Key Points
Genes associated with E-box-GATA enhancers are upregulated at specific time points during acute anemia recovery within erythroid precursors.
Anemia-activated Ssx2ip promotes erythroid progenitor and precursor cell cycle progression and proliferation.
Abstract
Acute anemia elicits broad transcriptional changes in erythroid progenitors and precursors. We previously discovered a cis-regulatory transcriptional enhancer at the sterile alpha motif domain-14 enhancer locus (S14E), defined by a CANNTG-spacer-AGATAA composite motif and occupied by GATA1 and TAL1 transcription factors, is required for survival in severe anemia. However, S14E is only 1 of dozens of anemia-activated genes containing similar motifs. In a mouse model of acute anemia, we identified populations of expanding erythroid precursors, which increased expression of genes that contain S14E-like cis elements. We reveal that several S14E-like cis elements provide important transcriptional control of newly identified anemia-inducing genes, including the Ssx-2 interacting protein (Ssx2ip). Ssx2ip expression was determined to play an important role in erythroid progenitor/precursor cell activities, cell cycle regulation, and cell proliferation. Over a weeklong course of acute anemia recovery, we observed that erythroid gene activation mediated by S14E-like cis elements occurs during a phase coincident with low hematocrit and high progenitor activities, with distinct transcriptional programs activated at earlier and later time points. Our results define a genome-wide mechanism in which S14E-like enhancers control transcriptional responses during erythroid regeneration. These findings provide a framework to understand anemia-specific transcriptional mechanisms, ineffective erythropoiesis, anemia recovery, and phenotypic variability within human populations.
Introduction
Erythropoiesis is controlled by extrinsic factors, including stem cell factor/Kit and erythropoietin (EPO)/EPO receptor signaling pathways, and intrinsic erythroid-expressed transcription factors (TFs).1,2 In acute anemia, unique signals and progenitor/precursor cells accelerate the physiological rate of erythropoiesis to restore homeostasis.3 Among the critical regulatory steps in resolving anemia, GATA-binding proteins (GATA1 and GATA2) coordinate hematopoietic and erythroid transcription intrinsically.4-7 Upregulation of the GATA factor target gene, Sterile alpha motif domain-14 (Samd14), is required for survival in acute anemia.4 To activate Samd14 expression in acute anemia, GATA factors use the Samd14 enhancer (S14E), delineated by an E-box (CANNTG) and GATA (A/T)GATA(G/A/C) sequence (E-box–GATA element).8,9 Other examples of E-box–GATA element control of erythropoiesis have been described at loci encoding the GATA2 TF;10,11 Kruppel-like factor-1 TF;12Epb4.2,13 a component of the erythroid cytoskeleton; Kit,14 a receptor tyrosine kinase; Runx1;15 and Smad1.16 Among what is predicted to be a large cohort of similar elements controlling vital cell functions and environmental stress responses,4,7 only a few E-box–GATA elements have been functionally tested.
Acute anemia provides a powerful model system to investigate proximal anemia-induced flux in transcriptional programs. Beyond the S14E at the Samd14 locus, cis elements sharing similar features to those of S14E may be required for anemia gene regulation.4,17,18 However, because regeneration involves extensive cellular remodeling of extramedullary sites (eg, spleen), 1 critical challenge to understanding the mechanisms of gene regulation in anemia requires deconvoluting cellular vs transcriptional changes in regenerative contexts. In this study, we used single-cell RNA sequencing (scRNA-seq) in a model of acute anemia to predict a network of S14E-like cis elements. We tested transcriptional control of prioritized S14E-like enhancers and linked these mechanisms to temporal changes in transcriptional activity over a time course of anemia recovery. By functionally evaluating candidates within a network of S14E-like elements, we identified new mechanisms promoting the resolution of acute anemia. These findings aid our understanding of transcriptional activation of anemia-response genes and unravel key factors involved in resolving anemia, particularly related to cell cycle.
Methods
scRNA-seq
Animal experiments received ethical approval of the University of Nebraska Medical Center Institutional Animal Care and Use Committee (IACUC). Hemolytic anemia was induced by phenylhydrazine (PHZ; Sigma) administered subcutaneously (100 mg/kg). We performed Seq-Well sequencing on Kit+ cells from 8 week-old female C57BL/6 mice (1, 3, or 7 days after PHZ treatment) as described by Gierahn et al.19 Whole transcriptome amplification libraries were quality checked using Bioanalyzer (UNMC Genomics Core Facility) and sequenced (Illumina NovaSeq).
scRNA-seq data analysis
After checking consistency (see supplemental Figure 1A-B), replicates were pooled and processed.20 Clusters were annotated using known markers in immunophenotypically defined hematopoietic populations.21 Single-cell regulatory network inference and clustering (SCENIC) analysis (hematopoietic stem/progenitor cells [HSPCs] and 5 erythroid clusters) was performed using R program.22 To define coexpression networks, we used GRNBoost, RcisTarget, and AUCell in R.22 To perform pseudotime analysis, we used Monocle3.23 For comparisons of cells isolated on days 1, 3, and 7 after PHZ treatment, samples were reclustered, yielding only 4 of the 5 erythroid clusters (E1 and E3-5). Timeclust function in TCseq24 was used for plot expression. Information regarding sequencing reads, mapping percentage, and gene coverage are shown in supplemental Table 1.
Cell isolation and culturing
Spleens from mice that underwent PHZ treatment were lineage-depleted (see supplemental Methods) to generate primary cell cultures. For colony assays (M3434, Stemcell Technologies), 2 × 104 retrovirally infected cells were mixed with 2 mL MethoCult (M3434) ∼16 hours after infection. Burst forming unit-erythroid (BFU-E) and colony forming unit-erythroid (CFU-E) were counted after days 5 and 2 in culture, respectively. Flow cytometric cell phenotyping and cell cycle and proliferation analyses were conducted on 3-day-old cultures of PHZ-treated and retrovirally infected erythroid precursors.
Plasmids and transfection
For mouse Ssx2ip knock down, short hairpin RNAs (shRNAs) targeting exons 6 and 13 were synthesized and cloned into pMSCV PIG (Addgene #21654). For human CRISPR interference experiments, 2 single-guide RNAs (sgRNAs) targeting E-box–GATA, 1 sgRNA targeting a nearby chromatin region, and nontargeting controls were synthesized and cloned into pLV hU6-sgRNA hUbC-dCas9-KRAB-T2a-GFP (Addgene #71237).
FAIRE
Formaldehyde assisted isolation of regulatory elements (FAIRE) was conducted as described by Simon et al.25 Cells were fixed with 1% formaldehyde and sonicated to shear the DNA. The sonicated size (200-300 bp) of DNA was checked via gel electrophoresis. After the phenol:chloroform extraction of protein-bound DNA, samples were analyzed via quantitative real-time polymerase chain reaction (RT-PCR) relative to input controls.
Statistical analysis
Wilcoxon signed rank test was used to test the significance of transcriptional changes in scRNA-seq data. Adjusted P value is based on Bonferroni correction. Statistical analysis for quantitative RT-PCR was performed using GraphPad Prism version 8 as indicated. The results are given as the mean ± standard error of the mean. Multiple independent cohorts were used in each experiment. Statistical comparisons between 2 groups were performed using two-tailed unpaired students t test, with a significance cutoff of P < .05.
Results
Anemia elicits transcriptional activation at loci containing S14E-like cis elements
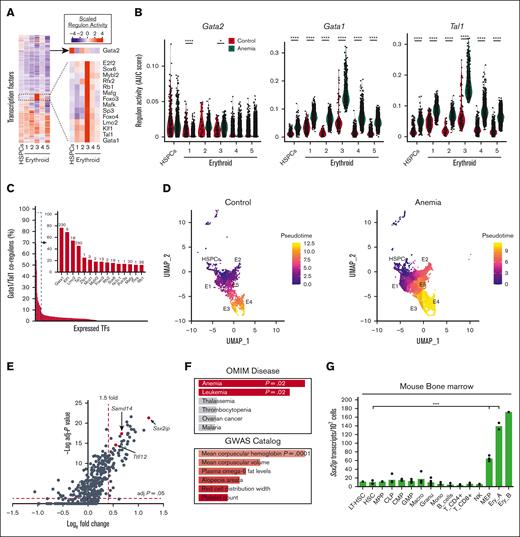
To identify anemia-induced changes in the numbers of phenotypically similar cells or transcript numbers per cell, we conducted scRNA-seq before and after acute anemia in mice. Kit+ cells were enriched from mouse spleen 3 days after treatment using the red blood cell lysing chemical PHZ or pretreatment control (Figure 1A). PHZ rapidly induces anemia and stimulates erythroid regeneration.26 A population of erythroid progenitors and precursors in spleen expands and differentiates. Compared with other anemia models with longer time courses, the rapid hemolysis and onset of anemia after PHZ injection permits investigation of anemia-induced transcriptional changes. Integrated analysis of control (7118 cells; N = 2 mice) and anemia (10 305 cells; N = 2 mice) scRNA-seq data revealed 18 cell clusters, visualized using uniform manifold approximation and projection (Figure 1B). Biological replicates from individual mice exhibited nearly identical clustering, indicating good reproducibility (supplemental Figure 1A-B). Cells expressing HSPC markers increased in anemic conditions (P < .0001). Five transcriptionally distinct erythroid clusters (termed as E1-E5) were identified with an increased frequency in anemia (P < .0001; Figure 1C). Nonerythroid cells decreased from 71.8% in controls to 35.8% in mice with anemia, consistent with previous finding about extramedullary erythropoiesis in mouse spleen.
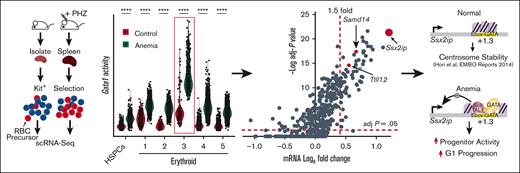
Enrichment of anemia-activated genes at loci containing E-box–GATA composite element sequences. (A) Experimental strategy for scRNA-seq of erythroid precursors from control and PHZ-induced anemic spleens. (B) UMAP representation of control (7118 cells) and PHZ-treated (10 305 cells) samples. Annotation indicates 18 distinct color-coded clusters. (C) Percentages of HSPCs and erythroid clusters relative to total cells analyzed in control and anemia samples. Cells not annotated as HSPC or erythroid are shown as “others.” Statistical significance between cell percentages in control and anemia determined using a Fisher exact test. (D) Dot plot depicting changes in normalized average expression (color of dot) and expression in a percentage of cells (size of dot) of hematopoietic and erythropoietic genes in HSPCs and erythroid cells. (E) Bar graph of Samd14 transcript levels in CD71+ cells from control and anemia samples (normalized expression of Tfrc > 0). Statistical significance calculated using Wilcoxon test. (F) The percentage of Samd14 expressing cells (depicted in a line graph) in control and anemia CD71+ cells. Statistical significance was determined using Fisher exact test. (G) Bar graph comparison of SLC15A37 and SOX6 expression in erythroid precursors from healthy controls and patients with aplastic anemia, alongside control and anemia data from PHZ-treated mouse samples. Statistical significance determined using unpaired Student t test. (H) In each cluster, ratio indicates the proportion of RNA transcripts annotated within 100 kb of CANNTG[N6-14]AGATAA (EGA) within all detected RNA transcripts (orange bars) or anemia-upregulated RNA transcripts (black bars; fold >1.5; adjusted P < .05). (I) In each cluster, ratio indicates the proportion of detected RNA transcripts within 100 kb of GATA/TAL-occupied EGA sequences (GSE51338) or GATA2 (GSE29193) and TAL1 (GSE36029) within all detected RNA transcripts (blue bars) or anemia-upregulated RNA transcripts (gray bars). Statistical significance was determined using Wilcoxon test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). RBC, red blood cell; UMAP, uniform manifold approximation and projection.
Enrichment of anemia-activated genes at loci containing E-box–GATA composite element sequences. (A) Experimental strategy for scRNA-seq of erythroid precursors from control and PHZ-induced anemic spleens. (B) UMAP representation of control (7118 cells) and PHZ-treated (10 305 cells) samples. Annotation indicates 18 distinct color-coded clusters. (C) Percentages of HSPCs and erythroid clusters relative to total cells analyzed in control and anemia samples. Cells not annotated as HSPC or erythroid are shown as “others.” Statistical significance between cell percentages in control and anemia determined using a Fisher exact test. (D) Dot plot depicting changes in normalized average expression (color of dot) and expression in a percentage of cells (size of dot) of hematopoietic and erythropoietic genes in HSPCs and erythroid cells. (E) Bar graph of Samd14 transcript levels in CD71+ cells from control and anemia samples (normalized expression of Tfrc > 0). Statistical significance calculated using Wilcoxon test. (F) The percentage of Samd14 expressing cells (depicted in a line graph) in control and anemia CD71+ cells. Statistical significance was determined using Fisher exact test. (G) Bar graph comparison of SLC15A37 and SOX6 expression in erythroid precursors from healthy controls and patients with aplastic anemia, alongside control and anemia data from PHZ-treated mouse samples. Statistical significance determined using unpaired Student t test. (H) In each cluster, ratio indicates the proportion of RNA transcripts annotated within 100 kb of CANNTG[N6-14]AGATAA (EGA) within all detected RNA transcripts (orange bars) or anemia-upregulated RNA transcripts (black bars; fold >1.5; adjusted P < .05). (I) In each cluster, ratio indicates the proportion of detected RNA transcripts within 100 kb of GATA/TAL-occupied EGA sequences (GSE51338) or GATA2 (GSE29193) and TAL1 (GSE36029) within all detected RNA transcripts (blue bars) or anemia-upregulated RNA transcripts (gray bars). Statistical significance was determined using Wilcoxon test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). RBC, red blood cell; UMAP, uniform manifold approximation and projection.
Anemia-induced expression changes were assessed based on transcripts per cell and number of cells expressing each transcript. To reveal functional distinctions, we compared expression patterns in erythroid clusters (supplemental Figure 1C). E1 cells expressed metal sequestering Mt1 and Mt2 genes. Erythroid commitment markers (eg, Slc4a1) and hemoglobin genes (eg, Hbaa1) were highest in E3 and E4 clusters, respectively. Because the GATA2 and GATA1 TFs regulate erythropoiesis at distinct stages,27 we then examined Gata2 and Gata1 expression. Gata2 was expressed highest in the HSPC-annotated cluster, and anemia led to an increase in the percentage of all Gata2-expressing Kit+ cells 4.9-fold compared with that in the controls. In erythroid-annotated clusters, anemia led to an increase in the percentage of Gata1-expressing cells 8.4-fold (Figure 1D). Because the erythroid precursors (CD71+Kit+) upregulate Samd14 via S14E in anemia,4 we isolated cells with >0 transcripts of the transferrin receptor gene (Tfrc encoding CD71) to mimic this population. Samd14 transcripts per cell were 6.4-fold higher in mice with anemia than in those without, confirming increased Samd14 in CD71+ cells (Figure 1E). Similarly, anemia led to an increase in the number of Samd14-expressing CD71+ cells (Figure 1F). Gene ontology (GO) of anemia upregulated genes in erythroid clusters included genes involved in DNA replication and cell cycle (supplemental Tables 2 and 3). Next, we compared PHZ-induced acute anemia to a human scRNA-seq data set generated from samples obtained from patients with aplastic anemia.28 A total of 121 transcripts were elevated more than twofold in GATA1- and TFRC-expressing erythroid precursors in patients with aplastic anemia compared with those in healthy controls (supplemental Figure 1D). In E3 cells, 36 of the 121 transcripts were PHZ-anemia upregulated. Upregulated genes in both data sets included the iron transporter mitoferrin (SLC25A27), SRY-box TF6 (SOX6; Figure 1G), and heme biosynthetic enzymes (supplemental Figure 1E).
Samd14 anemia activation requires an intronic Samd14 cis element (S14E).4 We hypothesized that a cohort of similar S14E-like elements, defined by an E-box–GATA sequence, GATA1 and T-cell acute lymphocytic leukemia protein 1 (TAL1) occupancy, and anemia activation, would reside near anemia-regulated gene loci. To discover E-box–GATA associated (EGA) genes throughout the mouse genome, E-box–GATA sequences were annotated to the nearest genes (<100 kb). Compared with all EGA transcripts in E1,3,4, and 5 clusters, a higher frequency were found at anemia upregulated genes (Figure 1H). Next, we limited the analysis to E-box–GATA sequences that were occupied by GATA1/2 and TAL1 in published data sets. The frequency of EGA anemia-activated genes was even further enriched (2.2-fold higher) compared with all detected transcripts (Figure 1I). De novo motif analysis of the region surrounding the E-box–GATA sequence did not help identify any other predictive motifs enriched at anemia-activated sites. Thus, cohorts of anemia-activated genes are near S14E-like elements.
Gene loci containing S14E-like cis elements are anemia-activated with Samd14 in similar cells, suggesting a common transcriptional mechanism. To investigate the anemia-instigated changes to TF activities in individual cells, we used SCENIC.22 SCENIC predicts TF targets by combining messenger RNA (mRNA) coexpression data with TF motif analysis to define a regulon activity score. SCENIC detected 297 active TF regulons in our data, including 14 with high regulon activity in E3 (Figure 2A). Among those 14, LIM domain only 2 (LMO2), LIM domain binding 1, and ETS-related gene are also known to colocalize on chromatin with GATA1 and TAL1.29 Notably, E3 is the same cluster in which we observed the highest enrichment of EGA-containing genes (Figure 1I). Unnormalized regulon scores in each cell were replotted and compared between anemia and control samples. Both Gata1 and Tal1 elicit higher regulon activity in anemia cells, particularly in E3 cells (Figure 2B).
Anemia increased regulon activity of EGA TFs. (A) Heatmap of scaled regulon activity scores based on the coexpression of predicted target genes and TF recognition motif enrichment, in HSPC and erythroid clusters. Inset: heatmap of GATA2 regulon activity and a cluster of regulons (including GATA1) with high activity in E3 cells. (B) Violin plot of GATA2, GATA1, and TAL1 regulon activity scores in every cell of HSPCs and erythroid clusters. Red, control; and blue, PHZ. Statistics were calculated using Wilcoxon test. (C) Bar graph depicting percentage of shared regulon activity between individual TFs (ranked per the order of shared activity) with the GATA1/TAL1 coregulons. All TFs with triregulon overlap of more than 10% are shown (inset). Complete list of TFs are given in supplemental Table 4. (D) UMAP representation of HSPC and erythroid cells from control and anemia samples that are color-coded based on pseudotime projections from Monocle3. (E) Volcano plot of all EGA GATA/TAL1-occupied genes in E3. Significantly upregulated genes were selected based on fold change >1.5 and adjusted P < .05. Red dots indicate Ssx2ip, Samd14, and Ttll12. (F) Enrichr analysis of 65 anemia upregulated genes associated with S14E-like enhancers, using data from the OMIM and GWAS catalog. (G) Bar graph quantitation of Ssx2ip expression level in sorted populations of hematopoietic cells isolated from mouse bone marrow, as described by Lara-Astiaso et al.21 Statistical significance was determined using two-tailed unpaired Student t test. ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001. CLP, common lymphoid progenitor; CMP, common myeloid progenitor; EryA, Ter119+CD71+FSChigh; EryB, Ter119+CD71+FSClow; HSC, hematopoietic stem cells; GMP, granulocyte monocyte progenitor; LT-HSC, long-term hematopoietic stem cells; MEP, megakaryocyte erythroid progenitor; MF, macrophage; MPP, multipotent progenitors; NK, natural killer cells; OMIM, Online Mendelian Inheritance in Man.
Anemia increased regulon activity of EGA TFs. (A) Heatmap of scaled regulon activity scores based on the coexpression of predicted target genes and TF recognition motif enrichment, in HSPC and erythroid clusters. Inset: heatmap of GATA2 regulon activity and a cluster of regulons (including GATA1) with high activity in E3 cells. (B) Violin plot of GATA2, GATA1, and TAL1 regulon activity scores in every cell of HSPCs and erythroid clusters. Red, control; and blue, PHZ. Statistics were calculated using Wilcoxon test. (C) Bar graph depicting percentage of shared regulon activity between individual TFs (ranked per the order of shared activity) with the GATA1/TAL1 coregulons. All TFs with triregulon overlap of more than 10% are shown (inset). Complete list of TFs are given in supplemental Table 4. (D) UMAP representation of HSPC and erythroid cells from control and anemia samples that are color-coded based on pseudotime projections from Monocle3. (E) Volcano plot of all EGA GATA/TAL1-occupied genes in E3. Significantly upregulated genes were selected based on fold change >1.5 and adjusted P < .05. Red dots indicate Ssx2ip, Samd14, and Ttll12. (F) Enrichr analysis of 65 anemia upregulated genes associated with S14E-like enhancers, using data from the OMIM and GWAS catalog. (G) Bar graph quantitation of Ssx2ip expression level in sorted populations of hematopoietic cells isolated from mouse bone marrow, as described by Lara-Astiaso et al.21 Statistical significance was determined using two-tailed unpaired Student t test. ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001. CLP, common lymphoid progenitor; CMP, common myeloid progenitor; EryA, Ter119+CD71+FSChigh; EryB, Ter119+CD71+FSClow; HSC, hematopoietic stem cells; GMP, granulocyte monocyte progenitor; LT-HSC, long-term hematopoietic stem cells; MEP, megakaryocyte erythroid progenitor; MF, macrophage; MPP, multipotent progenitors; NK, natural killer cells; OMIM, Online Mendelian Inheritance in Man.
Next, we questioned whether predicted target genes in Gata1 and Tal1 regulons overlapped with other TF regulons. This analysis identified TFs known to co-occupy chromatin with GATA factors (Kruppel-like factor 1 and Lmo2),9,30 and TFs not previously linked to GATA factor function (eg, Sox6 and Mafg; Figure 2C). Sox6 promotes erythropoiesis in anemia contexts,31 and regulon activity overlaps with GATA1 and TAL1 at 19 anemia-activated genes (supplemental Table 4). Genome-wide analysis of chromatin immunoprecipitation (ChIP)-seq in K562 cells reveals that the majority of GATA1-bound loci are also SOX6- and TAL1-occupied, suggesting cooperative function (supplemental Figure 2A). To establish E3 cells along the erythroid lineage, we performed a pseudotime analysis for HSPCs and all erythroid clusters using Monocle3,23 indicating that E3 cells were more differentiated than E1 and E5 cells but less differentiated than E4 cells (Figure 2D; supplemental Figure 2B). Together, E3 cells are likely middle-stage erythroid precursors with high GATA1 and TAL1 TF activities.
To identify S14E-like elements in E3 cells, GATA1 and TAL1 occupancy at E-box–GATA sites was determined using ChIP-seq data (see supplemental Methods). Of the 821 differentially expressed genes between control and anemia conditions, 65 were associated with GATA1/TAL1-occupied S14E-like elements, including the previously identified Samd14 transcript (Bonferroni-corrected P value < .05; fold change >1.5; Figure 2E). GO indicates that many of these genes are involved in DNA replication (supplemental Table 5). Cross referencing to the Online Mendelian Inheritance in Man database revealed that E3 anemia upregulated genes are associated with mutations commonly found in anemia and leukemia phenotypes, including SLC25A38, GLRX5, RAD51, and BRCA1, and polymorphisms in genes at these loci are associated with hematologic traits (Figure 2F). Intronic S14E-like elements, inferred based on chromatin accessibility/attributes and erythroid-specific gene expression, reside at loci containing SSX2-interacting protein (Ssx2ip) and Tubulin Tyrosine Ligase Like 12 (Ttll12) genes (Figure 2G; supplemental Figure 2C). Samd14, Gata1, Ssx2ip, and Ttll12 are similarly upregulated in a model of acute stress erythropoiesis induced by hypoxia32 (supplemental Figure 2D), suggesting that their upregulation is a common feature in acute anemia.
Anemia-activated E-box–GATA–containing cis elements coordinate gene regulation
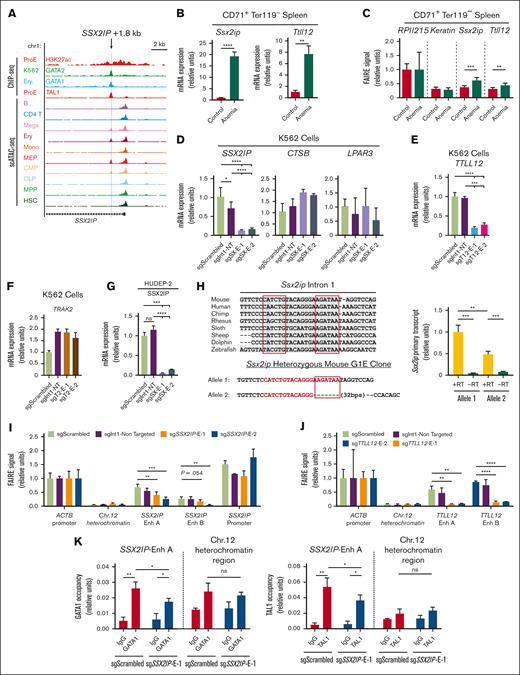
Anemia activation of Samd14 requires S14E characterized by erythroid-specific chromatin accessibility and H3K27 acetylation. Among S14E-associated cis elements located intronic to genes, the signature of Ssx2ip and Ttll12 putative enhancers in mouse and human data sets resembled S14E (Figure 3A; supplemental Figure 3A). The putative Ssx2ip enhancer is also SOX6 occupied in human K562 cells (supplemental Figure 3B). In CD71+ Ter119− fluorescence-activated cell sorting–purified mouse spleen cells, quantitative RT-PCR results showed that anemia increased Ssx2ip (19-fold; P < .0001) and Ttll12 (7.5-fold; P = .0014) mRNA levels (Figure 3B). FAIRE revealed that chromatin accessibility surrounding mouse intronic Ssx2ip (+ 1.3 kb) and Ttll12 (+ 2.7 kb) cis elements increased in anemia samples (Figure 3C). To test the activity of conserved elements at human SSX2IP (+ 1.8 kb) and TTLL12 (+ 3.4 kb) loci, we generated 2 cis element-targeting sgRNAs expressed in dead Cas9 fused to a Krüppel-associated box (dCas9-KRAB) lentiviral vectors. To control for enhancer-independent dCas9-mediated blockage of transcription, control sgRNAs target dCas9-KRAB at distinct sites in the same intron. In human K562 cells, sgRNA-dCas9-KRAB targeting the SSX2IP cis element reduced its expression compared with that of nontargeted (7.9-fold; P < .0001; sgSX-E-1) and intron-targeted controls (5.6-fold; P < .001; sgSX-E-1; Figure 3D). Cis element–targeted sgRNAs did not decrease the expressions of SSX2IP locus-adjacent genes, lysophosphatidic acid receptor 3 and Cathepsin-B. Similarly, TTLL12 transcription decreased by 5-fold (P < .001; sgT12-E-1) in K562 cells expressing TTLL12 cis element–targeted sgRNAs compared with intron-targeted controls (Figure 3E). Trafficking kinesin 2 (TRAK2) gene expression, which contained a GATA1/TAL1 occupied intronic E-box–GATA sequence in K562 cells, was uninhibited by sgRNA-dCas9-KRAB targeting, indicating that the cis element may be dispensable for TRAK2 expression in K562 cells (Figure 3F). Enhancer activity at the human SSX2IP + 1.8 site was confirmed in human erythroid progenitor cell line HUDEP-2 (Figure 3G). To independently establish transcriptional requirements for the Ssx2ip cis element, we generated a heterozygous-deleted G1E clone in which the GATA motif was edited out from only 1 allele (Figure 3H). Allele-specific Ssx2ip primary transcripts were 52% lower at the mutant vs wild-type alleles (Figure 3H). We then used FAIRE to quantitate open chromatin in dCas9-infected K562 cells. At SSX2IP and TTLL12, accessibility was lower in cells expressing dCas9-KRAB with an sgRNA targeted to SSX2IP + 1.8 or TTLL12 + 3.4 sites than that in cells with control-targeted sgRNAs (Figure 3I-J). Quantitative ChIP in dCas9-infected K562 cells revealed that cis element targeting decreased chromatin occupancy of GATA1 and TAL1 at the SSX2IP locus (Figure 3K). Thus, 2 erythroid-specific S14E-like enhancers drive the expression of the anemia-activated SSX2IP and TTLL12 genes, supporting a model in which a cohort of S14E-like elements regulate transcription in anemia conditions.
Anemia-activated cis elements are functional E-box–GATA enhancers. (A) Published data of chromatin occupancy and accessibility at the human SSX2IP locus shown in UCSC Genome Browser. Profiles from ChIP-sequencing of H3K27ac (GSM1278239) and TAL1 (GSM1278241) in human bone marrow derived proerythroblasts (ProE), GATA2 (GSM467648) in K562 cells, GATA1 (GSM935465) in human peripheral derived blood-erythroblast (Ery.), and single-cell ATAC-seq peak in human hematopoietic cell types (GSE74310). Light blue line indicates the E-box–GATA sequence. (B) Quantitation of Ssx2ip and Ttll12 mRNA in mouse CD71+ Ter119− spleen cells. Control, untreated WT mouse. Anemia, 3 days after PHZ treatment. (C) FAIRE signal at RPII215, Keratin, Ssx2ip, and Tttl12 loci in mouse mouse CD71+ Ter119− spleen cells. (D) Quantitation of human SSX2IP mRNA and its adjacent genes CTSB and LPAR3 mRNA in GFP+ K562 cells infected with CRISPRi/dCas9-KRAB lentivirus containing sgRNAs targeting Scrambled (sgScrambled), same-intron control (sgInt1-NT), and 2 distinct sequences in the SSX2IP + 1.8 (sgSX-E-1 and sgSX-E-2). (E) Quantitation of human TTLL12 mRNA in GFP+ K562 cells infected with CRISPRi/dCas9-KRAB lentivirus containing sgRNAs targeting Scrambled (sgScrambled), same-intron control (sgInt1-NT), and 2 distinct sequences in the TTLL12 + 3.4 (sgT12-E-1 and sgT12-E-2). (F) Quantitation of human TRAK2 mRNA in GFP+ K562 cells infected with CRISPRi/dCas9-KRAB lentivirus containing sgRNAs targeting Scrambled (sgScrambled), same-intron control (sgInt1-NT), and 2 distinct sequences in the TRAK2 + 3.4 (sgT2-E-1 and sgT2-E-2). (G) Quantitation of human SSX2IP mRNA in GFP+ human umbilical cord blood–derived erythroid progenitor 2 (HUDEP-2) cells infected with CRISPRi/dCas9-KRAB lentivirus containing sgRNAs targeting Scrambled (sgScrambled), same-intron targeting control (sgInt1-NT), and 2 distinct sequences in the TRAK2 + 1.8 (sgSX-E-1 and sgSX-E-2). (H) (Left) Evolutionary conservation of E-box–GATA sequence in mouse, human, and other species. Sequence validation of a CRISPR/Cas9-generated heterozygous deletion in mouse G1E cells. (Right) Allele-specific primary transcript expression at Ssx2ip in heterozygous mouse G1E cells. (I-J) FAIRE signal of CRISPRi in human K562 cells in ACTB-promoter open region control, Ch12 heterochromatin closed region control and (I) SSX2IP E-box–GATA region, SSX2IP promoter region and (J) TTLL12 E-box-GATA region. Enh A and B are 2 sets of primer targeting the same region. Error bars represent SD. ∗P < .05 (two-tailed unpaired Student t test). (K) Quantitative ChIP analysis of GATA1 and TAL1 in K562 cells expressing CRISPRi with sgScrambled or sgSsx2ip-E1 RNAs (n = 3). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. B, B cell; CD4 T, CD4+ T cell; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; CRISPRi, CRISPR interference; Ery, Ter119+ erythroid cell; GFP, green fluorescent protein; HSC, hematopoietic stem cells; Mega, megakaryocyte; MEP, megakaryocyte erythroid progenitor; Mono, monocytes; MPP, multipotential progenitors; −RT, no reverse transcriptase; SD, standard deviation; WT, wild-type.
Anemia-activated cis elements are functional E-box–GATA enhancers. (A) Published data of chromatin occupancy and accessibility at the human SSX2IP locus shown in UCSC Genome Browser. Profiles from ChIP-sequencing of H3K27ac (GSM1278239) and TAL1 (GSM1278241) in human bone marrow derived proerythroblasts (ProE), GATA2 (GSM467648) in K562 cells, GATA1 (GSM935465) in human peripheral derived blood-erythroblast (Ery.), and single-cell ATAC-seq peak in human hematopoietic cell types (GSE74310). Light blue line indicates the E-box–GATA sequence. (B) Quantitation of Ssx2ip and Ttll12 mRNA in mouse CD71+ Ter119− spleen cells. Control, untreated WT mouse. Anemia, 3 days after PHZ treatment. (C) FAIRE signal at RPII215, Keratin, Ssx2ip, and Tttl12 loci in mouse mouse CD71+ Ter119− spleen cells. (D) Quantitation of human SSX2IP mRNA and its adjacent genes CTSB and LPAR3 mRNA in GFP+ K562 cells infected with CRISPRi/dCas9-KRAB lentivirus containing sgRNAs targeting Scrambled (sgScrambled), same-intron control (sgInt1-NT), and 2 distinct sequences in the SSX2IP + 1.8 (sgSX-E-1 and sgSX-E-2). (E) Quantitation of human TTLL12 mRNA in GFP+ K562 cells infected with CRISPRi/dCas9-KRAB lentivirus containing sgRNAs targeting Scrambled (sgScrambled), same-intron control (sgInt1-NT), and 2 distinct sequences in the TTLL12 + 3.4 (sgT12-E-1 and sgT12-E-2). (F) Quantitation of human TRAK2 mRNA in GFP+ K562 cells infected with CRISPRi/dCas9-KRAB lentivirus containing sgRNAs targeting Scrambled (sgScrambled), same-intron control (sgInt1-NT), and 2 distinct sequences in the TRAK2 + 3.4 (sgT2-E-1 and sgT2-E-2). (G) Quantitation of human SSX2IP mRNA in GFP+ human umbilical cord blood–derived erythroid progenitor 2 (HUDEP-2) cells infected with CRISPRi/dCas9-KRAB lentivirus containing sgRNAs targeting Scrambled (sgScrambled), same-intron targeting control (sgInt1-NT), and 2 distinct sequences in the TRAK2 + 1.8 (sgSX-E-1 and sgSX-E-2). (H) (Left) Evolutionary conservation of E-box–GATA sequence in mouse, human, and other species. Sequence validation of a CRISPR/Cas9-generated heterozygous deletion in mouse G1E cells. (Right) Allele-specific primary transcript expression at Ssx2ip in heterozygous mouse G1E cells. (I-J) FAIRE signal of CRISPRi in human K562 cells in ACTB-promoter open region control, Ch12 heterochromatin closed region control and (I) SSX2IP E-box–GATA region, SSX2IP promoter region and (J) TTLL12 E-box-GATA region. Enh A and B are 2 sets of primer targeting the same region. Error bars represent SD. ∗P < .05 (two-tailed unpaired Student t test). (K) Quantitative ChIP analysis of GATA1 and TAL1 in K562 cells expressing CRISPRi with sgScrambled or sgSsx2ip-E1 RNAs (n = 3). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. B, B cell; CD4 T, CD4+ T cell; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; CRISPRi, CRISPR interference; Ery, Ter119+ erythroid cell; GFP, green fluorescent protein; HSC, hematopoietic stem cells; Mega, megakaryocyte; MEP, megakaryocyte erythroid progenitor; Mono, monocytes; MPP, multipotential progenitors; −RT, no reverse transcriptase; SD, standard deviation; WT, wild-type.
Ssx2ip promotes regenerative erythroid progenitor and precursor functions
In our search for S14E-like elements, we identified the Ssx2ip locus with an unknown role in erythropoiesis. Ssx2ip interacts with synovial sarcoma X proteins in nuclear extracts.33Ssx2ip also interacts with microtubules in the cytoplasm, contributing to centrioles/centrosomes structure.34 In prior work it was noted that cell surface Ssx2ip expression is a possible leukemia biomarker with a role at centrosomes during mitosis.35,36 To shed light on Ssx2ip function in erythroid regeneration, we conducted loss-of-function analysis in cells isolated from 3-day post-PHZ injected mice. Lineage-depleted spleens were infected with control or 2 shRNAs targeting Ssx2ip. Knockdown efficiency of shSsx2ip-1 and shSsx2ip-2 expressed in immunophenotypically defined CD71+Ter119+ cells after 3-day expansion was 1.7-fold and 7.2-fold, respectively (Figure 4A). To determine changes in erythroid cellularity after Ssx2ip knockdown, we used cell surface markers CD71 and Ter119 to delineate R1 to R5 stages of erythropoiesis (Figure 4B).37 Quantitation of cell percentages in each gate indicated that Ssx2ip knockdown decreased immature erythroid precursors R1 cells 4.4-fold (P = .001; shSsx2ip-2 compared with shControl), concomitant with an increased percentage of late stage R3, R4, and R5 cells (Figure 4C). In cells isolated from mouse primary bone marrow, we observed similar results (supplemental Figure 4A). No change in the differentiation was observed in HUDEP-2 cells infected with CRISPR interference/sgRNAs targeting the SSX2IP cis element (supplemental Figure 4B). Wright-Giemsa staining to assess cell morphology indicated that Ssx2ip knockdown mouse primary cell cultures contained smaller erythroblasts, indicating more mature cells than those in controls (Figure 4D). Within mouse R1 and R2 cells, we also noted a decrease in cell size, measured via forward scatter, in R1 and R2 progenitors/precursors (Figure 4E). As an alternative assessment, Ter119− and Ter119+ were sorted via magnetic bead isolation before flow cytometric analysis. Within Ter119− cells, shSsx2ip-2 knockdown decreased the percentage of CD71low cells by ∼50% (supplemental Figure 4C-D).
Ssx2ip knock down impairs the function of erythroid precursors in regenerative erythropoiesis. (A) Quantitation of Ssx2ip mRNA level in cells after retroviral infection with nontargeting shControl or 2 shRNAs targeting Ssx2ip transcript (shSsx2ip-1 shSsx2ip-2) cells. Sorted GFP+CD71+Ter119− and GFP+CD71+Ter119+ populations are shown. (B) Flow cytometric gating using anti-CD71 and anti-Ter119 antibodies in PHZ-treated, lineage-depleted spleen cells after retroviral infection with shControl or shSsx2ip-2. R1 to R5 represent distinct stages of erythroid maturation. (C) Quantitation from R1 to R5 percentages in knockdown cells (N = 3). (D) Wright-Giemsa staining of GFP sorted spleen cells infected with shControl or shSsx2ip-2. Arrows represent prominent cell morphologies in each culture. (E) Quantitation of forward scatter (FSC) relative MFI in R1 and R2 cell populations. (F) Representative histograms (left) and quantitation (right) of Kit fluorescence intensity in knockdown or control GFP+ R1 and R2 cells. (G) Quantitation of BFU-E (counted on day 5) and CFU-E (counted on day 2) in GFP sorted shControl or shSsx2ip-2-expressing cells cultured in methylcellulose media containing SCF and Epo (N = 4). (H) Violin plot of BFU-E colony size (mm2) in GFP sorted shControl or shSsx2ip-2-expressing cells cultured in methylcellulose media containing SCF and Epo (N = 6). Each dot represents 1 colony. Solid line indicated median and dashed line indicated quartiles. (I) mRNA expression of erythroid-related genes Kit, Gypa, and EpoR in GFP-sorted R2 or R3 cells infected with shControl or shSsx2ip-2–expressing retrovirus (N = 3). Error bars represent SD. ∗P < .05 (two-tailed unpaired Student t test). SCF, stem cell factor.
Ssx2ip knock down impairs the function of erythroid precursors in regenerative erythropoiesis. (A) Quantitation of Ssx2ip mRNA level in cells after retroviral infection with nontargeting shControl or 2 shRNAs targeting Ssx2ip transcript (shSsx2ip-1 shSsx2ip-2) cells. Sorted GFP+CD71+Ter119− and GFP+CD71+Ter119+ populations are shown. (B) Flow cytometric gating using anti-CD71 and anti-Ter119 antibodies in PHZ-treated, lineage-depleted spleen cells after retroviral infection with shControl or shSsx2ip-2. R1 to R5 represent distinct stages of erythroid maturation. (C) Quantitation from R1 to R5 percentages in knockdown cells (N = 3). (D) Wright-Giemsa staining of GFP sorted spleen cells infected with shControl or shSsx2ip-2. Arrows represent prominent cell morphologies in each culture. (E) Quantitation of forward scatter (FSC) relative MFI in R1 and R2 cell populations. (F) Representative histograms (left) and quantitation (right) of Kit fluorescence intensity in knockdown or control GFP+ R1 and R2 cells. (G) Quantitation of BFU-E (counted on day 5) and CFU-E (counted on day 2) in GFP sorted shControl or shSsx2ip-2-expressing cells cultured in methylcellulose media containing SCF and Epo (N = 4). (H) Violin plot of BFU-E colony size (mm2) in GFP sorted shControl or shSsx2ip-2-expressing cells cultured in methylcellulose media containing SCF and Epo (N = 6). Each dot represents 1 colony. Solid line indicated median and dashed line indicated quartiles. (I) mRNA expression of erythroid-related genes Kit, Gypa, and EpoR in GFP-sorted R2 or R3 cells infected with shControl or shSsx2ip-2–expressing retrovirus (N = 3). Error bars represent SD. ∗P < .05 (two-tailed unpaired Student t test). SCF, stem cell factor.
Ssx2ip-induced changes to erythroid cells in culture could reflect the following: (1) Ssx2ip promotion of early progenitor activity or survival, (2) Ssx2ip inhibition of survival or proliferation in late-stage erythroblasts, or (3) Ssx2ip opposing cell differentiation. To assess progenitor/precursor levels, we quantitated Kit+ cells in cultures via flow cytometry. In R1 and R2 cells, Ssx2ip knockdown led to a decrease in Kit+ percentages, and the magnitude of decrease between the 2 shRNAs correlated with knockdown efficiency (Figure 4F). To test progenitor activity, colony assays were performed on control and shSsx2ip-expressing cells 16 hours after infection. The more effective shSsx2ip-2 knockdown impaired BFU-E colony-forming ability and significantly decreased CFU-E numbers (Figure 4G). BFU-E size was twofold less in shSsx2ip-2-infected cells than that in control cells (Figure 4H). Consistent with decreased Kit+ cells, Ssx2ip knockdown lowered Kit mRNA (Figure 4I). Thus, Ssx2ip deficiency impaired erythroid precursor activity.
Although mRNA levels of erythroid-specific Hba-a2, Gypa, and Epor did not change after Ssx2ip knockdown in R3 cells, Ssx2ip knockdown in CD71+Ter119+ decreased the expression of cell cycle regulatory genes Cdk4, Cdk6, and Chek1 (Figure 5A). To investigate Ssx2ip in erythroid precursor cell cycle, bromodeoxyuridine (BrdU)-labeled Ter119− and Ter119+ cells were stained with anti-CD71, anti-BrdU, and DAPI (DNA) and gated for G1 (DAPIlowBrdU−), S (BrdU+), and G2/M (DAPIhighBrdU−) cell cycle phases (Figure 5A; supplemental Figure 4D). In Ter119− cells, Ssx2ip knockdown led to a slight decrease in the percentage of S-phase cells (56.0% vs 52.5%; P = .049) in Ter119−CD71low. However, in erythroid-committed Ter119+CD71low cells, Ssx2ip knockdown led to an increase in G1 (46.4% vs 76.8%; P < .001) and decrease in S-phase cells (42.8% vs 15.8%; P < .001; Figure 5C). Similarly, Ki67 labeling of Ssx2ip knockdown cells indicated decreased percentages of S-phase cells compared with that of controls (supplemental Figure 4E). To test Ssx2ip function in cell proliferation, we tracked the number of cell divisions in culture using a membrane-intercalating fluorescent dye. After 48 hours, cells expressing control shRNA underwent 6 or 7 cell divisions (Figure 5D). Ssx2ip knockdown led to a decrease in the number of cells with 6 divisions and increase in the number of cells with 7 and 8 divisions in R3 cells (Figure 5E). The proliferation index increased by 12.5% (shSsx2ip-1; P = .003) and 16.6% (shSsx2ip-2; P < .001) compared with that of shControl (Figure 5F). These results help establish a role for Ssx2ip in erythroid precursor cell cycle and proliferation.
Ssx2ip regulates erythroid progenitor cell cycle progression and proliferation. (A) mRNA expression of cell cycle regulatory genes Cdk4, Cdk6, and Chek1 in GFP-sorted CD71+Ter119− (R2) and CD71+Ter119+ (R3) cells infected with shControl or shSsx2ip-2-expressing retrovirus. (B) Representative flow cytometric gating of lineage-depleted spleen cells infected with shControl or shSsx2ip-2-expressing retrovirus, cultured for 3 days, and incubated with BrdU for 1 hour. Ter119+ cells were stained with DAPI, anti-CD71 and anti-BrdU antibodies. (C) Quantitation of percentage of G1/S/G2/M-phase cells (from Figure 5A) in CD71−Ter119−, CD71lowTer119−, CD71highTer119−, CD71highTer119+, CD71lowTer119+, and CD71−Ter119+ subpopulations. (D) Representative plots depicting ModFit analysis of cell proliferation generations (in different colors) after 48 hours using CellTrace Violet. R3 population is shown. (E) Quantitation of percentage of cells in each cell proliferation generation based on CellTrace Violet. (F) Proliferation index of R3 cells after Ssx2ip knockdown after 48 hours incubation with CellTrace Violet. Error bars represent SD. ∗P < .05 (two-tailed unpaired Student t test).
Ssx2ip regulates erythroid progenitor cell cycle progression and proliferation. (A) mRNA expression of cell cycle regulatory genes Cdk4, Cdk6, and Chek1 in GFP-sorted CD71+Ter119− (R2) and CD71+Ter119+ (R3) cells infected with shControl or shSsx2ip-2-expressing retrovirus. (B) Representative flow cytometric gating of lineage-depleted spleen cells infected with shControl or shSsx2ip-2-expressing retrovirus, cultured for 3 days, and incubated with BrdU for 1 hour. Ter119+ cells were stained with DAPI, anti-CD71 and anti-BrdU antibodies. (C) Quantitation of percentage of G1/S/G2/M-phase cells (from Figure 5A) in CD71−Ter119−, CD71lowTer119−, CD71highTer119−, CD71highTer119+, CD71lowTer119+, and CD71−Ter119+ subpopulations. (D) Representative plots depicting ModFit analysis of cell proliferation generations (in different colors) after 48 hours using CellTrace Violet. R3 population is shown. (E) Quantitation of percentage of cells in each cell proliferation generation based on CellTrace Violet. (F) Proliferation index of R3 cells after Ssx2ip knockdown after 48 hours incubation with CellTrace Violet. Error bars represent SD. ∗P < .05 (two-tailed unpaired Student t test).
Distinct activities of transcriptional networks/circuits revealed throughout anemia recovery timeline
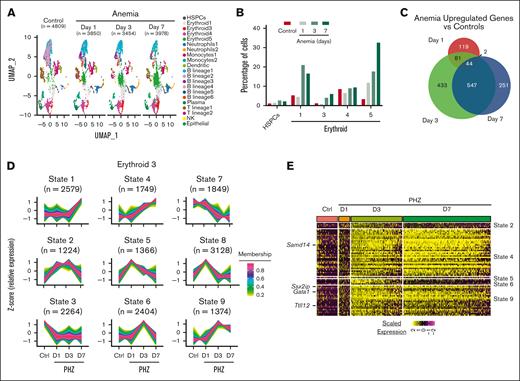
Three days after PHZ injection represents a time point during anemia response that is characterized by high BFU-E and CFU-E activity in the spleen.38 However, this time window captures neither progenitor cell activation, which occurs earlier, nor later stages of anemia recovery. Is transcription at S14E-associated loci elevated throughout recovery, or do distinct transcriptional states exist within stages of anemia recovery? To answer this, we generated scRNA-seq data in Kit+ spleen cells isolated from control mice, and early-, mid- and late-stage post-PHZ recovery mice on days 1, 3, and 7, respectively. (Figure 6A). The numbers of HSPCs and most erythroid clusters (E1 and E3-5) were elevated on days 3 and 7 after PHZ but not on day 1 (Figure 6B). Differential expression at each time point in post-PHZ recovery mice compared with controls in E3 cells (>1.5 fold; adjusted P < .05) revealed that 65% of upregulated genes on day 7 after PHZ were also upregulated on day 3, indicating sustained transcriptional activation of this cohort (Figure 6C). To probe activation patterns, we mapped the average expression of each transcript in E3 cells over time using TCseq.24 This analysis revealed 9 transcriptional states, including activation at early-stages (states 2, 5, and 8), midstage (states 4, 6, and 9), late-stage (state 1), and repression (states 3 and 7) (Figure 6D). Midstage activation states were observed in the Gata1 TF as well as the erythroid genes Samd14, Ssx2ip, and Ttll12 (Figure 6E). Approximately 86% of the 65 E3 anemia–activated E-box–GATA–associated genes were associated with midstage activation states 4 and 9 (Figure 6E). Although E3 cells upregulated a majority of genes on day 3 after PHZ, this was not the case in other cell populations. None of the 65 genes associated with S14E-like enhancers in E3 were state 1 (late-stage activation). However, in HSPCs and E1 cells, 27 and 26 of the 65 genes were state 1, respectively (supplemental Figure 5A). E4 and E5 cells were more like E3, albeit with fewer genes upregulated on day 3. Thus, distinct clusters exhibit differential patterns of gene activation in anemia responses. To determine the profile of cells with early-stage activation profiles, we mapped the highest 75 upregulated genes on day 1 after PHZ (supplemental Figure 5B; supplemental Table 6). Day 1 post-PHZ transcriptional activation was the highest in neutrophil-annotated cells but not erythroid cells. In addition to increased mRNA levels of neutrophil-associated genes, the percentage of neutrophils increased on days 1 and 3 after PHZ, followed by a decrease on day 7 (supplemental Figure 5C). Gene activation in neutrophils may reflect an immune response to acute anemia induced by PHZ.
Distinct activities of transcriptional networks/circuits revealed throughout anemia recovery timeline. (A) UMAP representation of scRNA-seq data generated from Kit+ spleen cells isolated from control mice and mice post-PHZ days 1, 3, and 7. Annotation indicates 21 distinct color-coded clusters. (B) Bar graph depicting HSPCs and erythroid-committed percentages relative to total Kit+ cells. (C) Venn diagram indicating the unique and shared upregulated genes in anemia vs control mice at indicated time points. (D) Clustered average expression over 4 time points of all E3-expressed genes. Each of 9 transcriptional states were determined by TCseq (z score represents relative vs average change in transcription level). Color bar of membership values indicate the degree to which data points conform to a transcriptional state. Number of transcripts conforming to each transcriptional state were shown in parentheses. (E) Heatmap of normalized expression level of 65 GATA/TAL1 occupied EGA genes in cells at each time points. Each column represents a single cell.
Distinct activities of transcriptional networks/circuits revealed throughout anemia recovery timeline. (A) UMAP representation of scRNA-seq data generated from Kit+ spleen cells isolated from control mice and mice post-PHZ days 1, 3, and 7. Annotation indicates 21 distinct color-coded clusters. (B) Bar graph depicting HSPCs and erythroid-committed percentages relative to total Kit+ cells. (C) Venn diagram indicating the unique and shared upregulated genes in anemia vs control mice at indicated time points. (D) Clustered average expression over 4 time points of all E3-expressed genes. Each of 9 transcriptional states were determined by TCseq (z score represents relative vs average change in transcription level). Color bar of membership values indicate the degree to which data points conform to a transcriptional state. Number of transcripts conforming to each transcriptional state were shown in parentheses. (E) Heatmap of normalized expression level of 65 GATA/TAL1 occupied EGA genes in cells at each time points. Each column represents a single cell.
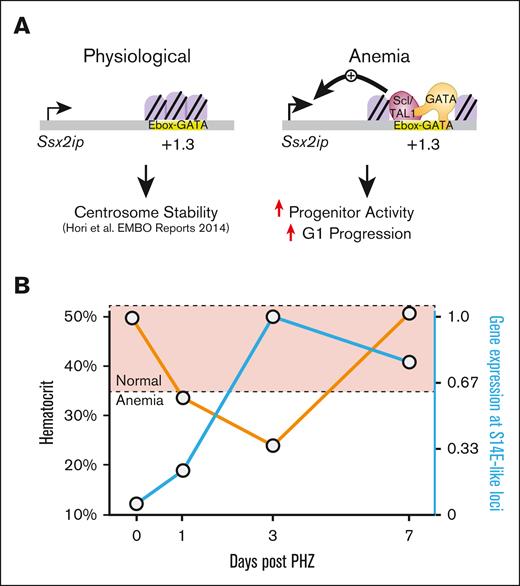
Overall, these findings revealed transcriptional activation kinetics in acute anemia at S14E-like genes. Ssx2ip activation via an S14E-like enhancer promoted erythroid precursor activity and cell cycle progression (Figure 7A). Expression of S14E-like EGA genes followed similar trajectories of anemia activation and inversely correlated with hematocrit levels in the peripheral blood (Figure 7B). By comparing the gene activation trajectories at multiple time points after anemia in cell types defined via scRNA-seq, our results support a staged recovery model in which an inflammatory signature precedes erythroid gene activation.
Transcriptional mechanisms of gene activation in regenerative erythropoiesis. (A) Ssx2ip activities are involved in regulating centrosome stability.35 In acute anemia, chromatin accessibility increased at an intronic GATA and TAL1-occupied E-box–GATA element in the Ssx2ip locus. Our model predicts that this element facilitates Ssx2ip upregulation in anemia. Ssx2ip knockdown in erythroid precursors isolated from anemic spleen revealed a role for Ssx2ip in colony formation and cell cycle regulation. (B) Expression levels of genes associated with S14E-like cis elements are inversely correlated with hematocrit levels in PHZ-induced models of acute anemia. Orange line, hematocrit levels; blue line, relative Ssx2ip expression.
Transcriptional mechanisms of gene activation in regenerative erythropoiesis. (A) Ssx2ip activities are involved in regulating centrosome stability.35 In acute anemia, chromatin accessibility increased at an intronic GATA and TAL1-occupied E-box–GATA element in the Ssx2ip locus. Our model predicts that this element facilitates Ssx2ip upregulation in anemia. Ssx2ip knockdown in erythroid precursors isolated from anemic spleen revealed a role for Ssx2ip in colony formation and cell cycle regulation. (B) Expression levels of genes associated with S14E-like cis elements are inversely correlated with hematocrit levels in PHZ-induced models of acute anemia. Orange line, hematocrit levels; blue line, relative Ssx2ip expression.
Discussion
In acute anemia, the required acceleration of erythropoiesis during recovery increases the strain on an already rapidly proliferating tissue. Foundational mechanistic work describes how erythroid precursors in anemia use unique signaling cues to ramp up erythropoiesis in multiple stages.1,3 Initial events after anemia involve the specification and amplification of stress progenitors and precursors that require signals resembling those of inflammatory stress,39,40 followed by EPO-induced erythroid precursor differentiation.41 Transcriptional changes induced by anemia during these stages involve broad changes to the cell cycle regulatory gene expression and an increase in the activity of specific subtypes of erythroid precursors.42,43 TFs including GATA1,44 GATA2,4,44 Smad5,26 and hypoxia inducible factor 1α45 mediate changes to gene expression programs in these contexts. To gain further insight into the anemia-induced transcriptional landscape, we interrogated a subset of anemia-activated genes containing shared chromatin features. Our analysis led to the identification of subtypes of erythroid precursors with unique predicted TF activities that increased per the specific anemia stress.
One of the necessary transcriptional changes in anemia involves upregulation of Samd14 via the S14E cis element.4 The presence of similar sequence and chromatin attributes found at other erythroid genes involved in anemia suggests that there may be shared mechanisms at other loci. However, predicting cis element activities based on bioinformatic criteria and the manner by which cis elements respond to environmental stimuli remains a complex challenge.46,47 We describe an approach for identifying cis elements with S14E-like functions using the anemia-stimulated activity of the S14E as a training set. This strategy predicted that several S14E-like elements exist throughout the genome, demarcated by an E-box–GATA sequence and GATA1 and TAL1 occupancy. This represents a functional subgroup of cis elements within a broader network of erythroid gene transcription. We predict that genes within this node may govern key processes related to anemia recovery. An in silico analysis of TFs with similar predicted anemia-induced regulatory networks with GATA factors helped identify Sox6, which promotes erythropoiesis in normal and stress contexts.31,48,49 SOX6 may cooperate with GATA factors at anemia-activated loci. Promising directions of this work involve distinguishing whether previously undescribed constituents of the S14E-like cohort function in erythropoiesis (steady state and/or stress) and establishing whether Ssx2ip and other constituents have analogous roles in chronic anemias.
SSX2IP (also called afadin DIL domain-interacting protein) maps to chromosome 1p22.3, a region prone to deletions, amplifications, and translocations in cancer.33,50 Following its discovery as an SSX2-interacting protein,33 cell surface SSX2IP levels were elevated in 1 of 3 acute myeloid leukemia samples.51 High SSX2IP expression also correlates with mitosis and abnormal elevation of cell cycle regulatory genes.52,53 SSX2IP interacts with centrosomes to control the orientation, organization, and length of interphase spindles during formation.35,54,55SSX2IP knockdown decreases mTORC1 activity, lysosome mobility, and cell proliferation.56 Our data suggest that SSX2IP controls erythroid precursor differentiation and proliferation. Taken together, this supports a mechanism through which GATA factor upregulation of SSX2IP promotes erythroid precursor self-renewal in anemia to facilitate recovery (Figure 7). Transcriptional control of SSX2IP, in a variety of contexts, may broadly affect the treatment of ineffective erythropoiesis and anemia recovery.
Acknowledgments
This work required support from Robert Lewis, Keith Johnson, and James Askew. Single-cell sequencing, Seq-Well reagents, and sequencing costs were provided by the Center for Molecular Target Discovery and Development, which is funded by the National Institutes of Health/National Institute of General Medical Sciences. The authors thank the UNMC Flow Cytometry Research Facility, supported by the Nebraska Research Initiative and The Fred and Pamela Buffett Cancer Center's National Cancer Institute Cancer support grant (P30 CA036727) as well as the UNMC DNA Sequencing Core, which receives partial support from the National Institute of General Medical Sciences INBRE – (P20GM103427-19). This study was supported by National Institutes of Health/National Heart, Lung, and Blood Institute (R01 HL155439-01) and the National Institute of Diabetes and Digestive and Kidney Diseases (K01DK113117-03). K.J.H received funding as a project leader in the Nebraska Center for Molecular Target Discovery and Development (1P20GM121316-01-A1), M.J.R. received grants from the National Institute of General Medical Sciences (R00GM127671 and R35GM147467), and Y.Z is supported by UNMC Graduate Studies Assistantship.
Authorship
Contribution: Y.Z. and K.J.H. designed the research and wrote the manuscript; Y.Z., V.R.D., S.R., and M.S. performed research; V.R.D., H.L.H., and M.J.R. contributed vital analytical tools; and Y.Z., M.J.R., and K.J.H. analyzed and interpreted data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kyle J. Hewitt, Department of Genetics, Cell Biology and Anatomy, University of Nebraska Medical Center, 985805 Nebraska Medical Center, Omaha, NE 68198; e-mail: kyle.hewitt@unmc.edu.
References
Author notes
scRNA-seq raw and processed files are deposited in the Gene Expression Omnibus database (accession number GSE212224).
Data are available on request from the corresponding author, Kyle J. Hewitt (kyle.hewitt@unmc.edu).
The full-text version of this article contains a data supplement.

![Enrichment of anemia-activated genes at loci containing E-box–GATA composite element sequences. (A) Experimental strategy for scRNA-seq of erythroid precursors from control and PHZ-induced anemic spleens. (B) UMAP representation of control (7118 cells) and PHZ-treated (10 305 cells) samples. Annotation indicates 18 distinct color-coded clusters. (C) Percentages of HSPCs and erythroid clusters relative to total cells analyzed in control and anemia samples. Cells not annotated as HSPC or erythroid are shown as “others.” Statistical significance between cell percentages in control and anemia determined using a Fisher exact test. (D) Dot plot depicting changes in normalized average expression (color of dot) and expression in a percentage of cells (size of dot) of hematopoietic and erythropoietic genes in HSPCs and erythroid cells. (E) Bar graph of Samd14 transcript levels in CD71+ cells from control and anemia samples (normalized expression of Tfrc > 0). Statistical significance calculated using Wilcoxon test. (F) The percentage of Samd14 expressing cells (depicted in a line graph) in control and anemia CD71+ cells. Statistical significance was determined using Fisher exact test. (G) Bar graph comparison of SLC15A37 and SOX6 expression in erythroid precursors from healthy controls and patients with aplastic anemia, alongside control and anemia data from PHZ-treated mouse samples. Statistical significance determined using unpaired Student t test. (H) In each cluster, ratio indicates the proportion of RNA transcripts annotated within 100 kb of CANNTG[N6-14]AGATAA (EGA) within all detected RNA transcripts (orange bars) or anemia-upregulated RNA transcripts (black bars; fold >1.5; adjusted P < .05). (I) In each cluster, ratio indicates the proportion of detected RNA transcripts within 100 kb of GATA/TAL-occupied EGA sequences (GSE51338) or GATA2 (GSE29193) and TAL1 (GSE36029) within all detected RNA transcripts (blue bars) or anemia-upregulated RNA transcripts (gray bars). Statistical significance was determined using Wilcoxon test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). RBC, red blood cell; UMAP, uniform manifold approximation and projection.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/20/10.1182_bloodadvances.2022009163/2/m_blooda_adv-2022-009163-gr1.jpeg?Expires=1769088257&Signature=NFopZ~AwHXyntCXQXUqunNbUVVYGjPOkgxcEMx7XkHGiwifi0DfiC1fhw5pK2IX2SoLUsqvQEAFKSuT~6uglAKiQcURaJTaoMVkOdE-TT2t~ACY2wjaECcv8bhkRA4-8QIXYeewjYVa-uV9hFM6CnhzGJa-0UDdBrZYFIwVjI~g1k09DcMvUpt2Z5BBAmAuMatbrHyAwAEPrqol1nCkqMS-obn337Jt8AnJc2djfk9L0DYlB8aApIiwaGiucmLyz1LUlpVGZm9dQ91YlGrHVt0rAbHU6-Fqkmw6t9jaVoCh-LIwSX5URutsSiyI9ekP5AJSfRW-hYmvHzwRjmdHY8g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)