TO THE EDITOR:
Marginal zone lymphoma (MZL) is an indolent subtype of B-cell non-Hodgkin lymphoma that made up roughly 7% of all newly diagnosed non-Hodgkin lymphoma cases in 2016.1-4 MZL is a heterogenous disease and is classified into 3 distinct subtypes: extranodal MZL of mucosa-associated lymphoid tissue (EMZL/MALT), splenic MZL (SMZL), and nodal MZL (NMZL).5 With a better understanding of the disease biology and owing to therapeutic advances, the survival of patients with MZL has improved over the past decade.6 Patients with MZL can have circulating lymphoma (CL) cells at diagnosis, and this is more commonly seen in SMZL.7,8 However, the prognostic relevance of CL at diagnosis is largely unknown. Therefore, we sought to evaluate the impact of CL at diagnosis on outcomes in patients with MZL across various academic centers in the United States.
This was a multicenter, retrospective cohort study of patients with MZL treated at 8 US medical centers. The study included patients who were aged ≥18 years, diagnosed with MZL on or after 1 January 2010, with stage III or IV disease and had peripheral blood (PB) immunophenotyping via flow cytometry performed at diagnosis. The study was approved by the institutional review boards at the participating sites and performed in compliance with the Declaration of Helsinki. To be eligible, patients must have received treatment for their MZL and had PB flow cytometry performed at diagnosis. CL was defined as detectable clonally restricted B cells in PB (in >1% analyzed cells) that matched the actual or expected immunophenotype of the tissue lymphoma immunophenotype. We collected variables known to be significantly associated with survival outcomes of MZL.9,10
The study population was divided into 2 groups: those with positive PB flow cytometry (CL+) and those with negative PB flow cytometry (CL–). The primary end point was to evaluate progression-free survival (PFS) in the 2 groups. Secondary end points included the evaluation of overall survival (OS), diagnosis-to-treatment interval (DTI), and cumulative incidence of histologic transformation (HT) in groups with or without CL at diagnosis. PFS was defined as the time from the start of first-line therapy until lymphoma relapse/progression or death from any cause, censoring from the last clinical assessment. OS was defined as the time from the start of first-line therapy until death or the last follow-up. DTI was defined as the time from diagnosis to initiation of systemic therapy.
Among 242 eligible patients with newly diagnosed advanced stage MZL, 113 (47%) had detectable CL at diagnosis. The most common MZL subtype was NMZL (36%), followed by SMZL (34%) and EMZL (29%). Within the CL+ group, the proportion of patients with CL was highest in SMZL (51%), followed by NMZL (27%) and EMZL (21%). Compared with patients in the CL– group, those with CL at diagnosis had more bone marrow involvement, elevated lactate dehydrogenase, and elevated beta 2 microglobulin. The most common treatment regimen in the entire cohort was rituximab monotherapy (n = 115), followed by immunochemotherapy (n = 98), and localized therapy (n = 21). Table 1 shows the baseline characteristics of the patient population according to the presence or absence of CL. Because patients receiving immunochemotherapy may be clinically a different patient population than those receiving rituximab monotherapy, we looked at the salient patient characteristics stratified by the presence or absence of CL in those receiving immunochemotherapy (n = 98; supplemental Table 1). The results were in line with the main analysis.
Baseline characteristics
| Characteristics . | Total (N = 242) . | CL+ (n = 113) . | CL– (n = 129) . | P value . |
|---|---|---|---|---|
| Age in y, median (range) | 65 (23-91) | 66 (23-91) | 65 (32-91) | .52 |
| Sex, n (%) | .20 | |||
| Male | 126 (52) | 64 (57) | 62 (48) | |
| Female | 116 (48) | 49 (43) | 67 (52) | |
| MZL subtype, n (%) | <.0001 | |||
| NMZL | 88 (36) | 31 (27) | 57 (44) | |
| SMZL | 83 (34) | 58 (51) | 25 (19) | |
| EMZL | 71∗ (29) | 24 (21) | 47 (36) | |
| ECOG PS, n (%) | .16 | |||
| 0-1 | 190 (93) | 91 (89) | 99 (96) | |
| ≥2 | 15 (7) | 11 (11) | 4 (4) | |
| Stage, n (%) | <.0001 | |||
| III | 39 (16) | 0 (0) | 39 (30) | |
| IV | 203 (84) | 113 (100) | 90 (70) | |
| B symptoms, n (%) | 49 (21) | 19 (18) | 30 (24) | .26 |
| BM involvement, n (%) | 137 (81) | 91 (91) | 46 (67) | <.0001 |
| Albumin <ULN (%) | 34 (15) | 19 (19) | 15 (13) | .26 |
| LDH >ULN, n (%) | 78 (37) | 47 (47) | 31 (28) | <.01 |
| B2M elevated, n (%) | 53 (78) | 33 (89) | 20 (65) | .02 |
| Monoclonal protein, n (%) | 61 (39) | 29 (37) | 32 (41) | .62 |
| Treatment modality, n (%) | .55 | |||
| R monotherapy | 115 (48) | 59 (52) | 56 (43) | |
| Immunochemotherapy | 98 (41) | 41 (36) | 57 (44) | |
| Local | 21 (9) | 9 (8) | 12 (9) | |
| Others | 13 (3) | 4 (3) | 9 (4) |
| Characteristics . | Total (N = 242) . | CL+ (n = 113) . | CL– (n = 129) . | P value . |
|---|---|---|---|---|
| Age in y, median (range) | 65 (23-91) | 66 (23-91) | 65 (32-91) | .52 |
| Sex, n (%) | .20 | |||
| Male | 126 (52) | 64 (57) | 62 (48) | |
| Female | 116 (48) | 49 (43) | 67 (52) | |
| MZL subtype, n (%) | <.0001 | |||
| NMZL | 88 (36) | 31 (27) | 57 (44) | |
| SMZL | 83 (34) | 58 (51) | 25 (19) | |
| EMZL | 71∗ (29) | 24 (21) | 47 (36) | |
| ECOG PS, n (%) | .16 | |||
| 0-1 | 190 (93) | 91 (89) | 99 (96) | |
| ≥2 | 15 (7) | 11 (11) | 4 (4) | |
| Stage, n (%) | <.0001 | |||
| III | 39 (16) | 0 (0) | 39 (30) | |
| IV | 203 (84) | 113 (100) | 90 (70) | |
| B symptoms, n (%) | 49 (21) | 19 (18) | 30 (24) | .26 |
| BM involvement, n (%) | 137 (81) | 91 (91) | 46 (67) | <.0001 |
| Albumin <ULN (%) | 34 (15) | 19 (19) | 15 (13) | .26 |
| LDH >ULN, n (%) | 78 (37) | 47 (47) | 31 (28) | <.01 |
| B2M elevated, n (%) | 53 (78) | 33 (89) | 20 (65) | .02 |
| Monoclonal protein, n (%) | 61 (39) | 29 (37) | 32 (41) | .62 |
| Treatment modality, n (%) | .55 | |||
| R monotherapy | 115 (48) | 59 (52) | 56 (43) | |
| Immunochemotherapy | 98 (41) | 41 (36) | 57 (44) | |
| Local | 21 (9) | 9 (8) | 12 (9) | |
| Others | 13 (3) | 4 (3) | 9 (4) |
B2M, beta 2 microglobulin; BM, bone marrow; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; R, rituximab; ULN, upper limit of normal.
Boldface values indicate statistical significance.
EMZL breakdown per primary site: lung = 15, gastro-intestinal = 13, oculo-adnexal = 13, salivary = 2, BM only = 2, breast = 2, >1 site = 14.
The median PFS (mPFS) was 6.9 years (95% confidence interval [CI], 3.73-9.44) in the CL+ group compared with 4.4 years (95% CI, 3.21-8.67) in the CL– group which was not statistically significant (P = .39; supplemental Figure 1). The 3- and 5-year PFS estimates were 67% (95% CI, 57-75) and 57% (95% CI, 46-67) in the CL+ group compared with 63% (95% CI, 53-71) and 48% (95% CI, 38-58), respectively, in the CL– group (P = .39; supplemental Figure 1). In the univariate Cox model, factors associated with inferior PFS included age (hazard ratio [HR], 1.02; P = .009) and low albumin (HR, 1.88; P = .02), whereas receipt of first-line immunochemotherapy (compared to rituximab monotherapy; HR, 0.41; P = .0001) was associated with superior PFS (supplemental Table 2). After adjusting for these factors in a multivariable Cox model, presence of CL was not associated with statistically significant inferior PFS (HR, 0.76; 95% CI, 0.47-1.23; P = .26; supplemental Table 2).
Considering the significant clinical and treatment differences between the histologic subtypes of MZL, we examined the association between the presence/absence of CL and PFS in each histology separately. There was no significant difference in mPFS (in years) in patients with SMZL (6.9 vs 3.4; P = .16), EMZL (7.4 vs 8.7; P = .4), and NMZL (7.2 vs 4.6; P = .26) between the CL+ and CL– groups (supplemental Figure 2). When evaluating the outcomes by first-line systemic therapy, there was no significant difference in mPFS (in years) between CL+ and CL– groups among those who received rituximab monotherapy (6.9 vs 3.2; P = .09; supplemental Figure 3A) or immunochemotherapy (9.4 vs 8.7; P = .24; supplemental Figure 3B).
We then evaluated the outcomes of first-line therapy based on the MZL subtype. There was no significant difference in mPFS (in years) between CL+ and CL– groups in NMZL who received rituximab monotherapy (7.2 vs 2.1; P = .08; supplemental Figure 4A) or immunochemotherapy (not reached [NR] vs 6.7; P = .59; supplemental Figure 4B). A similar trend was noted in patients with SMZL who received rituximab monotherapy (6.9 vs 3.4; P = .81; supplemental Figure 5A) or immunochemotherapy (9.4 vs NR; P = .84; supplemental Figure 5B). Although there was no significant difference in mPFS (in years) between CL+ and CL– groups in patients with EMZL receiving first-line rituximab monotherapy (2.7 vs 3.4; P = .35; supplemental Figure 6A,B), patients with CL had significantly inferior mPFS (in years) compared with those without CL among the recipients of immunochemotherapy (9.4 vs NR; P < .01; supplemental Figure 6B).
There was no significant difference in DTI between the CL+ and CL– groups (P = .79; supplemental Figure 7). There was a total of 8 HT events in the study, 1 in the CL+ group and 7 in the CL– groups. The cumulative incidence of HT was not significantly different between the CL+ and CL– groups with 3- and 5-year rates of HT of 0.9% vs 2.5% and 0.9% vs 3.6%, respectively (P = .07; supplemental Figure 8).
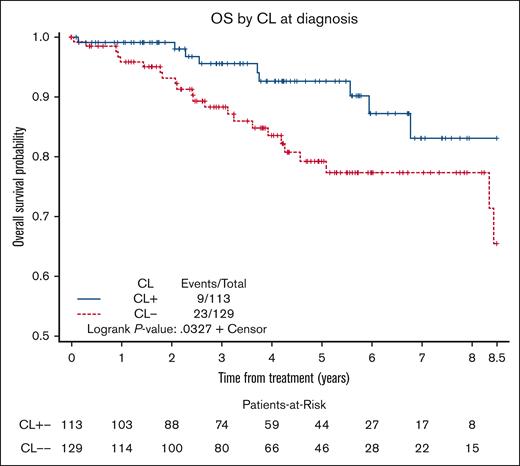
The median OS was NR in both CL+ and CL– groups. The 3- and 5-year OS estimates were 96% (95% CI, 89%-98%) and 93% (95% CI, 84-97) in the CL+ group compared with 88% (95% CI, 81-93) and 79% (95% CI, 69-86), respectively, in the CL– group (log-rank P = .03, Figure 1). In the univariate Cox model, factors associated with inferior OS included age (HR, 1.06; P = .0007), Eastern Cooperative Oncology Group performance status of ≥2 (HR, 4.67; P = .002), and low albumin (HR, 2.63; P = .02), whereas the presence of CL was associated with superior OS (HR, 0.44; P = .03). After adjusting for these factors in the multivariable Cox model, presence of CL remained associated with a significantly superior OS (HR, 0.36; 95% CI, 0.15-0.86; P = .02; supplemental Table 3).
OS in patients with MZL based on the presence or absence of CL at diagnosis.
In this multicenter retrospective cohort study, we evaluated the prognostic relevance of CL at diagnosis in patients with MZL and made several important observations. First, the presence of CL at diagnosis did not have a significant impact on PFS, regardless of the clinically determined SMZL and NMZL, however, PFS was actually numerically better among patients with CL. Second, the subset of patients with EMZL who had CL at diagnosis and received immunochemotherapy had inferior PFS compared with those without CL. Third, the presence of CL at diagnosis had better OS than that of no CL. Lastly, the presence of CL at diagnosis did not affect DTI or HT. To the best of our knowledge, this is the first in-depth study evaluating the effect of CL at diagnosis on outcomes in patients with MZL.
CL was previously investigated mainly in the context of SMZL, a disease that by definition involves the spleen, bone marrow, and, typically, blood to some extent. Examination of the blood by flow cytometry in MZL is not obtained consistently in clinical practice, so most prior studies did not consider it as a variable owing to the extent of missing data. Technically, the presence of lymphoma in the blood constitutes disseminated disease, that is, stage IV lymphoma, but blood involvement may often be inconspicuous, requiring flow cytometric analysis and may also be indistinguishable from a separate underlying monoclonal B-cell lymphocytosis (eg, in case of EMZL of mucosal sites). Our definition of CL assumes a biological relationship between MZL and circulating monoclonal lymphocytes in the blood, and we investigated CL as a prognostic factor. Other groups have explored factors that may influence treatment initiation and survival; however, CL was not included.8-10 We show that the presence of CL at diagnosis in patients with MZL was not associated with inferior PFS compared with those without CL in our study including the 3 MZL subtypes.
One exception to this general observation was the inferior PFS when using immunochemotherapy in patients with EMZL, which was driven by excellent outcomes (96% PFS at 5 years) among patients without CL who received immunochemotherapy. This may suggest that patients with EMZL with CL had somewhat different, perhaps more aggressive biology. Involvement of multiple mucosal sites has been identified as an alternative risk factor for worse survival in EMZL in the revised MALT-International Prognostic Index (IPI).11 In this context, CL may uncover this propensity for hematogenous spread, which can lead to shorter PFS. Alternatively, CL might be associated with unfavorable molecular or cytogenetic features that were not captured in our study. Currently, the guidelines from the National Comprehensive Cancer Network12 do not recommend PB flow cytometry for patients with newly diagnosed EMZL. However, our results suggest that in patients with EMZL for whom physicians are considering initiation of immunochemotherapy, obtaining PB flow cytometry to evaluate for CL may provide prognostic information. It might also help identify patients at risk for future recurrence despite seemingly localized EMZL.
In our study, we noted OS benefit among patients with MZL who had CL at diagnosis. Although the exact etiology is unclear, we postulate that patients with MZL with CL may be biologically different from those without CL, including their response to salvage therapies. More importantly, this underpins the need for a larger cohort to investigate this further. Previous studies that looked at the risk factors for high-grade transformation from MZL did not include the presence of CL at diagnosis.13-18 This study did not find a statistically significant difference between the 2 groups.
This study is subjected to the inherent limitations of a retrospective cohort including nonuniform selection of patients with MZL for the performance of PB flow cytometry at diagnosis and a small sample size of individual cohorts (such as MZL subtype and first-line systemic therapy) precluding the ability to perform multivariable analysis in these subgroups. Furthermore, we did not analyze the outcome between patients with CL and morphologic evidence of blood involvement vs those without. Of note, we only looked at the presence of CL as a prognostic factor and did not attempt to quantify the amount of CL present. Although there was no central pathology review, any ambiguous cases of low-grade B-cell lymphoma with plasmacytic differentiation not clearly fitting the MZL diagnosis were excluded.
In conclusion, in this study evaluating the prognostic relevance of CL in MZL, we found that the presence of CL at diagnosis did not impact the PFS negatively; on the contrary, it seemed to have numerically better PFS and was associated with significantly superior OS. In patients with EMZL treated with systemic therapies, especially immunochemotherapy, one may consider checking PB flow cytometry at diagnosis given the inferior PFS associated with CL in these patients. Future studies should explore the correlation of CL with other biological factors that impact the outcomes in patients with MZL.
Contribution: N.E. contributed to conception and design of the study; K.A., N.E., and R.L.W. analyzed the data; K.A. prepared the first draft of the manuscript; and all authors collected, assembled, and interpreted the data, provided critical and insightful comments, and gave final approval of the manuscript.
Conflict-of-interest disclosure: N.S.G. reports research funding from Genentech and Tessa Therapeutics, and honoraria/consulting/advisory board fees from ADC Therapeutics, Genentech, Kite, Novartis, Tessa Therapeutics, and Seattle Genetics. P.T. reports honoraria/consulting/advisory board fees from TG Therapeutics, ADC Therapeutics, Genentech, GenMab, and Lilly USA. B.C. reports research funding from Genentech, Acerta, Triphase, MorphoSys, Seagen, Millenium, Bristol Myers Squibb (BMS), and F. Hoffman-La Roche, and advisory board fees from Genentech and ADC Therapeutics. P.R.G. reports consultancy services to Kite Pharma, BMS, and Rafael Pharma, and serving on the advisory boards of Pharmacyclics LLC, ADC Therapeutics, Cellectar Biosciences, and Ono Pharma. R.K. received advisory board fees from BMS, Gilead Sciences/Kite Pharma, Janssen, Karyopharm, Pharmacyclics, MorphoSys, Epizyme, Genentech/Roche, EUSA, and Calithera; grants/research support from BMS, Takeda, BeiGene, Gilead Sciences/Kite, and Calithera; and speakers bureau fees from AstraZeneca, BeiGene, and MorphoSys. S.K.B. reports honoraria from Acrotech, Affimed, Daiichi Sankyo, Kyowa Kirin, Janssen, and Seagen. N.L.B. reports research funding from ADC Therapeutics, Autolus, BMS, Celgene, Forty Seven, Genentech, Immune Design, Janssen, Merck, Millennium, Pharmacyclics, Affirmed Therapeutics, Dynavax, Gilead, MedImmune, and Novartis, and consulting/ad board fees from Kite Pharma, Pfizer, ADC Therapeutics, Roche/Genentech, Seattle Genetics, BTG, and Acerta. A.J.O. reports funding from GenMab, Precision Bio, Adaptive Biotechnologies, Celldex, Acrotech Biopharma, Schrodinger, TG Therapeutics, and Genentech. N.E. reports research funding from BeiGene; speakers bureau fees from Incyte, BeiGene, and Novartis; and honoraria/consulting/advisory board fees from Merck, ADC Therapeutics, Lilly, Ipsen, and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Narendranath Epperla, Division of Hematology, Department of Medicine, The Ohio State University, Columbus, OH 43210; e-mail: narendranath.epperla@osumc.edu.
References
Author notes
All data are available on request from the corresponding author, Narendranath Epperla (Narendranath.Epperla@osumc.edu)
The full-text version of this article contains a data supplement.