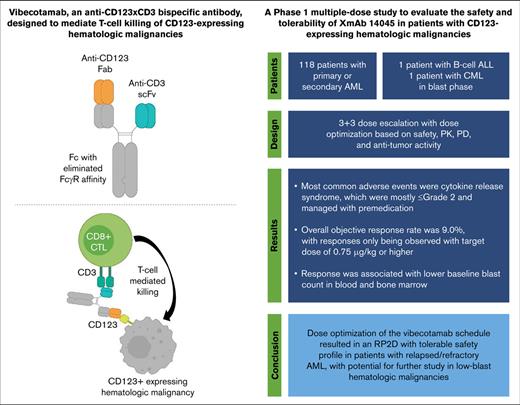
Key Points
A dose-optimized schedule of priming, step-up, and target vibecotamab doses mitigated CRS while achieving response.
Low blast-count AML and low PD-1 expression on CD4+ and CD8+ T cells were associated with response to vibecotamab.
Abstract
Acute myeloid leukemia (AML), an aggressive malignancy with unmet medical need, lacks immunotherapeutic options. CD123, the cellular receptor for interleukin-3, expressed in AML is an attractive target for tumor-specific therapy. Vibecotamab (XmAb14045), a humanized bispecific antibody, monovalently binds both CD3 and CD123 to recruit cytotoxic T cells to kill CD123+ tumor cells. This phase 1 study’s primary objectives were safety and tolerability and identification of a maximum tolerated dose/recommended dose for use as monotherapy in patients with relapsed/refractory AML. Identification of a recommended phase 2 vibecotamab dose comprised 3 step-up doses (Week 1), which were noted to reduce cytokine response syndrome (CRS), followed by weekly dosing (1.7 μg/kg, Cohort -1D). In 16 of 120 patients, at least 1 treatment-emergent adverse event was classified as a dose-limiting toxicity. CRS, the most common adverse event (59.2%), managed with premedication, were mostly ≤grade 2. A secondary objective was assessment of efficacy in patients with CD123-expressing leukemias. A total of 10 of 111 (9.0%) efficacy-evaluable patients with AML achieved an overall response of morphologic leukemia-free state or better with an overall objective response rate (ORR) of 9.0%. Response was only observed in patients receiving a target dose of 0.75 μg/kg or higher (n = 87) in which the efficacy-evaluable ORR was 11.5%. Response was associated with lower baseline blast counts in blood and bone marrow (<25%) suggesting potential benefit. This trial was registered at www.clinicaltrials.gov as #NCT02730312.
Introduction
Acute myeloid leukemia (AML), an aggressive malignancy resulting from the lack of differentiation and abnormal proliferation of hematopoietic stem cells, was diagnosed in ∼20 000 new cases in the United States in 2022. The median age at diagnosis was 68 years.1 These older patients may not benefit from intensive treatment approaches because of comorbidities and general frailty. For patients failing frontline therapy, prognosis is poor.2 The outcome of relapsed AML is dependent on a number of factors including age, duration of first remission, cytogenetics and molecular features, and performance of an allogeneic stem cell transplantation (SCT) during first complete response (CR).3 Previous trials using cytotoxic agents were largely unable to improve outcomes significantly,4 but advancements in molecularly targeted agents have improved outcomes in some patient subsets.5,6
CD123, an α subunit of interleukin-3 receptor (IL-3R), is found on a variety of hematologic cell types, including stem cells, plasmacytoid dendritic cells, monocytes, macrophages, B lymphocytes, and basophils.7
CD123 is expressed in high levels in AML, B-cell acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), hairy-cell leukemia (classic and variant), blastic plasmacytoid dendritic cell neoplasm (BPDCN), and myelodysplastic syndrome (MDS).8,9 AML that overexpresses CD123 seems to have constitutively higher proliferation rates, increased resistance to apoptosis induced by growth factor deprivation, increased cellularity at diagnosis, and a poorer prognosis; this may be because of the overexpressed IL-3R (and granulocyte-macrophage colony-stimulating factor receptor) being functional and leading to higher sensitivity to growth factor–induced proliferation responses.10 Because normal hematopoietic stem cells possess low expression of CD123, targeting CD123 may be a promising new strategy for patients with AML.7
Vibecotamab (also known as XmAb14045) is a humanized bispecific antibody (bsAb) that monovalently binds both CD3 and the tumor antigen CD123 to recruit cytotoxic T cells to kill CD123+ tumor cells. Vibecotamab was designed to maintain full-length humanized monospecific antibody properties, allowing development of stable molecules with a favorable in vivo half-life using standard antibody production methods. Monovalency for CD3 was a critical design constraint because bivalent binding of CD3 could potentially result in T-cell activation in the absence of CD123+ target cells. Vibecotamab in CD123-expressing malignancies has shown potent dose-dependent killing of CD123+ tumor cells in vitro and in vivo, and, in cynomolgus monkeys, demonstrates potency in depleting CD123+ basophils in both the peripheral blood and bone marrow.11 Vibecotamab is expected to strongly and specifically induce T-cell–mediated lysis of CD123-expressing tumor cells in patients.
This phase 1, first-in-human, dose-escalation study, investigated vibecotamab as a targeted monotherapy for AML, B-cell ALL, and CML.
Methods
Study design
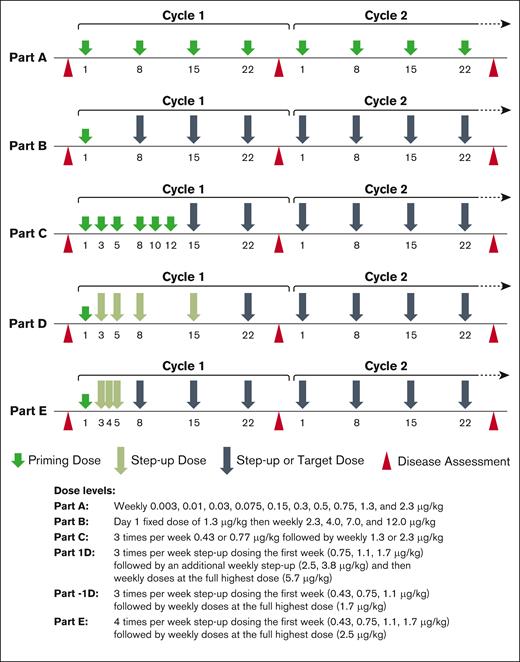
This study (#NCT02730312) comprised 5 parts (Figure 1). The primary objectives and end points were to determine the safety and tolerability profile and define a maximum tolerated dose/recommended dose. An additional end point was to determine a recommended phase 2 dose (RP2D). The secondary objectives were to characterize the pharmacokinetics (PKs) and immunogenicity of vibecotamab, and to preliminarily assess antitumor activity (measured by objective response rates [ORR], duration of response [DoR], progression-free survival [PFS], and overall survival [OS]). The study was conducted at participating centers in the United States for a duration of 60 months (18 August 2016 to 18 September 2021).
Dosing schedule by study part. Study design for Parts A to E, and respective dose and regimen.
Dosing schedule by study part. Study design for Parts A to E, and respective dose and regimen.
The study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice, and the protocol was approved by the institutional review board at participating sites. Written informed consent was provided by all patients before screening and enrollment. A dose-escalation review committee provided recommendations after review of safety data for each cohort. The study sponsor analyzed the data and conducted the statistical analyses. All authors had access to the primary clinical trial data on request.
Patients
A total of 120 adult patients were enrolled.
Patients who were eligible had primary or secondary AML, B-cell ALL, BPDCN, or CML in blast phase, relapsed or refractory disease with no available therapy, and an Eastern Cooperative Oncology Group performance status score of 0 to 2. Excluded from the study were patients with known uncontrolled central nervous system involvement by malignant disease; aspartate aminotransferase or alanine aminotransferase of >3.0 × upper limit of normal (ULN) at screening, bilirubin of >1.5 × ULN; and serum creatinine of >2.0 × ULN or estimated creatinine clearance of <40 mL/min calculated by either the Cockcroft-Gault or Modification of Diet in Renal Disease equations.
Treatment
Vibecotamab was administered IV as a 2-hour infusion followed by a 4-hour observation period. Premedication (dexamethasone, 10-20 mg IV; acetaminophen, 650 mg orally; and diphenhydramine, 25 mg orally/IV) was required before each vibecotamab dose for all patients who were enrolled after the 0.03 μg/kg dose in Part A (Figure 1). In the event of an infusion-related reaction (iRR) or cytokine release syndrome (CRS), the infusion was halted and the appropriate site-led management commenced as per guidelines.12,13
The initial study treatment was for 2 cycles (8 weeks). In the absence of unacceptable toxicity, patients received additional cycles of therapy if they had attained CR, complete remission with incomplete recovery of blood counts (CRi), morphologic leukemia-free state (MLFS), or partial response (PR) as defined by the 2017 European LeukemiaNet criteria for AML (as assessed by the investigator).14
Safety and tolerability assessments
Study assessments (history, physical examination, vital signs, Eastern Cooperative Oncology Group performance, 12-lead electrocardiogram, chemistry panel, hematology, coagulation, and urinalysis) were performed at screening, during treatment cycles 1 and 2, and after treatment, as appropriate. All adverse events (AEs) and serious adverse events (SAEs) were graded per National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03) except for CRS, which was graded by the Revised CRS Grading System13 with modification for hepatic toxicity per the Penn grading scale,15 and coded using the Medical Dictionary for Regulatory Activities 24.0 by system organ class (SOC), preferred term, severity, and relationship to vibecotamab.
PKs and immunogenicity assessments
Serum samples, before and after dose administration, were collected for PK assessments. For PK assessments and methods, refer to supplemental Appendix.
Pharmacodynamic assessments
The following tests were performed for pharmacodynamics (PD): IL-6 levels; CD123 expression by flow cytometry; T lymphocyte, B lymphocyte, and natural killer cell flow assay; and batch testing of bone marrow mononuclear cells and peripheral blood mononuclear cells by flow cytometry.
IL-6 cytokine levels in serum were measured by Luminex assay (R&D Systems, Minneapolis, MN).
Baseline and serial flow cytometry assessment of depletion of CD123-expressing cell populations (including tumor cells, leukemic stem cells, and basophils) during therapy, and degree of recovery at 4 weeks after therapy were evaluated.
Lymphocyte counts, CD3+ cells, CD4+ cells, and CD8+ cells in the blood were obtained at pretreatment, screening, and on day –1; during cycle 1 on days 1, 2, 4, 8, 15, and 22; during cycle 2 on days 1, 8, 15, and 22; at the end of treatment; and 14 and 28 days after the end of treatment.
Statistical analysis
The analysis for safety end points was performed using the safety analysis patient subset, which consisted of all patients who were enrolled in each cohort and received at least 1 infusion of any amount of vibecotamab.
AEs and SAEs were summarized separately by treatment cohort, and have been presented as the number and percentage of patients having any event, a related event, an event leading to withdrawal, or an event of grade ≥3.
The efficacy analysis subset of patients consisted of all patients who were enrolled in the study and received at least 1 infusion of any amount of vibecotamab. This analysis set was used to summarize ORR, DoR, PFS, and OS. For ORR, the point estimate, and corresponding confidence interval (CI) were calculated using the Clopper-Pearson exact method. DoR, PFS, and OS were analyzed using the Kaplan–Meier (K-M) method.
PK parameters were estimated from the serum concentration-time data using Phoenix WinNonlin version 8.0.0.3716 (Certara, Princeton, NJ).
For PD analyses, comparisons were performed using Wilcoxon rank-sum test.
Results
Patient characteristics
Most patients were male (n = 62 vs n = 58 female), with a median age of 64 years (Table 1).
Demographics of overall study population
| . | Part A (N = 55) . | Part B (N = 26) . | Part C (N = 14) . | Part D (N = 22) . | Part E (N = 3) . | Overall (N = 120) . |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| n | 55 | 26 | 14 | 22 | 3 | 120 |
| Median (min, max) | 61.0 (20, 85) | 68.0 (18, 74) | 63.0 (48, 83) | 70.5 (28, 84) | 74.0 (61, 78) | 64.0 (18, 85) |
| ≥65 | 23 (41.8) | 15 (57.7) | 6 (42.9) | 13 (59.1) | 2 (66.7) | 59 (49.2) |
| Sex, n (%) | ||||||
| Male | 32 (58.2) | 13 (50.0) | 5 (35.7) | 12 (54.5) | 0 | 62 (51.7) |
| Ethnicity, n (%) | ||||||
| Hispanic or Latino | 7 (12.7) | 3 (11.5) | 0 | 1 (4.5) | 0 | 11 (9.2) |
| Not Hispanic or Latino | 47 (85.5) | 23 (88.5) | 14 (100.0) | 21 (95.5) | 3 (100.0) | 108 (90.0) |
| Missing | 1 (1.8) | 0 | 0 | 0 | 0 | 1 (0.8) |
| Primary race, n (%) | ||||||
| Asian | 2 (3.6) | 1 (3.8) | 2 (14.3) | 1 (4.5) | 0 | 6 (5.0) |
| Black or African American | 0 | 1 (3.8) | 2 (14.3) | 1 (4.5) | 1 (33.3) | 5 (4.2) |
| White | 51 (92.7) | 21 (80.8) | 9 (64.3) | 19 (86.4) | 2 (66.7) | 102 (85.0) |
| Other | 2 (3.6) | 3 (11.5) | 1 (7.1) | 1 (4.5) | 0 | 7 (5.8) |
| . | Part A (N = 55) . | Part B (N = 26) . | Part C (N = 14) . | Part D (N = 22) . | Part E (N = 3) . | Overall (N = 120) . |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| n | 55 | 26 | 14 | 22 | 3 | 120 |
| Median (min, max) | 61.0 (20, 85) | 68.0 (18, 74) | 63.0 (48, 83) | 70.5 (28, 84) | 74.0 (61, 78) | 64.0 (18, 85) |
| ≥65 | 23 (41.8) | 15 (57.7) | 6 (42.9) | 13 (59.1) | 2 (66.7) | 59 (49.2) |
| Sex, n (%) | ||||||
| Male | 32 (58.2) | 13 (50.0) | 5 (35.7) | 12 (54.5) | 0 | 62 (51.7) |
| Ethnicity, n (%) | ||||||
| Hispanic or Latino | 7 (12.7) | 3 (11.5) | 0 | 1 (4.5) | 0 | 11 (9.2) |
| Not Hispanic or Latino | 47 (85.5) | 23 (88.5) | 14 (100.0) | 21 (95.5) | 3 (100.0) | 108 (90.0) |
| Missing | 1 (1.8) | 0 | 0 | 0 | 0 | 1 (0.8) |
| Primary race, n (%) | ||||||
| Asian | 2 (3.6) | 1 (3.8) | 2 (14.3) | 1 (4.5) | 0 | 6 (5.0) |
| Black or African American | 0 | 1 (3.8) | 2 (14.3) | 1 (4.5) | 1 (33.3) | 5 (4.2) |
| White | 51 (92.7) | 21 (80.8) | 9 (64.3) | 19 (86.4) | 2 (66.7) | 102 (85.0) |
| Other | 2 (3.6) | 3 (11.5) | 1 (7.1) | 1 (4.5) | 0 | 7 (5.8) |
max, maximum; min, minimum.
Of 120 patients enrolled, 118 had AML, 1 had ALL, and 1 had blast phase CML. No patients with BPDCN were enrolled.
Of the 118 patients with AML, at baseline, 33 (28.0%) had primary induction failure, 5 (4.2%) had status unspecified, all other patients had relapsed at last assessment before study start; 15 (12.7%) had therapy-related AML (Table 2), and 18 (15.3%) had a history of MDS. The median prior systemic therapies was 3, with 1 (0.8%) patient having undergone autologous SCT, and with 20 (16.7%) and 14 (11.7%) patients having had prior allogeneic SCT from related and unrelated donors, respectively.
Baseline characteristics of AML study population
| . | Part A (N = 54) . | Part B (N = 26) . | Part C (N = 14) . | Part D (N = 21) . | Part E (N = 3) . | Overall (N = 118) . |
|---|---|---|---|---|---|---|
| ECOG performance status, n (%)16 | ||||||
| 0 | 4 (7.4) | 1 (3.8) | 1 (7.1) | 3 (14.3) | 2 (66.7) | 11 (9.3) |
| 1 | 34 (63.0) | 19 (73.1) | 11 (78.6) | 13 (61.9) | 0 | 77 (65.3) |
| 2 | 16 (29.6) | 6 (23.1) | 2 (14.3) | 5 (23.8) | 1 (33.3) | 30 (25.4) |
| >2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Age at initial diagnosis, y | ||||||
| Median (min, max) | 60.3 (20, 84) | 67.7 (18, 75) | 62.5 (46, 83) | 67.8 (28, 84) | 74.3 (61, 77) | 63.6 (18, 84) |
| Time since initial diagnosis, mo | ||||||
| Median (min, max) | 13.03 (2.2, 206.2) | 8.34 (0.7, 46.4) | 8.31 (3.0, 58.3) | 12.58 (1.3, 44.8) | 10.81 (0.9, 16.6) | 10.76 (0.7, 206.2) |
| Bone marrow aspirate blast count (%) at baseline | ||||||
| Median (min, max) | 46.00 (4.0, 95.0) | 33.00 (1.8, 91.0) | 50.00 (8.0, 84.0) | 24.00 (6.0, 91.0) | 21.00 (16.0, 26.0) | 35.00 (1.8, 95.0) |
| Not reported | 1 (1.9) | 3 (11.5) | 3 (21.4) | 1 (4.8) | 1 (33.3) | 9 (7.6) |
| Bone marrow core blast count, %, at baseline | ||||||
| Median (min, max) | 46.00 (7.5, 90.0) | 38.50 (5.0, 91.0) | 43.00 (10.0, 80.0) | 30.00 (5.0, 90.0) | 25.00 (16.0, 30.0) | 34.00 (5.0, 91.0) |
| Not reported | 38 (70.4) | 12 (46.2) | 2 (14.3) | 4 (19.0) | 0 | 56 (47.5) |
| Therapy-related AML, n (%) | ||||||
| Yes | 4 (7.4) | 5 (19.2) | 2 (14.3) | 4 (19.0) | 0 | 15 (12.7) |
| No | 48 (88.9) | 21 (80.8) | 12 (85.7) | 17 (81.0) | 3 (100.0) | 101 (85.6) |
| Unknown | 2 (3.7) | 0 | 0 | 0 | 0 | 2 (1.7) |
| Disease status at last assessment before study start, n (%) | ||||||
| Primary induction failure | 16 (29.6) | 10 (38.5) | 2 (14.3) | 4 (19.0) | 1 (33.3) | 33 (28.0) |
| First relapse | 23 (42.6) | 10 (38.5) | 7 (50.0) | 8 (38.1) | 1 (33.3) | 49 (41.5) |
| Second relapse | 11 (20.4) | 4 (15.4) | 4 (28.6) | 7 (33.3) | 1 (33.3) | 27 (22.9) |
| Third relapse | 1 (1.9) | 2 (7.7) | 0 | 0 | 0 | 3 (2.5) |
| Fourth or more relapse | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (0.8) |
| Unspecified | 3 (5.6) | 0 | 1 (7.1) | 1 (4.8) | 0 | 5 (4.2) |
| AML, number of previous systemic therapies | ||||||
| Median | 3.00 | 2.00 | 3.00 | 3.00 | 1.00 | 3.00 |
| . | Part A (N = 54) . | Part B (N = 26) . | Part C (N = 14) . | Part D (N = 21) . | Part E (N = 3) . | Overall (N = 118) . |
|---|---|---|---|---|---|---|
| ECOG performance status, n (%)16 | ||||||
| 0 | 4 (7.4) | 1 (3.8) | 1 (7.1) | 3 (14.3) | 2 (66.7) | 11 (9.3) |
| 1 | 34 (63.0) | 19 (73.1) | 11 (78.6) | 13 (61.9) | 0 | 77 (65.3) |
| 2 | 16 (29.6) | 6 (23.1) | 2 (14.3) | 5 (23.8) | 1 (33.3) | 30 (25.4) |
| >2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Age at initial diagnosis, y | ||||||
| Median (min, max) | 60.3 (20, 84) | 67.7 (18, 75) | 62.5 (46, 83) | 67.8 (28, 84) | 74.3 (61, 77) | 63.6 (18, 84) |
| Time since initial diagnosis, mo | ||||||
| Median (min, max) | 13.03 (2.2, 206.2) | 8.34 (0.7, 46.4) | 8.31 (3.0, 58.3) | 12.58 (1.3, 44.8) | 10.81 (0.9, 16.6) | 10.76 (0.7, 206.2) |
| Bone marrow aspirate blast count (%) at baseline | ||||||
| Median (min, max) | 46.00 (4.0, 95.0) | 33.00 (1.8, 91.0) | 50.00 (8.0, 84.0) | 24.00 (6.0, 91.0) | 21.00 (16.0, 26.0) | 35.00 (1.8, 95.0) |
| Not reported | 1 (1.9) | 3 (11.5) | 3 (21.4) | 1 (4.8) | 1 (33.3) | 9 (7.6) |
| Bone marrow core blast count, %, at baseline | ||||||
| Median (min, max) | 46.00 (7.5, 90.0) | 38.50 (5.0, 91.0) | 43.00 (10.0, 80.0) | 30.00 (5.0, 90.0) | 25.00 (16.0, 30.0) | 34.00 (5.0, 91.0) |
| Not reported | 38 (70.4) | 12 (46.2) | 2 (14.3) | 4 (19.0) | 0 | 56 (47.5) |
| Therapy-related AML, n (%) | ||||||
| Yes | 4 (7.4) | 5 (19.2) | 2 (14.3) | 4 (19.0) | 0 | 15 (12.7) |
| No | 48 (88.9) | 21 (80.8) | 12 (85.7) | 17 (81.0) | 3 (100.0) | 101 (85.6) |
| Unknown | 2 (3.7) | 0 | 0 | 0 | 0 | 2 (1.7) |
| Disease status at last assessment before study start, n (%) | ||||||
| Primary induction failure | 16 (29.6) | 10 (38.5) | 2 (14.3) | 4 (19.0) | 1 (33.3) | 33 (28.0) |
| First relapse | 23 (42.6) | 10 (38.5) | 7 (50.0) | 8 (38.1) | 1 (33.3) | 49 (41.5) |
| Second relapse | 11 (20.4) | 4 (15.4) | 4 (28.6) | 7 (33.3) | 1 (33.3) | 27 (22.9) |
| Third relapse | 1 (1.9) | 2 (7.7) | 0 | 0 | 0 | 3 (2.5) |
| Fourth or more relapse | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (0.8) |
| Unspecified | 3 (5.6) | 0 | 1 (7.1) | 1 (4.8) | 0 | 5 (4.2) |
| AML, number of previous systemic therapies | ||||||
| Median | 3.00 | 2.00 | 3.00 | 3.00 | 1.00 | 3.00 |
ECOG, Eastern Cooperative Oncology Group performance status; max, maximum; min, minimum.
Baseline is defined as the last nonmissing result before administration of the first dose.
Pharmacodynamics
Safety and dose determination
CRS risk mitigation for priming and step-up dosing
Measurements of serum cytokine levels were taken at multiple time points during the first 24 hours after infusion to better define study drug-related CRS. Incidence and grade of CRS were observed to be highest on the first dose and on weekly step-up dosing. The averages of IL-6 peaks also correlated with higher priming doses and weekly dosing step-ups.
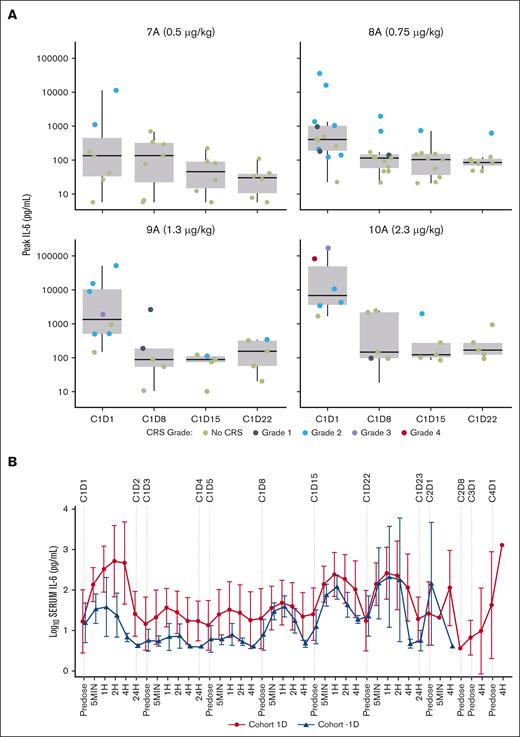
Peripheral T-cell activation, peak IL-6, and CRS manifestations occurring within 24 hours after the first dose were strongly dose related, with doses ≥0.7 μg/kg associated with an intolerable rate and severity of CRS (Figure 2).
Peak IL-6 levels seen in patients in dose escalation. (A) Summary of peak IL-6 levels and incidence/grade of CRS by fixed weekly dose. Peak IL-6 and CRS manifestations occurring within 24 hours after the first dose were strongly dose related, with doses ≥0.7 μg/kg associated with an intolerable rate and severity of CRS. (B) Summary of mean of log10 IL-6 levels in cohorts 1D and -1D. Frequent dosing within the first week was associated with enhanced tolerability and remarkable suppression of peak IL-6 levels. 7A, cohort 7 Part A; 8A, cohort 8 Part A; 9A, cohort 9 Part A; 10A, cohort 10, Part A.
Peak IL-6 levels seen in patients in dose escalation. (A) Summary of peak IL-6 levels and incidence/grade of CRS by fixed weekly dose. Peak IL-6 and CRS manifestations occurring within 24 hours after the first dose were strongly dose related, with doses ≥0.7 μg/kg associated with an intolerable rate and severity of CRS. (B) Summary of mean of log10 IL-6 levels in cohorts 1D and -1D. Frequent dosing within the first week was associated with enhanced tolerability and remarkable suppression of peak IL-6 levels. 7A, cohort 7 Part A; 8A, cohort 8 Part A; 9A, cohort 9 Part A; 10A, cohort 10, Part A.
For priming dose characteristics, the 0.43 μg/kg dose level was differentiated from the 0.7 μg/kg dose by exhibiting reduced incidence and severity of CRS and, consistent with this, exhibited reduced peak IL-6 elevation and reduced depth of T-cell activation and margination. Importantly, however, the biological activity for dose level 0.43 μg/kg was sufficient to strongly limit CRS manifestations and IL-6 levels after a second higher step-up dose given 48 hours later.
Response correlates
T-cell numbers and fitness/exhaustion, target CD123 expression level, and tumor burden (blast count) were explored for their relationship to clinical outcome and tumor response to therapy.
Tumor cell correlates of response: blast count
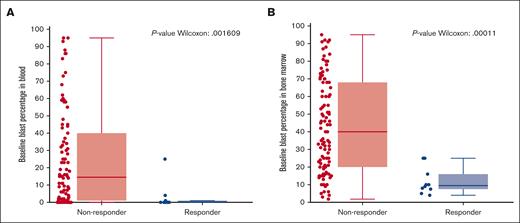
Blast numbers: response was highly associated with lower baseline blast count in the blood (P = .0016) and the bone marrow (P = .0001) (Figure 3). Baseline blast counts for all patients who responded to treatment were <25%, as measured either in the blood or the bone marrow; 8 of 10 responders had blast counts of <20%.
Responders associated with baseline blast percentage in the blood and bone marrow. Responders to the treatment were associated with a statistically significant lower absolute blast counts (<25%) at baseline in both the (A) blood and (B) bone marrow compared with that of nonresponders. For bone marrow, n = 111 and for peripheral blood, n = 104.
Responders associated with baseline blast percentage in the blood and bone marrow. Responders to the treatment were associated with a statistically significant lower absolute blast counts (<25%) at baseline in both the (A) blood and (B) bone marrow compared with that of nonresponders. For bone marrow, n = 111 and for peripheral blood, n = 104.
CD123 expression of baseline AML blasts in the marrow and blood was measured using median fluorescence intensity by flow cytometry. The surface level of CD123 expression on AML blasts was not associated with response (supplemental Figure 1). Nearly all patients had CD123+ expression on AML blasts within roughly a 10-fold level of expression, and responses were seen across this range. One notable patient had evidence of biphenotypic leukemia with disease including both CD123+ and CD123– cell populations pretreatment. On treatment, the patient demonstrated loss of the CD123+ population, indicating that response was dependent on CD123 expression (supplemental Figure 2).
T-cell response correlates of response: PD-1 expression
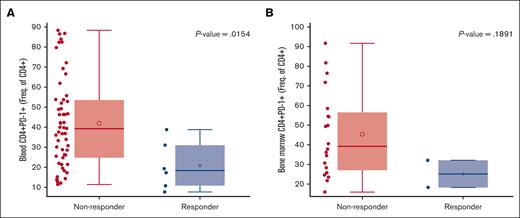
Responders were associated with low baseline programmed cell death protein 1 (PD-1) expression on peripheral CD4 T cells (P = .0154), and a similar trend was observed for CD8 T cells (P = .2213) (Figures 4 and 5).
Responders with baseline PD-1 expression on CD8 T cells. Responders had low baseline PD-1 expression in the (A) blood and (B) bone marrow CD8 T cells. Of 111 patients with response evaluable data, n = 61 for the blood and n = 22 for the bone marrow had expression data. Freq., frequency.
Responders with baseline PD-1 expression on CD8 T cells. Responders had low baseline PD-1 expression in the (A) blood and (B) bone marrow CD8 T cells. Of 111 patients with response evaluable data, n = 61 for the blood and n = 22 for the bone marrow had expression data. Freq., frequency.
Responders with baseline PD-1 expression on CD4 T cells. (A) Responders were associated with low baseline PD-1 expression on peripheral blood and (B) a similar trend was observed in the bone marrow CD4 T cells. Of 111 patients with response evaluable data, n = 61 for the blood and n = 22 for the bone marrow had expression data. Freq., frequency.
Responders with baseline PD-1 expression on CD4 T cells. (A) Responders were associated with low baseline PD-1 expression on peripheral blood and (B) a similar trend was observed in the bone marrow CD4 T cells. Of 111 patients with response evaluable data, n = 61 for the blood and n = 22 for the bone marrow had expression data. Freq., frequency.
Safety and tolerability
The most common treatment-emergent (TE) AEs (TEAEs) were CRS, chills, pyrexia, fatigue, anemia, and hypotension (supplemental Table 1).
In total, 105 (87.5%) patients experienced at least 1 TEAE of grade ≥3, the most common of which were anemia, febrile neutropenia, and pneumonia (Table 3).
TEAEs with CTCAE of grade 3 or higher occurring in 10% or more of patients, by SOC, preferred term, and maximum severity
| SOC preferred term, N (%) . | Part A (N = 55) . | Part B (N = 26) . | Part C (N = 14) . | Part D (N = 22) . | Part E (N = 3) . | Overall (N = 120) . |
|---|---|---|---|---|---|---|
| Number of patients with at least 1 TEAE of CTCAE, grade ≥3 | 46 (83.6) | 24 (92.3) | 12 (85.7) | 21 (95.5) | 2 (66.7) | 105 (87.5) |
| Grade 3 | 12 (21.8) | 10 (38.5) | 5 (35.7) | 11 (50.0) | 1 (33.3) | 39 (32.5) |
| Grade 4 | 32 (58.2) | 12 (46.2) | 4 (28.6) | 9 (40.9) | 1 (33.3) | 58 (48.3) |
| Grade 5 | 2 (3.6) | 2 (7.7) | 3 (21.4) | 1 (4.5) | 0 | 8 (6.7) |
| Blood and lymphatic system disorders | 27 (49.1) | 12 (46.2) | 9 (64.3) | 12 (54.5) | 1 (33.3) | 61 (50.8) |
| Anemia | 17 (30.9) | 3 (11.5) | 5 (35.7) | 5 (22.7) | 1 (33.3) | 31 (25.8) |
| Febrile neutropenia | 14 (25.5) | 7 (26.9) | 6 (42.9) | 3 (13.6) | 0 | 30 (25.0) |
| Leukopenia | 5 (9.1) | 0 | 4 (28.6) | 3 (13.6) | 0 | 12 (10.0) |
| Lymphopenia | 6 (10.9) | 2 (7.7) | 4 (28.6) | 6 (27.3) | 0 | 18 (15.0) |
| Infections and infestations | 25 (45.5) | 9 (34.6) | 5 (35.7) | 7 (31.8) | 1 (33.3) | 47 (39.2) |
| Pneumonia | 13 (23.6) | 3 (11.5) | 3 (21.4) | 1 (4.5) | 1 (33.3) | 21 (17.5) |
| Investigations | 27 (49.1) | 9 (34.6) | 5 (35.7) | 9 (40.9) | 1 (33.3) | 51 (42.5) |
| Lymphocyte count decreased | 10 (18.2) | 2 (7.7) | 0 | 1 (4.5) | 0 | 13 (10.8) |
| Neutrophil count decreased | 9 (16.4) | 4 (15.4) | 1 (7.1) | 0 | 1 (33.3) | 15 (12.5) |
| Platelet count decreased | 8 (14.5) | 2 (7.7) | 1 (7.1) | 2 (9.1) | 1 (33.3) | 14 (11.7) |
| White blood cell count decreased | 12 (21.8) | 2 (7.7) | 0 | 2 (9.1) | 0 | 16 (13.3) |
| Vascular disorders | 8 (14.5) | 2 (7.7) | 3 (21.4) | 6 (27.3) | 0 | 19 (15.8) |
| Hypertension | 6 (10.9) | 1 (3.8) | 2 (14.3) | 4 (18.2) | 0 | 13 (10.8) |
| SOC preferred term, N (%) . | Part A (N = 55) . | Part B (N = 26) . | Part C (N = 14) . | Part D (N = 22) . | Part E (N = 3) . | Overall (N = 120) . |
|---|---|---|---|---|---|---|
| Number of patients with at least 1 TEAE of CTCAE, grade ≥3 | 46 (83.6) | 24 (92.3) | 12 (85.7) | 21 (95.5) | 2 (66.7) | 105 (87.5) |
| Grade 3 | 12 (21.8) | 10 (38.5) | 5 (35.7) | 11 (50.0) | 1 (33.3) | 39 (32.5) |
| Grade 4 | 32 (58.2) | 12 (46.2) | 4 (28.6) | 9 (40.9) | 1 (33.3) | 58 (48.3) |
| Grade 5 | 2 (3.6) | 2 (7.7) | 3 (21.4) | 1 (4.5) | 0 | 8 (6.7) |
| Blood and lymphatic system disorders | 27 (49.1) | 12 (46.2) | 9 (64.3) | 12 (54.5) | 1 (33.3) | 61 (50.8) |
| Anemia | 17 (30.9) | 3 (11.5) | 5 (35.7) | 5 (22.7) | 1 (33.3) | 31 (25.8) |
| Febrile neutropenia | 14 (25.5) | 7 (26.9) | 6 (42.9) | 3 (13.6) | 0 | 30 (25.0) |
| Leukopenia | 5 (9.1) | 0 | 4 (28.6) | 3 (13.6) | 0 | 12 (10.0) |
| Lymphopenia | 6 (10.9) | 2 (7.7) | 4 (28.6) | 6 (27.3) | 0 | 18 (15.0) |
| Infections and infestations | 25 (45.5) | 9 (34.6) | 5 (35.7) | 7 (31.8) | 1 (33.3) | 47 (39.2) |
| Pneumonia | 13 (23.6) | 3 (11.5) | 3 (21.4) | 1 (4.5) | 1 (33.3) | 21 (17.5) |
| Investigations | 27 (49.1) | 9 (34.6) | 5 (35.7) | 9 (40.9) | 1 (33.3) | 51 (42.5) |
| Lymphocyte count decreased | 10 (18.2) | 2 (7.7) | 0 | 1 (4.5) | 0 | 13 (10.8) |
| Neutrophil count decreased | 9 (16.4) | 4 (15.4) | 1 (7.1) | 0 | 1 (33.3) | 15 (12.5) |
| Platelet count decreased | 8 (14.5) | 2 (7.7) | 1 (7.1) | 2 (9.1) | 1 (33.3) | 14 (11.7) |
| White blood cell count decreased | 12 (21.8) | 2 (7.7) | 0 | 2 (9.1) | 0 | 16 (13.3) |
| Vascular disorders | 8 (14.5) | 2 (7.7) | 3 (21.4) | 6 (27.3) | 0 | 19 (15.8) |
| Hypertension | 6 (10.9) | 1 (3.8) | 2 (14.3) | 4 (18.2) | 0 | 13 (10.8) |
Data are presented as number (percentage) of patients. The denominators for percentages are the number of patients treated in each group.
CTCAE, Common Terminology Criteria for Adverse Events.
Moreover, 90 (75.0%) patients experienced at least 1 TE SAE during the study. The most frequently reported TE SAEs were CRS, febrile neutropenia, pneumonia, sepsis, syncope, and pyrexia (supplemental Table 2).
Of 120 patients, 16 (13.3%) experienced at least 1 TEAE classified as a dose-limiting toxicity (DLT), with 13 (10.8%) grade 3, 2 (1.7%) grade 4, and 1 (0.8%) grade 5 in severity. TEAEs classified as DLTs of CRS, iRR, γ-glutamyl transferase increase, and hypertension were experienced by 2 (1.7%) patients each.
CRS, the most common toxicity, was managed with premedication. Premedication (IV steroids, diphenhydramine, and acetaminophen) was administered after symptoms consistent with CRS had been observed and was reserved for patients who developed CRS (grade 2 or higher) in prior dosing. Of 102 patients who experienced CRS/iRR-associated symptoms, 27 (22.5%) experienced grade 3; 4 (3.3%) experienced grade 4; and 1 (0.8%) experienced grade 5. Incidence and grade of CRS were observed to be highest at first dose and during weekly step-up dosing (Figure 6).
Distribution of CRS grade by dosing visit for Parts C and D. (A-B) Incidence and grade of CRS were observed to be highest on the first dose and on weekly step-up dosing. (C) CRS was limited to grade 1 and 2 events with more frequent dosing within the first week. For multiple CRS events for a patient at a dosing visit, the record with maximum CRS grade was used in the analysis. The denominator for percentages is the number of patients (n) dosed at each visit. C, cycle; D, day.
Distribution of CRS grade by dosing visit for Parts C and D. (A-B) Incidence and grade of CRS were observed to be highest on the first dose and on weekly step-up dosing. (C) CRS was limited to grade 1 and 2 events with more frequent dosing within the first week. For multiple CRS events for a patient at a dosing visit, the record with maximum CRS grade was used in the analysis. The denominator for percentages is the number of patients (n) dosed at each visit. C, cycle; D, day.
Of 120 patients, reasons for treatment discontinuations were: AEs (15%), physician decision (0.8%), progressive disease (30%), withdrawal by patient (7.5%), insufficient clinical response (35%), and death (2.5%).
Eight patients died because of TEAEs of pneumonia (3 patients), sepsis (1 patient), cerebral hemorrhage (1 patient), CRS (1 patient), acute pulmonary edema (1 patient), and enterococcal sepsis (1 patient). The fatal CRS event was considered related to vibecotamab. Treatment-related AEs (TRAEs) with SOC of nervous system disorders occurred in 23 patients (19.2%), with the most common nervous system disorder TRAE being headache (16 patients; 13.3%) followed by dizziness (4 patients; 3.3%). Only 1 treatment-related SAE with SOC of nervous system disorder occurred (encephalopathy, grade 3; 0.8%). No grade 5 TRAEs of SOC nervous system disorders occurred.
TRAEs with SOC of psychiatric disorders occurred in 11 patients (9.2%), with the most common psychiatric disorder TRAE being confusional state (5 patients; 4.2%) followed by delirium and mental status changes (3 patients each; 2.5%). No TR SAEs of SOC psychiatric disorders occurred.
Dose escalation and optimization
Safety, efficacy, and PD data were used to guide the dose-escalation strategy. In Part A, which was designed to assess the weekly administration of increasing fixed doses of vibecotamab (Figure 1), no DLTs were noted at dose levels 0.003 to 0.5 μg/kg. However, at dose levels >0.75 μg/kg, at least 1 DLT per cohort was reported. Notably, responses were reported at doses of ≥0.75 μg/kg, whereas no responses were seen at the lower doses. The combined safety and efficacy prompted selection of 1.3 μg/kg and 2.3 μg/kg as the priming and target dose, respectively, for Part B. A priming dose is a dose level that is tolerable with minimal or no serious CRS induction but could reduce the severity of CRS of subsequent higher and more efficacious step-up doses. Priming and step-up dose escalations continued until 1 DLT of grade-5 CRS occurred in the 12-μg/kg target dose cohort, resulting in a partial clinical hold; notably, the DLT occurred after administration of the 1.3 μg/kg priming dose.
To address safety concerns of high-grade CRS leading to death noted in Part B, more frequent dosing in the first 2 weeks at a lower priming dose was implemented in Part C. The increased frequency at a reduced priming dose of 0.43 μg/kg and target dose of 1.3 μg/kg resulted in a single DLT in the cohort. In the subsequent escalation cohort, at an increased priming dose of 0.77 μg/kg and target dose of 2.3 μg/kg, there were no DLTs and a response was observed.
Because more frequent intraweekly dosing improved tolerability and responses were consistently observed at target doses of ≥0.75 μg/kg, Parts D and E were designed to escalate the target dose while using frequent step-up dosing through the first week followed by weekly dosing with or without weekly step-ups to maintain the safety profile noted in Part C. In the first cohort of Part D (n = 19 patients), which incorporated a priming dose of 0.75 μg/kg with intraweekly and weekly step-up doses to a target dose of 5.7 μg/kg, 6 (31.6%), DLT events of grade 3 hypertension (2 patients), amylase increased, lipase increased, disseminated intravascular coagulation, and infusion site extravasation (1 patient each) were reported with an ORR of 11.1% (2/18 patients who were response evaluable).
To address the toxicity profile noted in the first cohort of Part D and to further characterize the impact of dose level and frequency on safety and efficacy, Part E included an additional dose in the first week and achieved weekly target dosing starting at the first week. In the first cohort of Part E, which had a priming dose of 0.43 μg/kg and a target dose of 2.5 μg/kg, 66.7% (2/3) of patients experienced a DLT with no response being reported. Notably, 1 patient who experienced a DLT of grade 3 iRR had subclinical pneumonia, which the investigator posited could have contributed to the DLT.
Given the safety and tolerability data from the first cohorts of Parts D and E, a lower dosing regimen with 3 step-up doses in the first week (0.43, 0.75, 1.1 μg/kg on days 1, 3, and 5, respectively) followed by weekly dosing at 1.7 μg/kg (cohort −1D) was implemented to reduce frequency and severity of CRS. Indeed, more frequent dosing within the first week was associated with enhanced tolerability and remarkable suppression of peak IL-6 levels, and CRS was limited to grade 1 and 2 events only (Figures 6 and 2B). No DLTs nor grade 3 CRS events were reported in this cohort, which was an improvement in the safety profile compared with prior cohorts. Likewise, an overall response of 33.3% (1 in 3 patients) was noted, which was consistent with the observations of increased responses at target dosages at ≥0.75 μg/kg in prior cohorts. Based on these data, the above dosing regimen was considered to have optimized efficacy while minimizing CRS severity and was declared the RP2D for vibecotamab.
Efficacy
Secondary objectives of the study included assessing preliminary antitumor activity of vibecotamab by ORR, DoR, PFS, and OS. The overall response to treatment was assessed based on the percentage reduction change from baseline in the bone marrow blast count. Patients with best overall response of PR or better were categorized as responders, the remaining patients who had response of stable disease or less, and patients with no response assessment were categorized as nonresponders.
Vibecotamab demonstrated preliminary antileukemic activity in a subset of patients. A total of 10 patients with AML were considered responders to the treatment, achieving a response of CR (3 patients), CRi (3patients), MLFS (3 patients), and PR (1 patient) with an ORR of 9.0% (Table 4). Notably, response was only observed in patients receiving a target dose of ≥0.75 μg/kg; the ORR for patients receiving a target dose (≥0.75 μg/kg) was 11.5% (10/87 patients who were response evaluable). The patient with ALL did not respond to vibecotamab therapy and the patient with CML did not have an evaluable response. Responders to the treatment (9 patients [1 patient had unknown data]) were associated with a statistically significant lower absolute blast counts (<25%) at baseline in both the blood and bone marrow (Table 5; Figure 3).
Best overall responses and ORRs for patients with AML
| Response category, N (%) . | Part A (N = 54) . | Part B (N = 26) . | Part C (N = 14) . | Part D (N = 21) . | Part E (N = 3) . | Overall (N = 118) . |
|---|---|---|---|---|---|---|
| Best overall response | ||||||
| CR | 2 (3.7) | 0 | 0 | 1 (4.8) | 0 | 3 (2.5) |
| CRi | 1 (1.9) | 1 (3.8) | 1 (7.1) | 0 | 0 | 3 (2.5) |
| MLFS | 1 (1.9) | 1 (3.8) | 0 | 1 (4.8) | 0 | 3 (2.5) |
| PR | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (0.8) |
| Stable disease | 27 (50.0) | 12 (46.2) | 7 (50.0) | 11 (52.4) | 0 | 57 (48.3) |
| Progressive disease | 9 (16.7) | 5 (19.2) | 5 (35.7) | 3 (14.3) | 1 (33.3) | 23 (19.5) |
| NE | 9 (16.7) | 5 (19.2) | 1 (7.1) | 4 (19.0) | 2 (66.7) | 21 (17.8) |
| ORR | 4/49 (8.2) | 2/24 (8.3) | 1/14 (7.1) | 3/21 (14.3) | 0/3 (0.0) | 10/111 (9.0) |
| 95% CI | 2.3, 19.6 | 1.0, 27.0 | 0.2, 33.9 | 3.0, 36.3 | 0.0, 70.8 | 4.4, 15.9 |
| Disease control∗rate | 31/49 (63.3) | 14/24 (58.3) | 8/14 (57.1) | 14/21 (66.7) | 0/3 (0.0) | 67/111 (60.4) |
| 95% CI | 48.3, 76.6 | 36.6, 77.9 | 28.9, 82.3 | 43.0, 85.4 | 0.0, 70.8 | 50.6, 69.5 |
| Time to treatment discontinuation | ||||||
| Number of events, n (%) | 54 (100) | 26 (100) | 14 (100) | 21 (100) | 3 (100) | 118 (100) |
| Median follow-up | 22.5 | 22.5 | 43.5 | 50.0 | 5.0 | 29.0 |
| Min, max follow-up | 1, 218 | 1, 134 | 15, 121 | 1, 96 | 1, 29 | 1, 218 |
| K-M median (95% CI) | 22.5 (22.0, 50.0) | 22.5 (15.0, 50.0) | 43.5 (22.0, 64.0) | 50.0 (22.0, 78.0) | 5.0 (1.0, NE) | 29.0 (22.0, 50.0) |
| 25%, 75% | 22.0, 78.0 | 8.0, 54.0 | 22.0, 64.0 | 22.0, 79.0 | 1.0, 29.0 | 22.0, 73.0 |
| Time to initial response (of PR or better) | ||||||
| Median (95% CI) | 26.5 (24.0, NE) | 24.5 (24.0, NE) | 26.0 (NE, NE) | 28.0 (25.0, NE) | NE (NE, NE) | 26.0 (24.0, 28.0) |
| 25%, 75% | 25.0, 39.5 | 24.0, 25.0 | 26.0, 26.0 | 25.0, 53.0 | NE, NE | 25.0, 28.0 |
| DoR for responders (d) | ||||||
| n | 4 | 2 | 1 | 3 | 0 | 10 |
| Number of events | 2 (50.0) | 2 (100) | 1 (100) | 1 (33.3) | — | 6 (60.0) |
| Unadjusted mean (SD) | 92.5 (38.48) | 78.0 (29.70) | 57.0 (−) | 65.0 (38.20) | — | 77.8 (33.38) |
| Unadjusted median | 103.0 | 78.0 | 57.0 | 52.0 | — | 76.0 |
| Min, max | 38, 126 | 57, 99 | 57, 57 | 35, 108 | — | 35, 126 |
| K-M median | 118.5 | 78.0 | 57.0 | 108.0 | — | 108.0 |
| Response category, N (%) . | Part A (N = 54) . | Part B (N = 26) . | Part C (N = 14) . | Part D (N = 21) . | Part E (N = 3) . | Overall (N = 118) . |
|---|---|---|---|---|---|---|
| Best overall response | ||||||
| CR | 2 (3.7) | 0 | 0 | 1 (4.8) | 0 | 3 (2.5) |
| CRi | 1 (1.9) | 1 (3.8) | 1 (7.1) | 0 | 0 | 3 (2.5) |
| MLFS | 1 (1.9) | 1 (3.8) | 0 | 1 (4.8) | 0 | 3 (2.5) |
| PR | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (0.8) |
| Stable disease | 27 (50.0) | 12 (46.2) | 7 (50.0) | 11 (52.4) | 0 | 57 (48.3) |
| Progressive disease | 9 (16.7) | 5 (19.2) | 5 (35.7) | 3 (14.3) | 1 (33.3) | 23 (19.5) |
| NE | 9 (16.7) | 5 (19.2) | 1 (7.1) | 4 (19.0) | 2 (66.7) | 21 (17.8) |
| ORR | 4/49 (8.2) | 2/24 (8.3) | 1/14 (7.1) | 3/21 (14.3) | 0/3 (0.0) | 10/111 (9.0) |
| 95% CI | 2.3, 19.6 | 1.0, 27.0 | 0.2, 33.9 | 3.0, 36.3 | 0.0, 70.8 | 4.4, 15.9 |
| Disease control∗rate | 31/49 (63.3) | 14/24 (58.3) | 8/14 (57.1) | 14/21 (66.7) | 0/3 (0.0) | 67/111 (60.4) |
| 95% CI | 48.3, 76.6 | 36.6, 77.9 | 28.9, 82.3 | 43.0, 85.4 | 0.0, 70.8 | 50.6, 69.5 |
| Time to treatment discontinuation | ||||||
| Number of events, n (%) | 54 (100) | 26 (100) | 14 (100) | 21 (100) | 3 (100) | 118 (100) |
| Median follow-up | 22.5 | 22.5 | 43.5 | 50.0 | 5.0 | 29.0 |
| Min, max follow-up | 1, 218 | 1, 134 | 15, 121 | 1, 96 | 1, 29 | 1, 218 |
| K-M median (95% CI) | 22.5 (22.0, 50.0) | 22.5 (15.0, 50.0) | 43.5 (22.0, 64.0) | 50.0 (22.0, 78.0) | 5.0 (1.0, NE) | 29.0 (22.0, 50.0) |
| 25%, 75% | 22.0, 78.0 | 8.0, 54.0 | 22.0, 64.0 | 22.0, 79.0 | 1.0, 29.0 | 22.0, 73.0 |
| Time to initial response (of PR or better) | ||||||
| Median (95% CI) | 26.5 (24.0, NE) | 24.5 (24.0, NE) | 26.0 (NE, NE) | 28.0 (25.0, NE) | NE (NE, NE) | 26.0 (24.0, 28.0) |
| 25%, 75% | 25.0, 39.5 | 24.0, 25.0 | 26.0, 26.0 | 25.0, 53.0 | NE, NE | 25.0, 28.0 |
| DoR for responders (d) | ||||||
| n | 4 | 2 | 1 | 3 | 0 | 10 |
| Number of events | 2 (50.0) | 2 (100) | 1 (100) | 1 (33.3) | — | 6 (60.0) |
| Unadjusted mean (SD) | 92.5 (38.48) | 78.0 (29.70) | 57.0 (−) | 65.0 (38.20) | — | 77.8 (33.38) |
| Unadjusted median | 103.0 | 78.0 | 57.0 | 52.0 | — | 76.0 |
| Min, max | 38, 126 | 57, 99 | 57, 57 | 35, 108 | — | 35, 126 |
| K-M median | 118.5 | 78.0 | 57.0 | 108.0 | — | 108.0 |
max, maximum; min, minimum; NE, not evaluable; SD, standard deviation.
Disease control is defined as the proportion of patients achieving a best overall response of CR, CRi, MLFS, PR, or stable disease.
Summary of responders with baseline disease characteristics
| Baseline value . | Responder (N = 10) . | Nonresponder (N = 101) . | Overall (N = 111) . |
|---|---|---|---|
| Blast percent in blood at baseline | |||
| n | 10 | 94 | 104 |
| Median (min, max) | 0.00 (0.0, 25.0) | 14.50 (0.0, 95.0) | 11.00 (0.0, 95.0) |
| Blast percent in bone marrow at baseline | |||
| n | 10 | 101 | 111 |
| Median (min, max) | 9.50 (4.0, 25.0) | 40.00 (1.8, 95.0) | 34.00 (1.8, 95.0) |
| PD-1 expression on CD8 cells in blood at baseline | |||
| n | 6 | 55 | 61 |
| Median | 25.25 (6.8, 71.4) | 37.80 (6.4, 95.6) | 36.50 (6.4, 95.6) |
| PD-1 expression on CD8 cells in the bone marrow at baseline | |||
| n | 2 | 20 | 22 |
| Median (min, max) | 34.65 (32.5, 36.8) | 42.60 (9.9, 82.5) | 41.30 (9.9, 82.5) |
| PD-1 expression on CD4 cells in the blood at baseline | |||
| n | 6 | 55 | 61 |
| Median (min, max) | 18.35 (7.7, 38.8) | 39.40 (11.5, 88.4) | 36.90 (7.7, 88.4) |
| PD-1 expression on CD4 cells in the bone marrow at baseline | |||
| n | 2 | 20 | 22 |
| Median (min, max) | 25.15 (18.2, 32.1) | 39.20 (15.9, 91.8) | 37.00 (15.9, 91.8) |
| Baseline value . | Responder (N = 10) . | Nonresponder (N = 101) . | Overall (N = 111) . |
|---|---|---|---|
| Blast percent in blood at baseline | |||
| n | 10 | 94 | 104 |
| Median (min, max) | 0.00 (0.0, 25.0) | 14.50 (0.0, 95.0) | 11.00 (0.0, 95.0) |
| Blast percent in bone marrow at baseline | |||
| n | 10 | 101 | 111 |
| Median (min, max) | 9.50 (4.0, 25.0) | 40.00 (1.8, 95.0) | 34.00 (1.8, 95.0) |
| PD-1 expression on CD8 cells in blood at baseline | |||
| n | 6 | 55 | 61 |
| Median | 25.25 (6.8, 71.4) | 37.80 (6.4, 95.6) | 36.50 (6.4, 95.6) |
| PD-1 expression on CD8 cells in the bone marrow at baseline | |||
| n | 2 | 20 | 22 |
| Median (min, max) | 34.65 (32.5, 36.8) | 42.60 (9.9, 82.5) | 41.30 (9.9, 82.5) |
| PD-1 expression on CD4 cells in the blood at baseline | |||
| n | 6 | 55 | 61 |
| Median (min, max) | 18.35 (7.7, 38.8) | 39.40 (11.5, 88.4) | 36.90 (7.7, 88.4) |
| PD-1 expression on CD4 cells in the bone marrow at baseline | |||
| n | 2 | 20 | 22 |
| Median (min, max) | 25.15 (18.2, 32.1) | 39.20 (15.9, 91.8) | 37.00 (15.9, 91.8) |
Baseline is defined as the last assessment that is nonmissing before first dose of vibecotamab.
max, maximum; min, minimum; SD, standard deviation.
Based on K-M estimates, the median DoR was 108.0 days (95% CI, 57.0-not evaluable). The DoR ranged between 38 to 95 days in patients who achieved CR (3 patients); between 57 to 126 days in patients who achieved CRi (3 patients); between 99 to 111 days in patients with MLFS (3 patients); and 35 days in 1 patient with PR.
Vibecotamab therapy in patients with AML demonstrated a K-M median PFS of 86 days (95% CI, 82.0-not evaluable) and a K-M median OS of 134 days (95% CI, 105.0-162.0).
PKs
After the first dose administration in Part A, the mean maximum plasma concentration ranged from 0.242 to 5.23 ng/mL whereas the mean area under the curve for a dosing interval ranged from 4.96 to 38.8 h × ng/mL for vibecotamab doses ranging from 0.03 to 2.3 μg/kg. Notably, mean terminal half-life was reported to range from 10 to 25 hours. Mean clearance ranged from 151 to 5440 mL/h and corresponding mean apparent volume of distribution during terminal phase ranged from 2.83 to 191 L. In line with the half-life of 10 to 25 hours, concentrations before dosing on the approach to steady state at 7 days after drug administration were below the limit of detection for the majority of the patients.
Discussion
Dose optimization of the vibecotamab schedule resulted in a RP2D with an acceptable and tolerable safety profile in patients with relapsed/refractory AML. No clear trends were observed in the incidence of patients with non-CRS TEAEs; however, CRS was observed in some patients, and dosing strategies played an important role in minimizing its occurrence. This RP2D schedule is currently being further explored in a phase 2 trial in patients with minimal residual disease (MRD)–positive AML and in those with MDS who failed hypomethylating agents.17,18
Emerging data from other CD3 bsAbs in clinical development established that CRS could be mitigated by using a lowered priming dose as a first dose. Use of clinical and PD data resulted in a first dose priming dose of 0.43 μg/kg, which met the criteria of exhibiting sufficient biological activity to suppress CRS manifestations in subsequent doses, while being a safe and tolerable dose with minimal CRS.
Initial weekly dosing frequency demonstrated a trend toward CRS reduction in subsequent doses. However, CRS was reduced further for the first 3 doses when administered in a once-every-other-day regimen. This was initially seen with split dosing, in which dosing of the first week (1.3 μg/kg weekly) was evenly divided into 3 equal doses (0.43 μg/kg × 3), and then instituting step-up dosing. Greater suppression of CRS was achieved with a 48-hour dosing interval than with a 72-hour interval.
The RP2D schedule of vibecotamab was derived from rational analysis of prior cohorts and used a low priming dose, every-other-day dosing in the first week to increase drug exposure early in treatment, and stable weekly dosing thereafter to mitigate CRS. Alongside the improved CRS profile, 1 MLFS response was seen in cohort −1D; this is consistent with previous cohorts, demonstrating responses at target doses of ≥0.75 μg/kg, and further supports selection of cohort −1D as the RP2D. Lastly, the short 2-hour IV infusion offers more convenience to patients compared with longer continuous infusion of CD123 × CD3 bsAbs19 currently in clinical trials.
The mean half-life range of ∼10 to 25 hours is unexpectedly short for an antibody20; it is speculated that CD123 expression on the endothelium acts as a drug sink for vibecotamab and would also contribute to the high rate and severity of CRS that can limit the dose escalation of vibecotamab. The short half-life of vibecotamab likely added challenges in addressing CRS via priming and step-up dosing typically used for CD3 T-cell engagers. In addition, the high-affinity CD3 portion of the bsAb and high level CD123 target in a high tumor burden disease like AML probably exacerbated CRS.
Based on the observation that response was seen only in patients with AML with a lower burden of disease (blasts of <25%), a high effector (T cell)-to-target (CD123-expressing tumor cell) ratio may be needed for efficacy. This important observation would suggest specific clinical scenarios in which patients with low blast burden are potentially more likely to benefit from vibecotamab therapy, for instance, in the eradication of MRD, after disease debulking with non–T cell toxic regimens, or in patients with high-risk MDS who have a blast burden of <20%. A follow-up phase 2 trial in MRD-positive AML and MDS populations is currently in progress to further characterize the safety and efficacy of the vibecotamab RP2D in these select populations.17,18 Assessment of early MRD is important in patients with AML,21 both in quantifying remission status depth and assessing risk of relapse.
Every-other-day step-up dosing was remarkably effective in suppressing CRS; further dose exploration could address whether even more aggressive step-up dosing would still be tolerable every 48 hours, and thereby achieve higher and earlier efficacious dose levels in the above recommended populations.
As tumor cytolytic killing by T cells could be related to the ratio of T cells to target, the number of T cells in the blood and bone marrow could also be a determinant of probability of response. As such, a classic effector-to-target ratio was explored, and although not statistically significant, there was a trend for a favorable outcome with increasing number of T cells (data not shown). It is conceivable that a cytokine therapy that increases total T-cell numbers may further increase the clinical activity of vibecotamab.
T-cell fitness was explored using PD-1 expression as a measure of T-cell exhaustion. Increasing expression of PD-1 at baseline was associated with an unfavorable outcome. This suggests the possibility that addition of an anti–PD-1 checkpoint blocker may improve clinical outcome.
Although the short drug half-life and limited higher dose exploration because of CRS made the determination of an RP2D challenging, novel approaches to dose optimization allowed exploration of higher doses through more frequent dosing (ie, every-other-day dosing) in the first week, which impressively mitigated CRS during step-ups and saw response. Important recommendations for further study of vibecotamab in MDS- and MRD-positive AML were made after observation of response in patients with low disease-burden AML.
Acknowledgments
This study was supported by Xencor, Inc in collaboration with Novartis. The authors thank all the patients for their participation in the study. Medical writing/editorial assistance during the preparation of this manuscript was provided to the authors by Aurelia Syngkon and Ruth Widmer and supported by Xencor, Inc.
Authorship
Contribution: This study was designed by the sponsor (Xencor, Inc) and the study was conducted by Xencor, Inc in collaboration with the investigators (F.R., A.M., A.B., J.F., W.S., R.M., N.S., M.Y., H.K., O.O., and A.P.). R.G., W.B.A., R.C., J.K., Y.D., H.L., and S.K. wrote the manuscript; F.R., A.M., A.B., J.F., W.S., and R.M. critically reviewed and revised the manuscript; and all authors agreed to be accountable for the accuracy and integrity of this work.
Conflict-of-interest disclosure: A.M. participated on the data safety and monitoring board for Daiichi Sankyo, Foghorn Therapeutics, and Jazz Pharmaceuticals; served on the scientific advisory board for Bristol Myers Squibb, AbbVie, Astellas Pharma Inc, Servier, Syndax, and Rigel Therapeutics; and served in a leadership or fiduciary role in the Beat AML Study for Leukemia and Lymphoma Society. W.S. received consulting fees from Amgen, AstraZeneca, Jazz, Kite, Pfizer, Kura, Syndax, and Servier; received honoraria from Jazz and Pfizer; and participated on the data safety monitoring board or advisory board for Newave. N.S. received grants or contracts from Takeda Oncology, Astellas Pharma, Xencor, Inc, Stemline Therapeutics Inc, and Next Cure; received consulting fees from Pfizer, GlaxoSmithKline, NKARTA, and Sanofi; and received payment or honoraria for lectures, presentations, etc. from Adaptive Biotechnologies, Novartis, Amgen, Takeda Oncology, Pfizer Inc, Astellas Pharma Inc, Sanofi, and BeiGene. M.Y. received payment or honoraria for lectures, presentations, etc. from Daiichi Sankyo, Pfizer, and Cardinal Health. O.O. received grants or contracts from AbbVie, Agios, Aprea, Astex, AstraZeneca, Bristol Myers Squibb, Calgene, CTI BioPharma, Daiichi, Incyte, Janssen, Kartos, Novartis, NS-Pharma, and Oncotherapy Sciences, and received honoraria for participation on a data safety monitoring board for Treadwell Therapeutics and on advisory boards for Bristol Myers Squibb, Calgene, Novartis, Rigel, Taiho, Kymera Therapeutics, and Blueprint Medicines. A.P. received research funding from Kronos Bio and Pfizer, and participated on the data safety monitoring board or served on an advisory board for AbbVie and Bristol Myers Squibb. R.G. and S.K. were former employees of Xencor, Inc. W.B.A., R.C., J.K., Y.D., and H.L. are full time employees of Xencor, Inc and are stockholders in Xencor, Inc. R.C. and Y.D. are listed on patents related to vibecotamab use in patients. The remaining authors declare no competing financial interests.
The current affiliation of S.K. is Clinical Development, Karyopharm Therapeutics, Boston, MA.
Correspondence: Farhad Ravandi, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: fravandi@mdanderson.org.
References
Author notes
Data are available on reasonable request from the corresponding author, Farhad Ravandi (fravandi@mdanderson.org).
Individual patient data will not be shared.
The full-text version of this article contains a data supplement.