TO THE EDITOR:
Erdheim-Chester disease (ECD) is a rare non–Langerhans cell histiocytosis belonging to the L-group of the 2016 revised classification for histiocytosis.1,2 ECD is characterized by a xanthomatous infiltration of various organs with foamy CD68+/CD1a– histiocytes.3 Cardiac involvement is present in half the patients and may lead to pericarditis, cardiac tamponade, and conduction disorders. The course of cardiac infiltrations after treatment and the factors associated with an improvement in cardiac involvement have never been analyzed in a large cohort study.4
The aim of this study was to use cardiac magnetic resonance (CMR) to investigate the course of cardiac involvement over time.
We retrospectively included patients with a biopsy-proven diagnosis of ECD referred to the internal medicine department of a French tertiary center and undergoing CMR imaging disclosing specific cardiac involvement between July 2005 and January 2020. In all cases, the ECD diagnosis was based on a typical clinical and radiological presentation, with confirmation based on typical pathology findings. We screened 96 patients with ECD and cardiac involvement, of whom 46 were included. A comparison of included and excluded populations is available in supplemental Table 1.
CMR imaging was performed at least twice for each patient. Data from the first and the last CMR scan obtained were compared to assess changes in overall cardiac involvement and for each lesion considered separately. Full imaging methods have been described previously.5
Cardiac involvement was defined as atrial infiltration (abnormal epicardial infiltration of the atria exceeding 3 mm) or a pericardial abnormality (including enhancement, infiltration, or thickening of the pericardium). Late gadolinium enhancement of atrial infiltration was collected if present.
The primary endpoint was the complete or partial regression of cardiac ECD lesions assessed by CMR imaging. Complete regression was considered to have occurred in cases in which the normalization was observed. Partial regression was considered to have occurred in cases in which infiltrations had shrunk or decreased. Cardiac involvement was considered stable in cases with persistent lesions. Progression was considered to have occurred if new abnormalities were detected or if the infiltrations present had increased in size.
We used the χ2 or Fisher exact test for categorical variables and the t test or Mann-Whitney-Wilcoxon tests for continuous variables. A survival analysis was performed to explore the association between the course of cardiac involvement and outcome. Statistical analysis was performed with R software, version 3.3.2.
The study was approved by the local ethics committee (Comité de Protection des Personnes d’Ile de France III ([#2011-A00447-34]) and was conducted in accordance with the Declaration of Helsinki.
We included 46 patients (male sex, 63%; median age, 63 years [53-69]). The median time between the first and last CMR images was 4 years (2-7). The BRAFV600E mutation was present in 43 patients (94%), MAP2K1 was mutated in 1 (2%), and mutational status was undetermined in 2 (4%). Patients received a median of 2 [1-3] treatments. Thirty-six (78%) received interferon alpha (standard or pegylated), 29 (63%) received the BRAF-inhibitor vemurafenib (8 as frontline therapy), and 6 (13%) received the MEK-inhibitor cobimetinib (2 as frontline therapy).
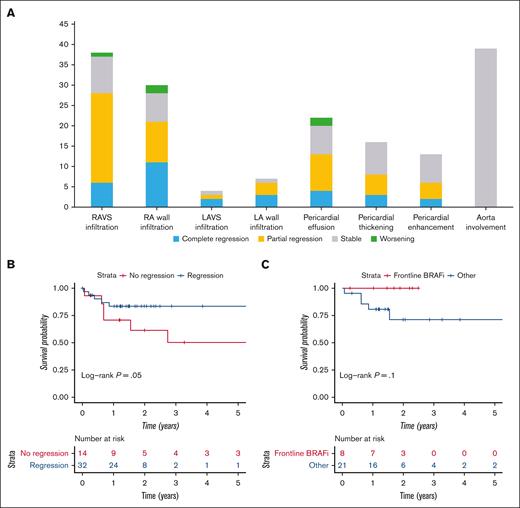
All 46 patients had cardiac involvement at baseline. Overall, complete regression was observed in 6 patients (13%), partial regression in 26 patients (56%), stable imaging in 12 patients (26%), and a worsening in 2 patients (5%) (Figure 1A). The right atrioventricular sulcus was infiltrated in 38 patients (83%) and resolved completely in 5 (16%) or partially in 22 (58%) (Figure 2). Pericardial effusion was present at baseline in 22 patients (48%) and resolved completely in 4 patients (18%), partially in 9 (43%), remained stable in 7 (33%), and worsened in 2 (10%).
Course of cardiac involvement in ECD and associated outcome. (A) Course of cardiac involvement in ECD according to the type of cardiac lesion. (B) Survival curve according to the course of cardiac involvement Kaplan-Meier survival curves for patients with regression (partial or complete) and without regression (stable or worsening) of their cardiac involvement during follow-up. (C) Survival curve according to treatment regimen. Kaplan-Meier survival for patients with and without vemurafenib as frontline therapy. The date of inclusion was taken as the date of last CMR. Follow-up was considered to end on the date of death or last medical visit. The last data were collected on 12 May 2021. The y-axis represents the number of patients. Median survival was 6 years, and the 5-year survival was 66%. HR, hazard ratio; LA, left atrium; LAVS, left atrioventricular sulcus; RA, right atrium; RAVS, right atrioventricular sulcus.
Course of cardiac involvement in ECD and associated outcome. (A) Course of cardiac involvement in ECD according to the type of cardiac lesion. (B) Survival curve according to the course of cardiac involvement Kaplan-Meier survival curves for patients with regression (partial or complete) and without regression (stable or worsening) of their cardiac involvement during follow-up. (C) Survival curve according to treatment regimen. Kaplan-Meier survival for patients with and without vemurafenib as frontline therapy. The date of inclusion was taken as the date of last CMR. Follow-up was considered to end on the date of death or last medical visit. The last data were collected on 12 May 2021. The y-axis represents the number of patients. Median survival was 6 years, and the 5-year survival was 66%. HR, hazard ratio; LA, left atrium; LAVS, left atrioventricular sulcus; RA, right atrium; RAVS, right atrioventricular sulcus.
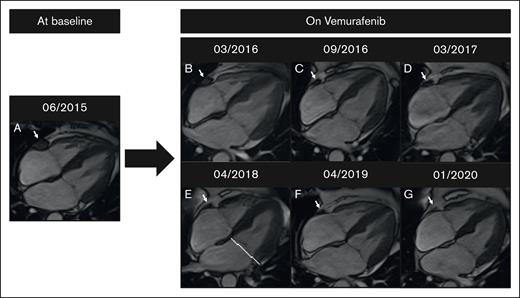
Change in CMR image results in a patient on vemurafenib. (A) Baseline. (B-G) Complete regression of right atrioventricular sulcus infiltration on vemurafenib over 4 years. CMR imaging was performed on a 1.5T MR scanner (Siemens Aera).
Change in CMR image results in a patient on vemurafenib. (A) Baseline. (B-G) Complete regression of right atrioventricular sulcus infiltration on vemurafenib over 4 years. CMR imaging was performed on a 1.5T MR scanner (Siemens Aera).
Three patients developed an ECD-related cardiac clinical event after the first imaging: 1 complete heart block in a patient after complete regression of atrial infiltration, 1 complete heart block in a patient with partial regression of an inter-atrial septum infiltration, and 1 tamponade in a patient with no regression of pericardial effusion during follow-up.
Patients with and without a regression of infiltration were compared (supplemental Table 2). The patients for whom a regression of infiltration was observed had a lower death rate (16% vs 64%; P = .002), a higher rate of atrial infiltration displaying late gadolinium enhancement (97% vs 71%; P = .01), a higher rate of hydronephrosis (50% vs 14%; P = .03), and a lower rate of interferon prescription (69% vs 100%, P = .02). A higher, but nonsignificant, rate of vemurafenib use was observed in patients with partial or complete regression (69% vs 54%; P = .3).
Rates of partial or complete regression in patients treated with interferon were 61% vs 100% in patients who were interferon-naïve (22/36 vs 10/10; P = .02). Patients who were interferon-naïve (n = 10) benefited from targeted therapy (vemurafenib, n = 8; cobimetinib, n = 2).
Rates of partial or complete regression in patients treated with vemurafenib was 76% vs 59% in patients who were vemurafenib-naïve (22/29 vs 10/17; P = .3). All 8 patients (100%) who received vemurafenib as frontline therapy exhibited regression of cardiac lesions.
None of the patients were lost to follow-up. Overall, 14 patients (30%) died. Causes of death are available in supplemental Table 3. The regression of cardiac involvement on follow-up imaging was significantly associated with better survival (hazard ratio = 0.3; 95% confidence interval, 0.1-0.99; P = .05) (Figure 1B). No deaths occurred in patients who received vemurafenib as frontline therapy vs 6 in patients who received vemurafenib as second-line or beyond (6/21 [29%] vs 0/8 [0%]; P = .1). Patients treated with vemurafenib as frontline therapy had a trend toward improved survival (hazard ratio = 10–9; 95% confidence interval, 0-NA; P = .1; Figure 1C) that did not reach statistical significance, possibly because of small sample size.
In total, the main results for this study are that a partial or complete resolution of cardiac involvement was (1) observed in 69% of patients with ECD, (2) seen in all patients with ECD who received a targeted argent as frontline therapy, and (3) associated with improved survival.
Comparison of treatment regimens is made difficult by the various types and durations of treatment received. The efficacy of both interferon and vemurafenib on cardiac infiltrations has been previously reported.6-10 In our series, all patients treated with vemurafenib as frontline therapy displayed regression of cardiac lesions, none of whom died during follow-up. Although nonsignificant, our data supports the current guidelines that advocate for treating cardiac manifestations with frontline targeted therapy.11
A regression of infiltration on imaging was significantly associated with improved survival. However, cardiac involvement was recently not found to be associated with lower survival.4,5,12,13 The resolution of cardiac involvement is probably a surrogate associated with other predictors of death rather than being directly linked to the risk of death itself.
Aortic infiltration did not resolve during follow-up and may be associated with fibrotic or atherosclerotic lesions for which ECD treatments have no substantial effects.
This study has limitations. First, it was a retrospective, single-center study. Second, we report the change in CMR imaging results as categories rather than quantitative measurements. Third, the time between the first and last CMR was long and varied between patients. Fourth, treatment regimens between the 2 CMR examinations were not standardized. Fifth, a selection bias is possible as only a subset of patients with ECD with cardiac involvement underwent follow-up imaging at our center. However, apart from the BRAFV600E mutation and BRAF-inhibitor treatment, the characteristics of the excluded and included populations did not differ on sensitivity analysis (supplemental Table 1).
To conclude, a regression of cardiac infiltration was observed in almost two-thirds of the patients with ECD and may be associated with a better outcome.
Contribution: L.-D.A., M.B., F.C.-A., and J.H. contributed to study design, data collection, statistical analysis conduction and interpretation, and manuscript writing; and J.-F.E., F.C., Z.A., and P.C. contributed to data collection and manuscript writing.
Conflict-of-interest disclosure: F.C.-A. and J.H. are investigators (F.C.-A. being the principal investigator) in an academic study on the efficacy of cobimetinib for treating histiocytosis (COBRAH, NCT 04007848).
Correspondence: Lévi-Dan Azoulay, Service de Médecine Interne 2, Hôpital Pitié-Salpêtrière, 47-83 Boulevard de l’hôpital, 75651 Paris CEDEX 13, France;.
References
Author notes
∗L.-D.A., M.B., and F.C.A. contributed equally to this study.
Data underlying this article will be shared on reasonable request to the corresponding author, Lévi-Dan Azoulay (levi-dan.azoulay@aphp.fr).
The full-text version of this article contains a data supplement.