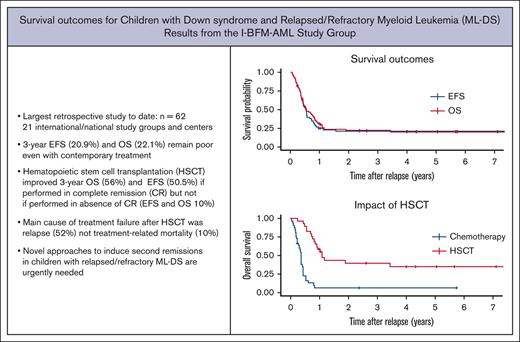
Key Points
Treatment outcomes for relapsed or refractory ML-DS remain dismal in a contemporary treatment period (3-year OS 22%).
Patients who had a long duration of first remission, achieved a second remission and underwent HSCT were most likely to survive.
Abstract
Children with Down syndrome (DS) are at a significantly higher risk of developing acute myeloid leukemia, also termed myeloid leukemia associated with DS (ML-DS). In contrast to the highly favorable prognosis of primary ML-DS, the limited data that are available for children who relapse or who have refractory ML-DS (r/r ML-DS) suggest a dismal prognosis. There are few clinical trials and no standardized treatment approach for this population. We conducted a retrospective analysis of international study groups and pediatric oncology centers and identified 62 patients who received treatment with curative intent for r/r ML-DS between year 2000 to 2021. Median time from diagnosis to relapse was 6.8 (range, 1.1-45.5) months. Three-year event-free survival (EFS) and overall survival (OS) were 20.9 ± 5.3% and 22.1 ± 5.4%, respectively. Survival was associated with receipt of hematopoietic stem cell transplantation (HSCT) (hazard ratio [HR], 0.28), duration of first complete remission (CR1) (HR, 0.31 for > 12 months) and attainment of remission after relapse (HR, 4.03). Patients who achieved complete remission (CR) before HSCT, had an improved OS and EFS of 56.0 ± 11.8% and 50.5 ± 11.9%, respectively compared to those who underwent HSCT without CR (3-year OS and EFS of 10.0 ± 9.5%). Treatment failure after HSCT was predominantly because of disease recurrence (52%) followed by treatment-related mortality (10%). The prognosis of r/r ML-DS remains dismal even in the current treatment period and serve as a reference point for current prognostication and future interventional studies. Clinical trials aimed at improving the survival of patients with r/r ML-DS are needed.
Introduction
Young children with Down syndrome (DS) have a 150-fold increased risk of developing myeloid leukemia.1 Myeloid leukemia associated with DS (ML-DS) has distinct clinical and biological features, such as a younger age of onset (<4 years), predominantly megakaryocytic blast phenotype2-7 and hypersensitivity of blasts to chemotherapeutic agents including, but not limited to, cytarabine.4,8,9 ML-DS is thought to have evolved from a subclone of transient abnormal myelopoiesis (TAM),10-13 the preleukemic accumulation of megakaryoblasts present in newborns with DS. Although DS-specific somatic mutations of GATA1 initiate TAM, cooperating mutations of cohesin complex genes and epigenetic regulators or signal transducers promote progression to ML-DS.3,14-21 A clinically striking feature of primary ML-DS is a significantly more favorable prognosis compared to that of acute myeloid leukemia (AML) in children without DS (5-year event-free survival [EFS]∼90%4,5,22-26 compared to 49% to 62% in pediatric non-DS AML27-31). In marked contrast, the few data that are available from individual study groups and previous treatment periods suggest that patients with relapsed or refractory ML-DS (r/r ML-DS) may have a very low probability of survival.4,5,26,32 It is not known whether the prognosis is uniformly poor with current treatment approaches and which factors are associated with survival.
The purpose of our study, therefore was to estimate the probability of survival for r/r ML-DS; to describe the spectrum of therapeutic interventions including hematopoietic stem cell transplantation (HSCT) and use of experimental agents; and to establish a contemporary reference point for prognostication and future intervention trials.
Methods
Patients
This project was coordinated through the International Berlin-Frankfurt-Münster AML Study Group (I-BFM-AML-SG). A total of 12 national pediatric AML study groups and 9 pediatric oncology centers in the United States and Canada identified eligible patients from their databases and medical records, respectively. Pediatric oncology centers in the United States and Canada were contacted directly (see Appendix for a list of participating study groups and centers). Patients treated with curative intent for r/r ML-DS between 1 January 2000 and 1 January 2021 were eligible. Diagnosis of primary ML-DS required the presence of constitutional trisomy 21 (or trisomy 21 mosaicism) and either age <4 years or, if older, the documentation of a somatic GATA1 mutation in the leukemic blasts. The Research Ethics Board at The Hospital for Sick Children, Toronto, Canada, and the institutional review boards of participating centers approved the study.
Data collection
Data regarding demographics (age, sex, and disease status), blasts (immunophenotype, cytogenetic, GATA1 mutational status, and sequencing results if available), time and site of relapse, intent and type of treatment, outcome (response to therapy, survival status, and treatment-related adverse events), use of HSCT and experimental agents, were collected using a standardized case report form.
Definitions and statistical analysis
Diagnosis of r/r ML-DS was based on the clinical, molecular, and histopathological assessment by participating study groups and centers (no additional central review was performed). Complete remission (CR) was defined as <5% morphological blasts in the bone marrow with regenerating normal hematopoietic cells. Overall survival (OS) was defined as the time from the diagnosis of r/r ML-DS until death from any cause. EFS was defined as the time from the diagnosis of first relapse until second relapse or death. For patients with refractory ML-DS, the duration of CR was considered zero. Survival probabilities were computed using Kaplan-Meier estimates,33 and cumulative incidence of relapse was calculated using the Aalen-Johansen estimator.34 Factors associated with OS and EFS were evaluated in a multivariable Cox proportional hazard model and described using hazard ratios (HR) with 95% confidence intervals (CIs). HSCT was treated as a time-dependent variable and also evaluated using a separate univariate Cox proportional hazard model. All tests were 2-tailed with a P< .05 considered significant. Analyses were performed using R (version 4.0.5).35
Results
Patient characteristics
A total of 78 patients with r/r ML-DS were identified. Sixteen patients were excluded from the analysis owing to incomplete data (n = 1), diagnostic uncertainty (age at diagnosis of primary myeloid leukemia >4 years without documentation of a somatic GATA1 mutation, n = 6), or treatment of r/r ML-DS without curative intent (n = 9). A total of 62 patients were included in the analysis. Patient characteristics are shown in Table 1. Most patients received ≥4 cycles of chemotherapy during treatment for primary ML-DS including an average of 2 doses of intrathecal chemotherapy. Relapse of ML-DS occurred after a median interval of 6.8 months from initial diagnosis (range, 1.1-45.5 months) and 82% of events occurred within 12 months of initial diagnosis. Relapses almost exclusively occurred in the bone marrow (98% of patients) with no central nervous system relapses. Cytogenetic data were available in 31 patients from both the blasts of primary and r/r ML-DS. At diagnosis of r/r ML-DS there was no increase in monosomy 7. Numerical or structural abnormalities of chromosome 17 were absent in all patients at primary diagnosis but present in 7 patients at the time of r/r ML-DS diagnosis (Table 2). Nine patients (15%) had refractory ML-DS (Table 1). Approximately half of all patients with r/r ML-DS (n = 33, 53%) were treated with chemotherapy alone and remaining half (n = 29, 47%) also underwent HSCT.
Patient characteristics and outcomes
| Patient characteristics . | n . | % . |
|---|---|---|
| 62 | ||
| Primary ML-DS | ||
| Age (mo) | 23.4 (7.3-63.8)∗ | |
| Male/female | 1.29 (35/27) | |
| Constitutional trisomy 21 | 57 | 92 |
| Trisomy 21 mosaicism | 5 | 8 |
| History of transient abnormal myelopoiesis | ||
| Yes | 19 | 31 |
| No | 30 | 48 |
| Not available | 13 | 21 |
| WBC (×109/L) | 6 (2-88)∗ | |
| Extramedullary involvement | ||
| CNS | 1 | 2 |
| Bone | 1 | 2 |
| Liver/spleen | 1 | 2 |
| Blast cytogenetics‡ | ||
| Structurally complex, not monosomal | 18 | 29 |
| Structurally complex and monosomal | 4 | 6 |
| Monosomal, not structurally complex | 2 | 3 |
| Other structural abnormalities | 7 | 11 |
| Acquired trisomy (other than chromosome 21) | 6 | 10 |
| No acquired aberration | 8 | 13 |
| Not available | 17 | 27 |
| GATA1 mutation | ||
| Present | 36 | 58 |
| Absent/not available | 26 | 42 |
| Treatment included at least 1 course of high-dose cytarabine | 51 | 82 |
| Cumulative anthracycline dose (doxorubicin equivalents, mg/m2) | 195† | |
| Doses of intrathecal chemotherapy | 2† | |
| Relapsed/refractory ML-DS | ||
| Refractory | 9 | 15 |
| Relapse | 53 | 85 |
| Time from diagnosis of primary ML-DS to first relapse (mo) | 6.8 (1-45)∗ | |
| Time from first remission to relapse (mo) | 5.5 (0-43)∗ | |
| Blast percentage in the bone marrow | 23.5† | |
| Site of relapse | ||
| Bone marrow | 61 | 98 |
| Extramedullary§ | 1 | 2 |
| Number of relapse events | ||
| One | 42 | 68 |
| More than 1 | 11 | 18 |
| Second remission after treatment for first relapse | ||
| No | 34 | 55 |
| Yes | 28 | 45 |
| Treatment | ||
| Chemotherapy alone | 33 | 53 |
| Chemotherapy and HSCT | 29 | 47 |
| Outcomes | ||
| Alive | 14 | 23 |
| in remission | 14 | 23 |
| time interval since first relapse (mo) | 56.1 (2.6-145)∗ | |
| Died | 48 | 77 |
| Likely reason for death | ||
| progressive disease | 42 | 68 |
| treatment-related mortality | 6 | 10 |
| time interval since first relapse to death (mo) | 5.1 (0.4-41)∗ | |
| 3-year overall survival (%) | 22.1 ± 5.4 (95% CI, 13.7-35.8) | |
| 3-year event-free survival (%) | 20.9 ± 5.3 (95% CI, 12.7-34.3) | |
| 3-year cumulative incidence of relapse (%) | 79.1 ± 5.3 (95% CI, 65.7-87.3) | |
| Patient characteristics . | n . | % . |
|---|---|---|
| 62 | ||
| Primary ML-DS | ||
| Age (mo) | 23.4 (7.3-63.8)∗ | |
| Male/female | 1.29 (35/27) | |
| Constitutional trisomy 21 | 57 | 92 |
| Trisomy 21 mosaicism | 5 | 8 |
| History of transient abnormal myelopoiesis | ||
| Yes | 19 | 31 |
| No | 30 | 48 |
| Not available | 13 | 21 |
| WBC (×109/L) | 6 (2-88)∗ | |
| Extramedullary involvement | ||
| CNS | 1 | 2 |
| Bone | 1 | 2 |
| Liver/spleen | 1 | 2 |
| Blast cytogenetics‡ | ||
| Structurally complex, not monosomal | 18 | 29 |
| Structurally complex and monosomal | 4 | 6 |
| Monosomal, not structurally complex | 2 | 3 |
| Other structural abnormalities | 7 | 11 |
| Acquired trisomy (other than chromosome 21) | 6 | 10 |
| No acquired aberration | 8 | 13 |
| Not available | 17 | 27 |
| GATA1 mutation | ||
| Present | 36 | 58 |
| Absent/not available | 26 | 42 |
| Treatment included at least 1 course of high-dose cytarabine | 51 | 82 |
| Cumulative anthracycline dose (doxorubicin equivalents, mg/m2) | 195† | |
| Doses of intrathecal chemotherapy | 2† | |
| Relapsed/refractory ML-DS | ||
| Refractory | 9 | 15 |
| Relapse | 53 | 85 |
| Time from diagnosis of primary ML-DS to first relapse (mo) | 6.8 (1-45)∗ | |
| Time from first remission to relapse (mo) | 5.5 (0-43)∗ | |
| Blast percentage in the bone marrow | 23.5† | |
| Site of relapse | ||
| Bone marrow | 61 | 98 |
| Extramedullary§ | 1 | 2 |
| Number of relapse events | ||
| One | 42 | 68 |
| More than 1 | 11 | 18 |
| Second remission after treatment for first relapse | ||
| No | 34 | 55 |
| Yes | 28 | 45 |
| Treatment | ||
| Chemotherapy alone | 33 | 53 |
| Chemotherapy and HSCT | 29 | 47 |
| Outcomes | ||
| Alive | 14 | 23 |
| in remission | 14 | 23 |
| time interval since first relapse (mo) | 56.1 (2.6-145)∗ | |
| Died | 48 | 77 |
| Likely reason for death | ||
| progressive disease | 42 | 68 |
| treatment-related mortality | 6 | 10 |
| time interval since first relapse to death (mo) | 5.1 (0.4-41)∗ | |
| 3-year overall survival (%) | 22.1 ± 5.4 (95% CI, 13.7-35.8) | |
| 3-year event-free survival (%) | 20.9 ± 5.3 (95% CI, 12.7-34.3) | |
| 3-year cumulative incidence of relapse (%) | 79.1 ± 5.3 (95% CI, 65.7-87.3) | |
median and range
median
structurally complex karyotype defined as ≥3 chromosomal aberrations, including at least 1 structural aberration
chloroma
Cytogenetics of primary and relapsed/refractory ML-DS blasts
| Cytogenetic abnormalities . | Primary ML-DS (n = 31) . | Relapsed/refractory ML-DS (n = 31) . | |
|---|---|---|---|
| Trisomy 8 | 8 | 10 | |
| Trisomy 21, acquired | 6 | 6 | |
| Chromosome 17 | Monosomy 17 | 0 | 5 |
| del(17p) | 0 | 2 | |
| Chromosome 7 | Monosomy 7 | 3 | 4 |
| i(7)(q10) | 3 | 4 | |
| del(7p) | 1 | 1 | |
| Cytogenetic abnormalities . | Primary ML-DS (n = 31) . | Relapsed/refractory ML-DS (n = 31) . | |
|---|---|---|---|
| Trisomy 8 | 8 | 10 | |
| Trisomy 21, acquired | 6 | 6 | |
| Chromosome 17 | Monosomy 17 | 0 | 5 |
| del(17p) | 0 | 2 | |
| Chromosome 7 | Monosomy 7 | 3 | 4 |
| i(7)(q10) | 3 | 4 | |
| del(7p) | 1 | 1 | |
Among 31 patients who had paired karyotypes available from both time-points, 18 gained abnormalities, 2 lost abnormalities, and 11 showed no change at the time of diagnosis of r/r ML-DS compared to those at the primary diagnosis. The table summarizes the subset of changes present in more than 1 patient.
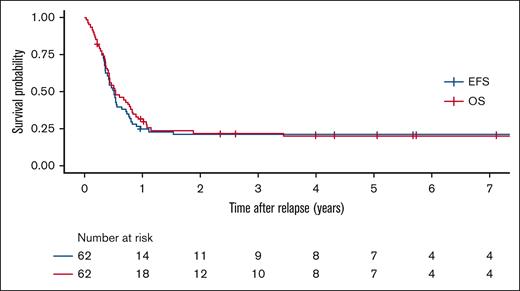
Outcomes
Across all treatment attempts, 28 patients (45%) achieved remission. Of those, 10 patients developed a subsequent relapse. The probability of OS, EFS and cumulative incidence of relapse (± standard error) at 3 years of the entire cohort of patients with r/r ML-DS were 22.1% (±5.4%), 20.9% (±5.3%), and 79.1% (± 5.3%), respectively (Figure 1). In the patients who did not survive, the median interval from relapse or refractory disease to death was 5.1 (range, 0.4-41.0) months. In multivariable analysis (n = 61), duration of first remission (CR1), achievement of a second remission (CR2), and use of HSCT were significantly associated with improved OS and EFS (Table 3). Patients who achieved CR after relapse (CR2) had improved survival (3-year OS, 46.4 ± 9.9%; and 3-year EFS, 43.3 ± 9.7%), compared with patients who failed to achieve CR2 (3-year OS and EFS of 2.94 ± 2.9%) (supplemental Figure 1). Age at primary diagnosis of ML-DS, sex, GATA1 mutational status, history of transient abnormal myelopoiesis, disease status (relapse vs. refractory ML-DS), number of relapses, cumulative dose of anthracyclines and use of high-dose cytarabine during treatment for primary disease were not associated with probability of survival in univariate analysis (supplemental Table 1).
Survival outcomes of patients with relapsed or refractory ML-DS. Overall survival (red), and event-free survival (blue)
Survival outcomes of patients with relapsed or refractory ML-DS. Overall survival (red), and event-free survival (blue)
Multivariable analysis of prognostic factors for relapsed or refractory ML-DS
| Variable . | n 61 . | Overall survival . | Event-free survival . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| Duration of CR1 | .029 | .013 | |||
| 1 d–5.99 mo | 29 | ref | ref | ||
| 6-12 mo | 18 | 0.49 (0.24-0.99) | 0.40 (0.20-0.83) | ||
| >12 mo | 5 | 0.31 (0.09-1.06) | 0.28 (0.08-0.95) | ||
| 0 (refractory) | 9 | 0.38 (0.16-0.89) | 0.36 (0.15-0.86) | ||
| Treated with HSCT | <.001 | .002 | |||
| No | 32 | ref | ref | ||
| Yes | 29 | 0.28 (0.14-0.54) | 0.35 (0.18-0.69) | ||
| Achieved second remission after treatment for first relapse | <.001 | <.001 | |||
| Yes | 27 | ref | ref | ||
| No | 34 | 4.03 (1.93-8.41) | 3.58 (1.68-7.62) | ||
| Variable . | n 61 . | Overall survival . | Event-free survival . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| Duration of CR1 | .029 | .013 | |||
| 1 d–5.99 mo | 29 | ref | ref | ||
| 6-12 mo | 18 | 0.49 (0.24-0.99) | 0.40 (0.20-0.83) | ||
| >12 mo | 5 | 0.31 (0.09-1.06) | 0.28 (0.08-0.95) | ||
| 0 (refractory) | 9 | 0.38 (0.16-0.89) | 0.36 (0.15-0.86) | ||
| Treated with HSCT | <.001 | .002 | |||
| No | 32 | ref | ref | ||
| Yes | 29 | 0.28 (0.14-0.54) | 0.35 (0.18-0.69) | ||
| Achieved second remission after treatment for first relapse | <.001 | <.001 | |||
| Yes | 27 | ref | ref | ||
| No | 34 | 4.03 (1.93-8.41) | 3.58 (1.68-7.62) | ||
REF reference value.
Chemotherapy
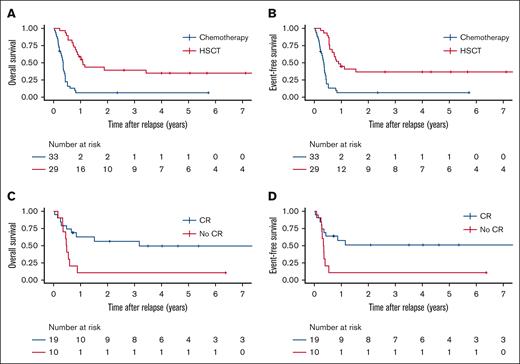
A range of chemotherapy regimens were used (median number of cycles 2, range, 1-7; supplemental Table 2). Among 33 patients treated with chemotherapy alone, 27 (82%) did not achieve remission and died of progressive leukemia after a median of 4.1 months from the diagnosis of r/r ML-DS (supplemental Table 3). Three patients (9%) died of infection or sepsis. The 3 patients who survived after treatment with chemotherapy alone, received an average of 3 cycles of chemotherapy (consisting of standard dose cytarabine and anthracycline). Both 3-year OS and EFS for patients treated with chemotherapy alone was 6.4% (±4.3%) (Figure 2A-B; supplemental Table 3). Most patients (45%) received a course of high-dose cytarabine combined with fludarabine (FLAG) with or without an anthracycline or high-dose cytarabine with or without an anthracycline during the first treatment attempt for r/r ML-DS (supplemental Table 2). FLAG was also the most commonly used regimen during the second treatment attempt followed by high-dose cytarabine combined with etoposide (with or without anthracycline). Among the 14 survivors (23% of all patients, including those treated with HSCT), cytarabine (high/standard dose), anthracycline ± fludarabine had been used in 13 patients during the first or second treatment attempt. Novel agents were used infrequently. Three patients received venetoclax during their first treatment attempt and did not achieve remission. Four patients were treated with hypomethylating agents (azacytidine or decitabine) after change to a palliative intent.
Survival outcomes of patients with relapsed or refractory ML-DS according to treatment. (A) OS and (B) EFS for patients treated with chemotherapy alone or chemotherapy followed by hematopoietic stem cell transplantation (HSCT, analyzed time-dependent covariate). (C) OS and (D) EFS for patients with relapsed or refractory ML-DS according to remission status before HSCT.
Survival outcomes of patients with relapsed or refractory ML-DS according to treatment. (A) OS and (B) EFS for patients treated with chemotherapy alone or chemotherapy followed by hematopoietic stem cell transplantation (HSCT, analyzed time-dependent covariate). (C) OS and (D) EFS for patients with relapsed or refractory ML-DS according to remission status before HSCT.
HSCT
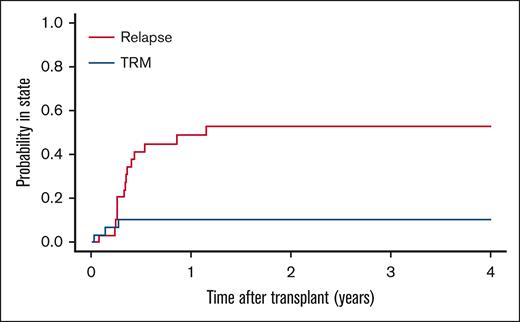
There were 29 patients (47%) who received HSCT (supplemental Table 4). The median interval from diagnosis of relapse to transplantation was 3.6 (range, 2.3-7.1) months. A second remission after treatment for first relapse was achieved in 22 patients (76%) and maintained at the time of HSCT in 19 patients (66%). Out of 10 patients (34%) who were tested before HSCT for minimal residual disease, 6 were positive according to local criteria. A variety of donor types, stem cell sources, and preparative regimens were used. After a median follow-up of 60 months (range, 11-145), 11 patients (38%) were alive with no evidence of disease, 15 patients (52%) had died of recurrent leukemia, and 3 patients (10%) experienced treatment-related adverse events (infection, 1; graft-versus-host disease, 1; pulmonary hypertension, 1) (Figure 3). Of the 19 patients who were in morphological remission before HSCT, 6 patients (32%) relapsed after HSCT and died of progressive disease. Of the 10 patients who were not in remission at the time of HSCT, 9 patients (90%) relapsed and died of progressive leukemia.
Cause of treatment failure after HSCT for relapsed or refractory ML-DS. Cumulative incidence of subsequent relapse and treatment-related mortality.
Cause of treatment failure after HSCT for relapsed or refractory ML-DS. Cumulative incidence of subsequent relapse and treatment-related mortality.
Compared to patients treated with chemotherapy alone those who underwent HSCT (treated as a time-dependent covariate) had a higher 3-year OS and EFS of 39.8% (±9.3%) and 36.7% (±9.2%), respectively, with an HR of 0.48 (OS as a function of HSCT, 95% CI, 0.23-1.02; P = .057) favoring HSCT (Figure 2A-B). Patients who underwent HSCT in the absence of CR had a significantly lower 3-year OS (10% ± 9.5% vs 56.0% ± 11.8%; HR = 3.12 [95% CI, 1.19-8.16; P = .023]) and EFS (10% ± 9.5% vs 50.5 ± 11.9%; HR = 3.09 [95% CI, 1.19-8.07; P = .023]) than those who received transplant at the time of CR (Figure 2C-D). Treatment period (years 2000-2010 vs 2010-2022) had no significant association with OS and EFS (supplemental Figure 2).
Discussion
Although recent trials have established the excellent prognosis of primary ML-DS (EFS, 83% to 89%),4,5,24,26 even when treated with chemotherapy of reduced intensity,4 it is less clear whether patients with r/r ML-DS respond to salvage therapy. A retrospective study of 26 patients recruited over a 10-year period by the JPLSG in Japan observed initial remission in 50% of patients with r/r ML-DS which proved long lasting in 4 of 5 patients treated with chemotherapy alone, suggesting response to relapse chemotherapy.32 The 3-year OS was 25.9 ± 8.5%.32 In contrast, in the ML-DS study conducted in 2006 by the BFM, NOPHO, and DCOG study groups, 7 of 9 patients who relapsed, died.5 Similarly, COG studies for primary ML-DS reported an OS of 20% (at 5-year, AAML0431, recalculated for patients with relapse,4 T. Alonzo, personal communication) and 16% (at 1 year, AAML1531)36 for patients with r/r ML-DS. Most patients who relapsed, did so within the first year from initial diagnosis of ML-DS32,36 and duration of first remission was inversely correlated with OS.32,36
A reliable estimate of the salvageability of patients with relapsed ML-DS is key for prognostic counseling of families and clinical decision making. It also affects the design of clinical trials for primary ML-DS because a low salvageability after relapse would limit further efforts to reduce treatment intensity of primary therapy in order to lower the high treatment-related mortality observed in early studies.37 Because there are neither reported clinical trials nor a standard treatment protocol for r/r ML-DS, we collected data from international AML study groups and cooperating pediatric oncology centers for the largest cohort of patients with r/r ML-DS reported to date. We found that even during the current treatment period (2000-2021) the probability of survival for patients with r/r ML-DS remains strikingly low (3-year OS, 22.1%). This result sets up a difficult dichotomy for families of children diagnosed with primary ML-DS, who now have to be informed that although responders have a much better prognosis than patients with non-DS AML, those who develop r/r ML-DS have a very low probability of survival, in fact lower than that of children with r/r non-DS AML (3-year OS, 40%) and more comparable to early relapse of non-DS AML.38,39
There was no standard chemotherapy regimen for treatment of r/r ML-DS. Extrapolating from the treatment of AML relapse in patients without DS39 most patients were treated with a cytarabine combined with fludarabine–based regimen during the first or second treatment attempt for r/r ML-DS whereas the use of anthracyclines and etoposide was variable. Experimental agents, such as venetoclax, were used sparingly (5%) and hypomethylating agents (azacytidine/decitabine) were only used with palliative intent (supplemental Table 2). In contrast to reports describing a favorable response of patients with r/r ML-DS who achieve a second remission to further treatment with chemotherapy alone (4 survivors among 5 patients32), in our cohort only 6 of 33 patients achieved a second remission after chemotherapy alone and only 3 of them survived (supplemental Table 3). In keeping with this report, failing to achieve a second remission was a significant prognostic factor for OS (HR, 4.03, Table 2; supplemental Figure 1), highlighting the need to include patients with r/r-ML-DS among those eligible for early phase AML relapse trials and to develop an international study to evaluate novel agents for this highly aggressive form of leukemia.
Few data are available regarding the role of HSCT in the treatment of r/r ML-DS. A retrospective study by the JPLSG showed that only 2 of 8 patients who received HSCT while in CR survived.32 Four of 6 patients who underwent HSCT in the absence of morphological remission died of recurrent disease.32 In a CIBMTR registry study of 28 patients with r/r ML-DS who underwent transplant, with half the cohort in second remission, 3-year disease free survival was only 14% with subsequent relapse being the main cause of treatment failure (61% at 3 years).40 In our cohort, 29 patients who underwent HSCT had improved survival. The proportion of patients who died of progressive disease was higher in those treated with chemotherapy alone compared with those treated with HSCT (82% vs 52%) suggesting that currently available chemotherapy is not sufficient to eradicate the r/r ML-DS clone. Prognosis remained particularly dismal for patients proceeding to HSCT with active disease (9 of 10 relapsed after HSCT; Figure 2C-D), arguing against this approach. There was no apparent difference in treatment-related mortality between those treated with chemotherapy alone and those undergone HSCT (10% vs 9%; supplemental Tables 3 and 4). The higher OS of patients treated with HSCT in our study may be attributable to a lower rate of treatment-related mortality (10% vs 25%40) because the proportion of patients who were in remission before HSCT was similar (66% vs 62%40). However, even the patients who underwent HSCT in morphologic remission had ∼30% risk of relapse after HSCT, highlighting both the need for new treatments to achieve a deeper remission before HSCT and the opportunity to evaluate assessment tools, such as minimal residual disease.
Although, to the best of our knowledge, we report outcomes for the largest cohort of children with r/r ML-DS to date, we need to highlight the limitations of our study. Because relapse of ML-DS is rare even an international and multicenter data collection resulted in a small sample size that does not allow for subgroup analysis. Race or ethnicity was not assessed in our study. Lack of randomized use of chemotherapy vs transplant introduces confounding by indication, because some patients who were treated with chemotherapy alone may have been HSCT candidates before death from relapse. To avoid immortal-time bias, we used time of HSCT as a time-dependent covariate. Furthermore, we found significant heterogeneity of chemotherapy and HSCT approaches. Although capturing this heterogeneity, as a call for action, was an objective of this study it also is a caveat for the conclusions drawn. Elucidating the molecular mechanisms of ML-DS relapse is another interesting goal that was outside the scope of this study.
In summary, we conclude that the probability of survival for patients with r/r ML-DS is low, in striking contrast to the prognosis of both primary ML-DS and relapsed AML in children without DS, and that those patients who underwent HSCT had a higher probability of survival but only if remission could be achieved before the procedure. Treatment for r/r ML-DS must aim for attainment of CR, followed by HSCT. Results provide practitioners with information for prognostic counseling of families and trial designers with a reference point for future intervention studies.
Acknowledgments
The authors thank the International Berlin-Frankfurt-Münster AML Study Group for their support and help establishing contact with regional AML study groups and centers, the tireless people who helped to obtain ethics board approvals and put data transfer agreements in place, and the individuals who carefully completed the data entry.
Authorship
Contribution: N.R. and J.H. designed the study, organized and performed research, collected and analyzed the data, and wrote the manuscript; B.M. performed statistical analysis; A.H. analyzed cytogenetic data; L.S. designed the study; K.N., S.R., E.A., T.S., P.M., A.V., K.R.R., C.A., R.S.K., D.C., K.J., A.K., W.B., A.N., M.O., S.C., A.C., N.A.-C., B.G., M.R.V., H.A., J.B., E.A.K., F.L., D.H., J.-H.K., H.H., provided clinical data; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Johann Hitzler, Division of Haematology/Oncology, The Hospital for Sick Children, 555 University Ave, Toronto, ON, M5G 1X8 Canada; e-mail: johann.hitzler@sickkids.ca.
References
Author notes
Data are available on request from corresponding author, Johann Hitzler (johann.hitzler@sickkids.ca).
The full-text version of this article contains a data supplement.