and P.T., J.G. and S.J.K. critically revised the manuscript and edited and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jihyun Song, Division of Hematology, Huntsman Cancer Institute, School of Medicine, University of Utah, Salt Lake City, UT 84112; e-mail: jihyun.song@utah.edu; and Josef T. Prchal, Division of Hematology, Huntsman Cancer Institute, School of Medicine, University of Utah, Salt Lake City, UT 84112; e-mail: josef.prchal@hsc.utah.edu.
References
Author notes
Any data or resources in the manuscript will be shared upon request: josef.prchal@hsc.utah.edu.
The full-text version of this article contains a data supplement.