TO THE EDITOR:
VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome is a disease caused by somatic UBA1 (ubiquitin like modifier activating enzyme 1) mutations in hematopoietic progenitor cells, leading to a plethora of inflammatory symptoms and hematologic conditions.1
Systemic inflammation involves different sites such as skin, eyes, blood vessels, cartilage, and lungs. Additionally, a number of hematologic problems occur, including macrocytic anemia, thrombocytopenia, and hematologic malignancies such as myelodysplastic syndrome.2
Here, we describe, to our knowledge, the first case of a male patient with chronic myeloid leukemia (CML) and VEXAS syndrome. We give a detailed review of blood, bone marrow, and molecular genetics, including clonal evolution over a period of 8 years.
In 2014, a 48-year-old White man with no relevant prior medical history presented with painful erythematous cutaneous nodules on arms and legs as well as episcleritis. A blood count showed a mild normocytic anemia of 120 g/L (134-140 g/L) and leukopenia of 1.7 × 109/L (3 × 109/L -9.6 × 109/L), with a predominant neutropenia of 0.5 × 109/L (1.4 × 109/L -8 × 109/L). Blood chemistry analysis revealed mild C-reactive protein elevation from 5 to 10 mg/L (normal; <5 mg/L) and an elevated blood sedimentation rate of 40 mm/h (normal, <15 mm/h). The kidney, liver, and lactate dehydrogenase parameters were within the normal range. Active infectious diseases such as cytomegalovirus, Epstein-Barr virus, parvovirus B19, hepatitis B/C, and HIV were ruled out. Immunological testing for antineutrophil cytoplasmatic antibody (ANCA) and anti-nuclear antibodies (ANA) antibodies showed negative results.
A bone marrow aspirate showed increased marrow cellularity with overall increased and left-shifted myelopoiesis. The myelopoietic cells showed increased vacuoles. No elevation in myeloblast numbers was detected (Figure 1). Cytogenetic analysis of bone marrow was normal (46, XY). The bone marrow was considered to be reactive to an inflammatory stimulus. A skin biopsy showed septal panniculitis. Taking together the symptoms and skin biopsy, the diagnosis of erythema nodosum was established.
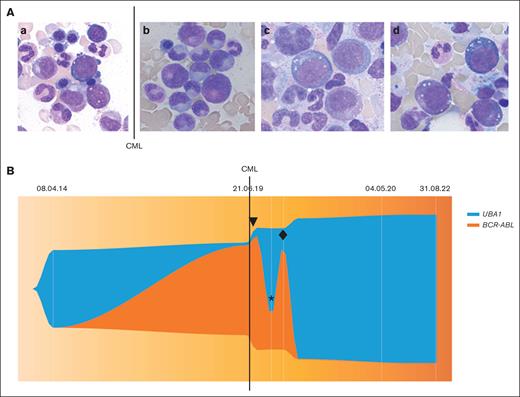
Bone marrow aspirate and molecular analysis. (A) Bone marrow aspiration cytology (hematoxylin and eosin staining; original magnification ×1000). a) April 2014, showing vacuoles in myelomonocytic precursor cells and erythroblast. (b) June 2019, showing no vacuolation in myelomoncytic precursor cells at CML diagnosis. (c) January 2020 and (d) March 2022: showing vacuoles in myelomonocytic precursor cells and erythroblasts. (B) Visualization of clonal dynamics (performed using R v. 4.3.1 and package fishplot version 0.23) between UBA1 variant p.Met41Val allele burden (variant allele frequency %) and BCR-ABL:ABL1 ratios (International Scale (IS) %). Line, CML diagnosis in June 2019; ▼ imatinib initiation in July 2019; ∗ imatinib withdrawal in November 2019; ♦ nilotinib initiation in February 2020.
Bone marrow aspirate and molecular analysis. (A) Bone marrow aspiration cytology (hematoxylin and eosin staining; original magnification ×1000). a) April 2014, showing vacuoles in myelomonocytic precursor cells and erythroblast. (b) June 2019, showing no vacuolation in myelomoncytic precursor cells at CML diagnosis. (c) January 2020 and (d) March 2022: showing vacuoles in myelomonocytic precursor cells and erythroblasts. (B) Visualization of clonal dynamics (performed using R v. 4.3.1 and package fishplot version 0.23) between UBA1 variant p.Met41Val allele burden (variant allele frequency %) and BCR-ABL:ABL1 ratios (International Scale (IS) %). Line, CML diagnosis in June 2019; ▼ imatinib initiation in July 2019; ∗ imatinib withdrawal in November 2019; ♦ nilotinib initiation in February 2020.
Until 2017, the patient developed various new symptoms, such as bilateral hilar lymphadenopathy and anemia of varying degree with progressive macrocytosis (Figure 2). Repetitive treatment with high-dose oral prednisone led to complete resolution of symptoms. However, several subsequent attempts to taper prednisone were unsuccessful.
Correlation of blood count, C-reactive peptide, and molecular markers. (A) Hemoglobin count (g/L), mean corpuscular volume MCV (fl), and c-reactive peptide CRP (mg/L) from April 2014 to August 2022. Line, June 2019 CML diagnosis. (B) UBA1 variant p.Met41Val allele burden (variant allele frequency %) from April 2014 to August 2022 and BCR-ABL:ABL1 ratios (International Scale (IS) %) from June 2019 to August 2022. Line, CML diagnosis in June 2019; ▼ imatinib initiation in July 2019; ∗ imatinib withdrawal in November 2019; ♦ nilotinib initiation in February 2020.
Correlation of blood count, C-reactive peptide, and molecular markers. (A) Hemoglobin count (g/L), mean corpuscular volume MCV (fl), and c-reactive peptide CRP (mg/L) from April 2014 to August 2022. Line, June 2019 CML diagnosis. (B) UBA1 variant p.Met41Val allele burden (variant allele frequency %) from April 2014 to August 2022 and BCR-ABL:ABL1 ratios (International Scale (IS) %) from June 2019 to August 2022. Line, CML diagnosis in June 2019; ▼ imatinib initiation in July 2019; ∗ imatinib withdrawal in November 2019; ♦ nilotinib initiation in February 2020.
In 2019, at the age 51 years, the patient was referred to the hematology department, with a newly detected elevated white blood cell count of 80 × 109/L. The white blood cell differential showed neutrophilia and elevated precursors, basophilia, monocytosis, and eosinophilia. Furthermore, a mild normocytic anemia of 120g/L was detected. Genetic testing confirmed the suspicion of CML with presence of the Philadelphia chromosome (t(9;22)(q34;q11.2)). Bone marrow aspirate showed hypercellular marrow with marked granulocytic proliferation but no vacuoles, dysplasia, or blast count elevation (Figure 1).
At CML diagnosis, the patient had practically no clinical symptoms of his previous inflammatory state, including normal c-reactive protein (CRP)(Figure 2), requiring almost no glucocorticoids. We initiated medication with the tyrosine kinase inhibitor (TKI) imatinib.
Three months after starting imatinib treatment, painful cutaneous nodules, known to the patient, reoccurred in combination with CRP elevation and fevers. We suspected a flare of the erythema nodosum owing to imatinib treatment. We thus paused TKI treatment and reinitiated high-dose prednisone and subsequently replaced imatinib with nilotinib. All attempts to taper prednisone led to clinical worsening, with development of polyarthritis, intermittent microhematuria, and bipulmonary ground glass opacities with reduced diffusing capacity for carbon monoxide over time.
In 2022, because of unclear and emerging new inflammatory symptoms, we suspected VEXAS syndrome. Reevaluation of bone marrow aspirates from 2014, 2019, and 2020, and a new bone marrow analysis (Figure 1) showed hypercellularity and vacuoles in myeloid precursors and erythroblasts in all samples, except in the sample at the time of CML diagnosis (2019). In concordance, a UBA1 c.121A>G (p.Met41Val) mutation was detected. We thus retrospectively analyzed all available DNA and RNA samples from peripheral blood and bone marrow from 2014 to 2022.
The UBA1 mutation could be traced back until 2014. The allele burden of the UBA1 variant p.Met41Val was determined with a custom-targeted DNA sequencing panel, which covers UBA1 exon 3 (VariantPlex, ArcherDx, Boulder, CO).
BCR-ABL could be analyzed since 2019 until now, because no RNA material was available before. BCR-ABL1:ABL1 ratios were determined by quantitative polymerase chain reaction using the Ipsogen BCR-ABL1 Mbcr Kit (Qiagen). BCR-ABL1:ABL1 ratios were converted using a laboratory-specific conversion factor to express results on the international scale.4
We found the UBA1 mutation being present since 2014, with reduction of allele burden at diagnosis of CML, and again rising rapidly after treatment initiation with TKIs, being highest since a major molecular response of CML (Figure 1).
In summary, we established the diagnosis of VEXAS syndrome with concomitant CML in this middle-aged male patient with multiple inflammatory symptoms, recurrent hypercellular bone marrow with vacuoles, macrocytic anemia, and detectable somatic UBA1 mutation (UBA1mut).
Co-occurrence of VEXAS with hematologic neoplasms is a known phenomenon, especially with myelodysplastic syndrome and plasma cell dyscrasia.1,2 A few case reports described VEXAS in combination with essential thrombocytemia.5,6
Recently, it has been shown that typical clonal hematopoiesis, driven by mutations in genes such as DNMT3A and TET2, co-occurred with UBA1mut in up to 60% of patients. Furthermore, the data suggest UBA1mut being the dominant clone.7 In addition, a case report showed UBA1mut dominance over a CALR mutant clone.6 To our knowledge, this is the first reported case of CML and concomitant VEXAS syndrome. The patient’s history and laboratory analysis suggest competition of the UBA1mut and CML clones, with CML being the dominant clone.
Arguments in favor of this hypothesis are the opposing UBA1mut and BCRL-ABL kinetics (Figure 1 and 2) showing a reduced allele frequency of UBA1mut at CML diagnosis with high BCR-ABL allele frequency and UBA1mut expansion after CML treatment and major molecular response of CML. Although these might reflect relative changes, the clinical course with improvement of inflammatory symptoms and very low CRP levels at CML diagnosis and the subsequent increase of inflammation upon therapeutic CML control strongly argue for clonal competition (Figure 1). Furthermore, the bone marrow aspirate at CML diagnosis showed no vacuoles in myeloid and erythroid precursors as an indicator for VEXAS (Figure 1). Another aspect worth mentioning is the change of hemoglobin and mean corpuscular volume values during disease course especially since diagnosis of CML (Figure 2); at CML diagnosis, the patient had a mild normocytic anemia. Before CML diagnosis and after initiation of TKI treatment, we documented a worsening over time with the blood count becoming more and more macrocytic, a typical phenomenon in VEXAS syndrome.2
In summary, this unique case highlights 2 competing hematologic diseases. This case and previous cases of concomitant clones in VEXAS syndrome indicate that dominance depends, as expected, on the strength of the driver. It remains unclear whether UBA1mut, its inflammatory microenvironment, or epigenetic mechanisms influenced the initiation and/or expansion of a BCR-ABL clone.
Finding the adequate steroid-sparing treatment regimen for VEXAS syndrome remains a clinical challenge. We considered allogeneic hematopoietic stem cell transplant,8-10 but because of the high no-relapse mortality and a well-controlled CML, we decided against it. Other evaluated therapeutic agents with promising results are the JAK inhibitor ruxolitinib,11 azacitidine,12 anti–interleukin-6 (anti–IL-6; tocilizumab),13,14 and anti–IL-1RA (anakinra).15 Indeed, we recently started treatment with anti–IL-6 therapy (tocilizumab), which is currently showing encouraging control of inflammation.
Acknowledgment: The authors thank the patient for consenting to this case report.
Contribution: N.D. and K.Z. collected data and wrote the manuscript; K.Z. contributed analytical tools; and N.D., K.Z., M.R., M.O.B., M.G.M., and S.B. analyzed and interpreted the data.
Conflict-of-interest disclosure: M.O.B. reports research grant from Novartis foundation for biomedical research; congress participation support from Bayer, GlaxoSmithKline (GSK), Novartis, and Merck Sharp & Dohme (MSD); and speaker fee from Amgen, Novartis, MSD, Mepha, and Vifor. The remaining authors declare no competing financial interest.
Correspondence: Nadia Djerbi, Department of Medical Oncology and Hematology, University Hospital Zurich–University of Zurich, Raemistrasse 100, 8091 Zurich, Switzerland; e-mail: nadia.djerbi@usz.ch.
References
Author notes
Data are available on request from the corresponding author, Nadia Djerbi (nadia.djerbi@usz.ch).