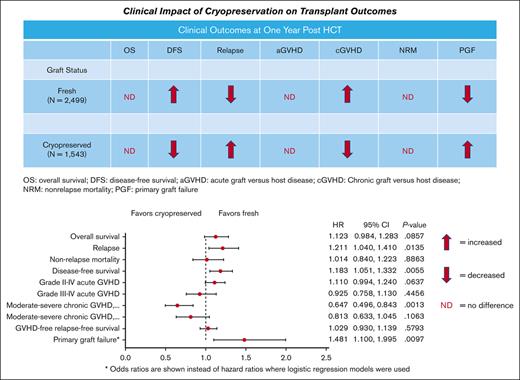
Key Points
There was no significant impact of graft cryopreservation on the OS, severe acute GVHD, or nonrelapse mortality 1 year after HCT.
Cryopreservation resulted in high odds of primary graft failure, a low risk of chronic GVHD, and a small increase in relapse risk.
Abstract
At the onset of the COVID-19 pandemic, the National Marrow Donor Program mandated the cryopreservation of hematopoietic cell grafts from volunteer unrelated donors because of numerous patient and donor safety concerns and logistical hurdles. Using the Center for International Blood and Marrow Transplant Research outcomes database, we report the impact of cryopreservation on overall survival (OS) and other outcomes within 1 year after hematopoietic cell transplantation (HCT). We analyzed 1543 recipients of cryopreserved allografts receiving HCT at US centers during the first 6 months of the pandemic and compared them with 2499 recipients of fresh allografts during a 6-month period in 2019. On multivariable regression analysis, we observed no difference in the OS (P = .09), nonrelapse mortality (P = .89), graft-versus-host disease (GVHD), or GVHD- and relapse-free survival (P = .58) in recipients of cryopreserved vs fresh allografts. Disease-free survival (DFS) was lower in the cryopreserved allograft recipients (P = .006) because of a higher risk of relapse (P = .01) compared with the fresh allograft recipients. Primary graft failure was higher (P = .01), and the risk of chronic GVHD was lower (P = .001) with cryopreservation compared with fresh grafts. In conclusion, although there was no negative impact of cryopreservation on OS, relapse was higher, and DFS was lower than that with no cryopreservation. Fresh grafts are recommended as the pandemic-related logistical hurdles resolve. Cryopreservation should be considered an option for patients when fresh grafts are not feasible.
Introduction
The impact of graft cryopreservation on recipient outcomes after an allogeneic hematopoietic cell transplantation (HCT) remains unclear. Given the absence of well-controlled prospective data, clinical decision-making has relied mostly on retrospective analyses from single centers or observational data derived from patient registries. These studies have numerous limitations, including small numbers, variations in supportive care at single centers, possible reporting biases, and inconsistent ascertainment of the factors influencing the decision to cryopreserve.1-8 Consequently, the data are conflicting; some studies suggest a deleterious impact on clinical outcomes, whereas others have found no significant negative impact.
At the outset of the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), substantial risks arose with the use of fresh donor products. Delivery after conditioning could not be guaranteed because of logistical challenges posed by travel bans, flight delays, cancellations, and rerouting of couriers.9 Moreover, donors were at risk for SARS-CoV-2 infection, necessitating last-minute cancellations of graft collection. Under these circumstances, the National Marrow Donor Program (NMDP), along with several other global donor registries, imposed a temporary requirement for cryopreservation of most unrelated donor (URD) products during the first 6 months of the pandemic.9 Many transplantation programs followed suit with related donor products. These policy changes were consistent with recommendations from national and international societies and accrediting bodies.10-12 Therefore, the pandemic provided a unifying rationale for the provision of cryopreserved allografts and an opportunity to better understand the true impact of cryopreservation on HCT clinical outcomes. To address this question, we used the Center for International Blood and Marrow Transplant Research (CIBMTR) outcome database to evaluate the impact of cryopreservation on patients receiving HCT at US transplantation centers during the first 6 months of the SARS-CoV-2 pandemic and compared this with the effects among patients receiving fresh products in the previous year.
Methods
Study objectives
The primary objective of this cohort study was to compare the overall survival (OS) of recipients of cryopreserved vs fresh allografts. Secondary objectives included comparison of disease-free survival (DFS), relapse, nonrelapse mortality (NRM), hematopoietic engraftment, primary and secondary graft failure, acute graft-versus-host disease (aGVHD), chronic GVHD (cGVHD), GVHD- and relapse-free survival (GRFS), donor chimerism, receipt of a booster or second HCT, and use of donor lymphocyte infusion (DLI) for any purpose.
Data sources
Patient data were reported to the CIBMTR. Observational studies using this database were performed in compliance with all applicable federal regulations pertaining to the protection of human participants. All the participants provided written informed consent. Protected health information used in the performance of such research was collected and maintained with the researcher acting as a public health authority, per the researcher’s capacity, under the health insurance portability and accountability act privacy rule. Additional data concerning the graft origin, transit times, graft-cell doses, and the proportion of grafts planned for cryopreservation in URD HCT recipients were obtained from the NMDP. Analysis of the impact of point of collection (POC) and product transit time was limited to recipients of URD grafts because there were almost no related donor products collected internationally. Domestic vs international POC was used as a surrogate measure to approximate the product transit time before cryopreservation. This was defined as the time from the end of collection (either bone marrow [BM] harvest or apheresis) to the beginning of cryopreservation processing at the transplantation center. We limited the analysis of international products to those collected from the United Kingdom, the European Union, or Israel, because the number of products obtained from other international regions during the first 6 months of the pandemic was low. The study was approved by the NMDP’s Institutional Review Board, which is the board of records for the CIBMTR database protocols. This study followed the reporting guidelines of the strengthening of reporting of observational studies in epidemiology.13
Patient selection
We selected US HCT recipients who had undergone transplantations using cryopreserved allografts during the first 6 months of the pandemic (from 1 March 2020 to 31 August 2020). Further selection included sufficient posttransplantation data reported and patients from centers reporting good standing per the CIBMTR quality metrics, with >85% data completion rates. The comparison cohort included recipients of fresh allografts who underwent transplantation between 1 March 2019 and 31 August 2019 and reported to the CIBMTR with the same selection restrictions as those described earlier.
Definition of study end points
The primary study outcome was OS, defined as the time from HCT to death from any cause and was censored at the time of last follow-up or 365 days, whichever came first. aGVHD was defined as the occurrence of grade 2 or 4 or grade 3 or 4 within 100-days of transplantation, based on the clinical severity in accordance with the criteria published by Przeprioka et al.14 Relapse represented the time from HCT to clinical evidence of disease recurrence and was censored at the time of last follow-up. NRM was defined as the time to death without clinical evidence of disease recurrence. DFS was defined as the time to treatment failure due to death or clinical evidence of relapse. Relapse, DFS, and NRM were censored at the time of the last follow-up or at 365 days, whichever occurred first. GRFS was a composite variable defined as lack of grade III-IV aGVHD (censored at 100 days), moderate-or-severe cGVHD (censored at 365 days), clinical evidence of relapse within malignant diseases (censored at 365 days) and death (censored at 365 days). Neutrophil recovery was defined as the time to achieve an absolute neutrophil count (ANC) ≥500 mm3 for 3 consecutive days after HCT, whereas platelet recovery was defined as achieving a platelet count of 20 000 by day 100 after HCT. The need for a subsequent transplantation or DLI after HCT because of poor graft function or graft failure, whichever occurred first, was defined as a composite engraftment outcome. Primary graft failure was defined as the failure to achieve neutrophil recovery by day 28 after transplantation. Secondary graft failure was defined as the recipient meeting the criteria for initial engraftment but subsequently developing a loss of a previously functioning graft because of the development of at least 2 lines of cytopenia. The intensity of allogeneic HCT–conditioning regimens was categorized as myeloablative conditioning or reduced-intensity/nonmyeloablative conditioning using consensus criteria.15
Statistical methods
Descriptive statistics included medians and ranges for continuous variables and frequencies for categorical variables. Death was considered a competing risk for all outcomes except OS, DFS, and GRFS. Relapse and NRM were competing risks to each other.
The cumulative incidence function was used to estimate the probability of neutrophil and platelet recovery, NRM, relapse, aGVHD grades II-IV, and aGVHD grades III-IV, accounting for competing risks. OS, DFS, and GRFS probabilities were calculated using the Kaplan-Meier estimator. Logistic regression was used to obtain odds ratios (ORs) for primary graft failure, secondary graft failure, and subsequent HCT/DLI, indicating a poor graft function, primary graft failure, or secondary graft failure. Because of the differences in the year of transplantation resulting in differential length of follow-up among cohorts, follow-up was truncated at 365 days after HCT in both groups.
Multivariable regression analyses for OS, aGVHD, relapse, DFS, NRM, moderate/severe cGVHD, and GRFS were performed using the Cox proportional hazards model. All variables were tested for the affirmation of the proportional hazards assumption. Factors violating the proportional hazards assumption were adjusted via stratification. Multivariable regression analyses for primary graft failure and platelet recovery were performed using a logistic regression model. Backward elimination was used to select adjusted covariates and develop multivariable models for primary and secondary outcomes, and the main effect of cryopreservation was included in the models. P < .05 was considered significant. Interactions between the main effect of cryopreservated vs fresh allografts as well as other significant factors were also tested. All P values were raw and 2-sided. Statistical analyses were performed using SAS version 9.4 (Cary, NC).
Results
Baseline characteristics
The patient characteristics are presented in Table 1. The completeness of follow-up at 1 year for the groups with fresh and cryopreserved grafts was 96% and 95%, respectively. Recipients of cryopreserved allografts were older, more likely to receive a URD graft and a peripheral blood stem cell (PBSC) graft, more likely to receive post-transplant ation cyclophosphamide for GVHD prophylaxis, and less likely to undergo HCT because of a nonmalignant condition, compared with recipients of fresh allografts.
Baseline patient characteristics based on the type of graft
| Characteristic . | Fresh . | Cryopreserved . |
|---|---|---|
| No. of patients | 2499 | 1543 |
| Age at transplantation (y), n (%) | ||
| Median (min-max) | 55 (0-79) | 58 (0-82) |
| 0-9 | 209 (8) | 61 (4) |
| 10-17 | 159 (6) | 57 (4) |
| 18-29 | 249 (10) | 116 (8) |
| 30-39 | 182 (7) | 143 (9) |
| 40-49 | 266 (11) | 185 (12) |
| 50-59 | 436 (17) | 290 (19) |
| 60-69 | 746 (30) | 503 (33) |
| 70+ | 252 (10) | 188 (12) |
| Race, n (%) | ||
| White | 2016 (81) | 1317 (85) |
| Black or African American | 201 (8) | 91 (6) |
| Asian | 109 (4) | 45 (3) |
| Native Hawaiian or other Pacific Islander | 6 (0) | 3 (0) |
| American Indian or Alaska Native | 10 (0) | 7 (0) |
| More than 1 race | 22 (1) | 5 (0) |
| Missing | 135 (5) | 75 (5) |
| Ethnicity, n(%) | ||
| Hispanic or Latino | 311 (12) | 164 (11) |
| Not Hispanic or Latino | 2100 (84) | 1298 (84) |
| Missing∗ | 88 (4) | 81 (5) |
| HCT-CI, n (%) | ||
| 0-2 | 1272 (51) | 842 (55) |
| 3+ | 1206 (48) | 688 (45) |
| Missing | 21 (1) | 13 (1) |
| Karnofsky/Lansky performance score, n (%) | ||
| 0-80 | 1011 (40) | 660 (43) |
| 90-100 | 1429 (57) | 850 (55) |
| Missing | 59 (2) | 33 (2) |
| Disease, n (%) | ||
| AML | 921 (37) | 588 (38) |
| ALL | 338 (14) | 251 (16) |
| MDS | 480 (19) | 301 (20) |
| Lymphoma | 163 (7) | 121 (8) |
| Other leukemias | 128 (5) | 77 (5) |
| Other malignancies | 180 (7) | 111 (7) |
| SAA | 104 (4) | 40 (3) |
| Nonmalignant diseases | 185 (7) | 54 (3) |
| Disease status, n (%) | ||
| CR1/2 or early MDS | 1184 (47) | 787 (51) |
| Other disease status, other malignancy, or nonmalignant disease | 1315 (53) | 756 (49) |
| DRI | ||
| Low | 133 (5) | 68 (4) |
| Intermediate | 1113 (44) | 790 (51) |
| High | 447 (18) | 259 (17) |
| Very high | 51 (2) | 30 (2) |
| Missing | 118 (5) | 121 (8) |
| Nonmalignant disease | 289 (12) | 94 (6) |
| Not applicable | 384 (14) | 181 (12) |
| Conditioning intensity, n (%) | ||
| MAC | 1031 (41) | 645 (42) |
| RIC | 924 (37) | 593 (38) |
| NMA | 248 (10) | 209 (14) |
| Missing | 7 (0) | 2 (0) |
| N/A, not malignant disease | 289 (12) | 94 (6) |
| Conditioning intensity, n (%) | ||
| MAC | 1031 (41) | 645 (42) |
| RIC/NMA | 1172 (47) | 802 (52) |
| Nonmalignant, no drugs, or missing | 296 (12) | 96 (6) |
| GVHD Prophylaxis, n (%) | ||
| Ex vivo T-cell depletion | 38 (2) | 9 (1) |
| CD34 selection | 27 (1) | 0 (0) |
| Post-Cy | 767 (31) | 579 (38) |
| Tac based | 1448 (58) | 859 (56) |
| CsA based | 175 (7) | 56 (4) |
| Other | 18 (1) | 20 (1) |
| Missing | 26 (1) | 20 (1) |
| ATG/alemtuzumab use, n (%) | ||
| No | 1778 (71) | 1155 (75) |
| Yes | 721 (29) | 388 (25) |
| Donor, n (%) | ||
| HLA-identical sibling | 378 (15) | 150 (10) |
| Other related | 467 (19) | 200 (13) |
| Well-matched unrelated (8/8) | 1449 (58) | 1037 (67) |
| Mismatched unrelated | 205 (8) | 150 (10) |
| Missing | 0 (0) | 6 (0) |
| Donor center location, n (%) | ||
| Domestic | 703 (28) | 588 (38) |
| International | 956 (38) | 639 (41) |
| No donor center, related donor | 839 (34) | 314 (20) |
| Missing | 1 (0) | 2 (0) |
| No. of PBSC collection days | ||
| One-day collection | 1023 (41) | 886 (57) |
| Two-day collection | 218 (9) | 184 (12) |
| N/A, BM | 665 (26) | 182 (12) |
| Missing, related donor | 593 (24) | 291 (19) |
| Donor/recipient sex match, n (%) | ||
| M-M | 1013 (41) | 547 (35) |
| M-F | 589 (24) | 392 (25) |
| F-M | 463 (19) | 294 (19) |
| F-F | 417 (17) | 286 (19) |
| Missing | 17 (1) | 24 (2) |
| Donor/recipient CMV serostatus, n (%) | ||
| +/+ | 816 (33) | 473 (31) |
| +/− | 303 (12) | 186 (12) |
| −/+ | 676 (27) | 492 (32) |
| −/− | 686 (27) | 380 (25) |
| Missing | 18 (1) | 12 (1) |
| Graft type, n (%) | ||
| BM | 665 (27) | 182 (12) |
| Peripheral blood | 1834 (73) | 1361 (88) |
| CD34 for PB, or TNC for BM above or below median, n (%) | ||
| ≤median | 1244 (50) | 972 (63) |
| >median | 1255 (50) | 571 (37) |
| TNC (×108/kg) for BM | ||
| n | 584 | 131 |
| Median (min-max) | 3.1 (0.01-767.5) | 2.5 (0.04-13.1) |
| 5th-95th pctl | 0.9-8.5 | 0.9-6.7 |
| CD34(×106/kg) for peripheral blood | ||
| n | 1810 | 1199 |
| Median (min-max) | 6.3 (0.02-83855.4) | 5.7 (0.01-3478.4) |
| 5th-95th pctl | 2.6-16.1 | 2.5-12.9 |
| Y of transplant, n (%) | ||
| 2019 | 2499 (100) | 0 (0) |
| 2020 | 0 (0) | 1543 (100) |
| Follow-up (d) - median (range) | 373 (27-524) | 372 (22-540) |
| Characteristic . | Fresh . | Cryopreserved . |
|---|---|---|
| No. of patients | 2499 | 1543 |
| Age at transplantation (y), n (%) | ||
| Median (min-max) | 55 (0-79) | 58 (0-82) |
| 0-9 | 209 (8) | 61 (4) |
| 10-17 | 159 (6) | 57 (4) |
| 18-29 | 249 (10) | 116 (8) |
| 30-39 | 182 (7) | 143 (9) |
| 40-49 | 266 (11) | 185 (12) |
| 50-59 | 436 (17) | 290 (19) |
| 60-69 | 746 (30) | 503 (33) |
| 70+ | 252 (10) | 188 (12) |
| Race, n (%) | ||
| White | 2016 (81) | 1317 (85) |
| Black or African American | 201 (8) | 91 (6) |
| Asian | 109 (4) | 45 (3) |
| Native Hawaiian or other Pacific Islander | 6 (0) | 3 (0) |
| American Indian or Alaska Native | 10 (0) | 7 (0) |
| More than 1 race | 22 (1) | 5 (0) |
| Missing | 135 (5) | 75 (5) |
| Ethnicity, n(%) | ||
| Hispanic or Latino | 311 (12) | 164 (11) |
| Not Hispanic or Latino | 2100 (84) | 1298 (84) |
| Missing∗ | 88 (4) | 81 (5) |
| HCT-CI, n (%) | ||
| 0-2 | 1272 (51) | 842 (55) |
| 3+ | 1206 (48) | 688 (45) |
| Missing | 21 (1) | 13 (1) |
| Karnofsky/Lansky performance score, n (%) | ||
| 0-80 | 1011 (40) | 660 (43) |
| 90-100 | 1429 (57) | 850 (55) |
| Missing | 59 (2) | 33 (2) |
| Disease, n (%) | ||
| AML | 921 (37) | 588 (38) |
| ALL | 338 (14) | 251 (16) |
| MDS | 480 (19) | 301 (20) |
| Lymphoma | 163 (7) | 121 (8) |
| Other leukemias | 128 (5) | 77 (5) |
| Other malignancies | 180 (7) | 111 (7) |
| SAA | 104 (4) | 40 (3) |
| Nonmalignant diseases | 185 (7) | 54 (3) |
| Disease status, n (%) | ||
| CR1/2 or early MDS | 1184 (47) | 787 (51) |
| Other disease status, other malignancy, or nonmalignant disease | 1315 (53) | 756 (49) |
| DRI | ||
| Low | 133 (5) | 68 (4) |
| Intermediate | 1113 (44) | 790 (51) |
| High | 447 (18) | 259 (17) |
| Very high | 51 (2) | 30 (2) |
| Missing | 118 (5) | 121 (8) |
| Nonmalignant disease | 289 (12) | 94 (6) |
| Not applicable | 384 (14) | 181 (12) |
| Conditioning intensity, n (%) | ||
| MAC | 1031 (41) | 645 (42) |
| RIC | 924 (37) | 593 (38) |
| NMA | 248 (10) | 209 (14) |
| Missing | 7 (0) | 2 (0) |
| N/A, not malignant disease | 289 (12) | 94 (6) |
| Conditioning intensity, n (%) | ||
| MAC | 1031 (41) | 645 (42) |
| RIC/NMA | 1172 (47) | 802 (52) |
| Nonmalignant, no drugs, or missing | 296 (12) | 96 (6) |
| GVHD Prophylaxis, n (%) | ||
| Ex vivo T-cell depletion | 38 (2) | 9 (1) |
| CD34 selection | 27 (1) | 0 (0) |
| Post-Cy | 767 (31) | 579 (38) |
| Tac based | 1448 (58) | 859 (56) |
| CsA based | 175 (7) | 56 (4) |
| Other | 18 (1) | 20 (1) |
| Missing | 26 (1) | 20 (1) |
| ATG/alemtuzumab use, n (%) | ||
| No | 1778 (71) | 1155 (75) |
| Yes | 721 (29) | 388 (25) |
| Donor, n (%) | ||
| HLA-identical sibling | 378 (15) | 150 (10) |
| Other related | 467 (19) | 200 (13) |
| Well-matched unrelated (8/8) | 1449 (58) | 1037 (67) |
| Mismatched unrelated | 205 (8) | 150 (10) |
| Missing | 0 (0) | 6 (0) |
| Donor center location, n (%) | ||
| Domestic | 703 (28) | 588 (38) |
| International | 956 (38) | 639 (41) |
| No donor center, related donor | 839 (34) | 314 (20) |
| Missing | 1 (0) | 2 (0) |
| No. of PBSC collection days | ||
| One-day collection | 1023 (41) | 886 (57) |
| Two-day collection | 218 (9) | 184 (12) |
| N/A, BM | 665 (26) | 182 (12) |
| Missing, related donor | 593 (24) | 291 (19) |
| Donor/recipient sex match, n (%) | ||
| M-M | 1013 (41) | 547 (35) |
| M-F | 589 (24) | 392 (25) |
| F-M | 463 (19) | 294 (19) |
| F-F | 417 (17) | 286 (19) |
| Missing | 17 (1) | 24 (2) |
| Donor/recipient CMV serostatus, n (%) | ||
| +/+ | 816 (33) | 473 (31) |
| +/− | 303 (12) | 186 (12) |
| −/+ | 676 (27) | 492 (32) |
| −/− | 686 (27) | 380 (25) |
| Missing | 18 (1) | 12 (1) |
| Graft type, n (%) | ||
| BM | 665 (27) | 182 (12) |
| Peripheral blood | 1834 (73) | 1361 (88) |
| CD34 for PB, or TNC for BM above or below median, n (%) | ||
| ≤median | 1244 (50) | 972 (63) |
| >median | 1255 (50) | 571 (37) |
| TNC (×108/kg) for BM | ||
| n | 584 | 131 |
| Median (min-max) | 3.1 (0.01-767.5) | 2.5 (0.04-13.1) |
| 5th-95th pctl | 0.9-8.5 | 0.9-6.7 |
| CD34(×106/kg) for peripheral blood | ||
| n | 1810 | 1199 |
| Median (min-max) | 6.3 (0.02-83855.4) | 5.7 (0.01-3478.4) |
| 5th-95th pctl | 2.6-16.1 | 2.5-12.9 |
| Y of transplant, n (%) | ||
| 2019 | 2499 (100) | 0 (0) |
| 2020 | 0 (0) | 1543 (100) |
| Follow-up (d) - median (range) | 373 (27-524) | 372 (22-540) |
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; ATG, anti-thymocyte globulin; CMV, cytomegalovirus; CsA, cyclosporine A; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; NMA, non-myeloablative; pctl, percentile; RIC, reduced intensity conditioning; SAA, severe aplastic anemia; TNC, total nucleated cell count.
Includes NA, nonresident of the United States: n = 24 in the group with fresh grafts, and n = 8 in the group with cryopreserved grafts.
Univariable analyses
OS, DFS, and NRM
Univariate analysis of clinical outcomes based on the type of graft are presented in Table 2. One-year OS was 74.6% in the group with cryopreserved products (95% confidence interval [CI], 72.3.-76.8) vs 76.9% in the group with fresh products (95% CI, 75.2.-78.6; P = .064). One-year DFS was 63.2% in the group with cryopreserved grafts (95% CI, 60.5-65.8) vs 66.9% in the group with fresh grafts (95% CI, 64.9-68.9; P = .027). One-year NRM was 14.7% in the group with cryopreserved grafts (95% CI, 12.8.-16.7) vs 13.9% in the group with fresh grafts (95% CI, 12.4-15.4; P = .068).
Univariate analysis of outcomes based on the type of graft
| Outcomes . | Fresh (N = 2499) . | Cryopreserved (N = 1543) . | P value . | ||
|---|---|---|---|---|---|
| N . | Prob (95% CI) . | N . | Prob (95% CI) . | ||
| Neutrophil recovery (BM only) | 659 | 180 | .598 | ||
| 14 d | 25.9 (22.7-29.4)% | 23.9 (17.9-30.4)% | |||
| 30 d | 95.3 (93.5-96.8)% | 95 (91.3-97.7)% | |||
| Neutrophil recovery (PB only) | 1821 | 1351 | < .001 | ||
| 14 d | 46.2 (43.9-48.5)% | 34.6 (32.1-37.2)% | < .001 | ||
| 30 d | 97.3 (96.5-98)% | 95.5 (94.3-96.5)% | .009 | ||
| Neutrophil recovery (all patients) | 2480 | 1531 | < .001 | ||
| 14 d | 40.8 (38.9-42.7)% | 33.4 (31-35.8)% | < .001 | ||
| 30 d | 96.7 (96-97.4)% | 95.4 (94.3-96.4)% | .041 | ||
| Platelet recovery (BM only) | 640 | 173 | .217 | ||
| 14 d | 5.9 (4.2-7.9)% | 6.4 (3.2-10.5)% | |||
| 30 d | 65 (61.3-68.7)% | 61.8 (54.5-69)% | |||
| 60 d | 92.5 (90.3-94.4)% | 86.7 (81.2-91.4)% | |||
| 100 d | 94.5 (92.6-96.2)% | 92.5 (88-96)% | |||
| Platelet recovery (PB only) | 1802 | 1329 | < .001 | ||
| 14 d | 20.2 (18.4-22.1)% | 13.2 (11.4-15)% | < .001 | ||
| 30 d | 82.8 (81-84.5)% | 73.1 (70.7-75.5)% | < .001 | ||
| 60 d | 93.1 (91.9-94.3)% | 91.1 (89.5-92.6)% | .041 | ||
| 100 d | 94.9 (93.9-95.9)% | 92.8 (91.4-94.1)% | .016 | ||
| Platelet recovery (all patients) | 2442 | 1502 | < .001 | ||
| 14 d | 16.5 (15-18)% | 12.4 (10.8-14.1)% | < .001 | ||
| 30 d | 78.1 (76.5-79.7)% | 71.8 (69.5-74.1)% | < .001 | ||
| 60 d | 93 (91.9-93.9)% | 90.6 (89.1-92)% | .010 | ||
| 100 d | 94.8 (93.9-95.7)% | 92.8 (91.4-94)% | .011 | ||
| aGVHD II-IV | 2313 | 1424 | .115 | ||
| 14 d | 1.1 (0.7-1.5)% | 1.6 (1-2.3)% | |||
| 30 d | 12.8 (11.4-14.1)% | 15.5 (13.7-17.4)% | |||
| 60 d | 28.1 (26.2-29.9)% | 32 (29.6-34.5)% | |||
| 100 d | 32.7 (30.8-34.6)% | 36 (33.5-38.5)% | .042 | ||
| aGVHD III-IV | 2288 | 1413 | .233 | ||
| 14 d | 0.3 (0.1-0.6)% | 0.8 (0.4-1.3)% | |||
| 30 d | 4.6 (3.8-5.5)% | 4.8 (3.8-6)% | |||
| 60 d | 9 (7.9-10.2)% | 8.7 (7.3-10.2)% | |||
| 100 d | 10.4 (9.2-11.7)% | 9.8 (8.3-11.4)% | .504 | ||
| cGVHD (moderate/severe) | 2461 | 1509 | .025 | ||
| 180 d | 9.6 (8.4-10.8)% | 7.9 (6.6-9.4)% | .082 | ||
| 365 d | 19.8 (18.2-21.4)% | 16.9 (15-18.9)% | .023 | ||
| Relapse∗ | 2162 | 1298 | .004 | ||
| 100 d | 7.6 (6.5-8.7)% | 8.1 (6.7-9.6)% | .591 | ||
| 180 d | 13.7 (12.3-15.2)% | 15.3 (13.4-17.3)% | .217 | ||
| 365 d | 19.2 (17.6-20.9)% | 22.2 (19.9-24.5)% | .042 | ||
| NRM∗ | 2162 | 1298 | .068 | ||
| 100 d | 5.5 (4.5-6.5)% | 7.9 (6.5-9.4)% | |||
| 180 d | 9.6 (8.4-10.9)% | 10.6 (9-12.4)% | |||
| 365 d | 13.9 (12.4-15.4)% | 14.7 (12.8-16.7)% | |||
| DFS∗ | 2162 | 1298 | < .001 | ||
| 100 d | 87 (85.5-88.3)% | 84 (82-86)% | .020 | ||
| 180 d | 76.6 (74.8-78.4)% | 74.1 (71.7-76.4)% | .093 | ||
| 365 d | 66.9 (64.9-68.9)% | 63.2 (60.5-65.8)% | .027 | ||
| OS | 2499 | 1543 | .064 | ||
| 100 d | 93.3 (92.3-94.2)% | 91.3 (89.8-92.7)% | |||
| 180 d | 86.8 (85.4-88.1)% | 84.6 (82.7-86.4)% | |||
| 365 d | 76.9 (75.2-78.6)% | 74.6 (72.3-76.8)% | |||
| GRFS | 2220 | 1247 | .287 | ||
| 100 d | 79.4 (77.7-81.1)% | 76.4 (74-78.7)% | |||
| 180 d | 65.5 (63.5-67.5)% | 62.8 (60-65.4)% | |||
| 365 d | 51.1 (49-53.2)% | 50 (47.2-52.8)% | .518 | ||
| Outcomes . | Fresh (N = 2499) . | Cryopreserved (N = 1543) . | P value . | ||
|---|---|---|---|---|---|
| N . | Prob (95% CI) . | N . | Prob (95% CI) . | ||
| Neutrophil recovery (BM only) | 659 | 180 | .598 | ||
| 14 d | 25.9 (22.7-29.4)% | 23.9 (17.9-30.4)% | |||
| 30 d | 95.3 (93.5-96.8)% | 95 (91.3-97.7)% | |||
| Neutrophil recovery (PB only) | 1821 | 1351 | < .001 | ||
| 14 d | 46.2 (43.9-48.5)% | 34.6 (32.1-37.2)% | < .001 | ||
| 30 d | 97.3 (96.5-98)% | 95.5 (94.3-96.5)% | .009 | ||
| Neutrophil recovery (all patients) | 2480 | 1531 | < .001 | ||
| 14 d | 40.8 (38.9-42.7)% | 33.4 (31-35.8)% | < .001 | ||
| 30 d | 96.7 (96-97.4)% | 95.4 (94.3-96.4)% | .041 | ||
| Platelet recovery (BM only) | 640 | 173 | .217 | ||
| 14 d | 5.9 (4.2-7.9)% | 6.4 (3.2-10.5)% | |||
| 30 d | 65 (61.3-68.7)% | 61.8 (54.5-69)% | |||
| 60 d | 92.5 (90.3-94.4)% | 86.7 (81.2-91.4)% | |||
| 100 d | 94.5 (92.6-96.2)% | 92.5 (88-96)% | |||
| Platelet recovery (PB only) | 1802 | 1329 | < .001 | ||
| 14 d | 20.2 (18.4-22.1)% | 13.2 (11.4-15)% | < .001 | ||
| 30 d | 82.8 (81-84.5)% | 73.1 (70.7-75.5)% | < .001 | ||
| 60 d | 93.1 (91.9-94.3)% | 91.1 (89.5-92.6)% | .041 | ||
| 100 d | 94.9 (93.9-95.9)% | 92.8 (91.4-94.1)% | .016 | ||
| Platelet recovery (all patients) | 2442 | 1502 | < .001 | ||
| 14 d | 16.5 (15-18)% | 12.4 (10.8-14.1)% | < .001 | ||
| 30 d | 78.1 (76.5-79.7)% | 71.8 (69.5-74.1)% | < .001 | ||
| 60 d | 93 (91.9-93.9)% | 90.6 (89.1-92)% | .010 | ||
| 100 d | 94.8 (93.9-95.7)% | 92.8 (91.4-94)% | .011 | ||
| aGVHD II-IV | 2313 | 1424 | .115 | ||
| 14 d | 1.1 (0.7-1.5)% | 1.6 (1-2.3)% | |||
| 30 d | 12.8 (11.4-14.1)% | 15.5 (13.7-17.4)% | |||
| 60 d | 28.1 (26.2-29.9)% | 32 (29.6-34.5)% | |||
| 100 d | 32.7 (30.8-34.6)% | 36 (33.5-38.5)% | .042 | ||
| aGVHD III-IV | 2288 | 1413 | .233 | ||
| 14 d | 0.3 (0.1-0.6)% | 0.8 (0.4-1.3)% | |||
| 30 d | 4.6 (3.8-5.5)% | 4.8 (3.8-6)% | |||
| 60 d | 9 (7.9-10.2)% | 8.7 (7.3-10.2)% | |||
| 100 d | 10.4 (9.2-11.7)% | 9.8 (8.3-11.4)% | .504 | ||
| cGVHD (moderate/severe) | 2461 | 1509 | .025 | ||
| 180 d | 9.6 (8.4-10.8)% | 7.9 (6.6-9.4)% | .082 | ||
| 365 d | 19.8 (18.2-21.4)% | 16.9 (15-18.9)% | .023 | ||
| Relapse∗ | 2162 | 1298 | .004 | ||
| 100 d | 7.6 (6.5-8.7)% | 8.1 (6.7-9.6)% | .591 | ||
| 180 d | 13.7 (12.3-15.2)% | 15.3 (13.4-17.3)% | .217 | ||
| 365 d | 19.2 (17.6-20.9)% | 22.2 (19.9-24.5)% | .042 | ||
| NRM∗ | 2162 | 1298 | .068 | ||
| 100 d | 5.5 (4.5-6.5)% | 7.9 (6.5-9.4)% | |||
| 180 d | 9.6 (8.4-10.9)% | 10.6 (9-12.4)% | |||
| 365 d | 13.9 (12.4-15.4)% | 14.7 (12.8-16.7)% | |||
| DFS∗ | 2162 | 1298 | < .001 | ||
| 100 d | 87 (85.5-88.3)% | 84 (82-86)% | .020 | ||
| 180 d | 76.6 (74.8-78.4)% | 74.1 (71.7-76.4)% | .093 | ||
| 365 d | 66.9 (64.9-68.9)% | 63.2 (60.5-65.8)% | .027 | ||
| OS | 2499 | 1543 | .064 | ||
| 100 d | 93.3 (92.3-94.2)% | 91.3 (89.8-92.7)% | |||
| 180 d | 86.8 (85.4-88.1)% | 84.6 (82.7-86.4)% | |||
| 365 d | 76.9 (75.2-78.6)% | 74.6 (72.3-76.8)% | |||
| GRFS | 2220 | 1247 | .287 | ||
| 100 d | 79.4 (77.7-81.1)% | 76.4 (74-78.7)% | |||
| 180 d | 65.5 (63.5-67.5)% | 62.8 (60-65.4)% | |||
| 365 d | 51.1 (49-53.2)% | 50 (47.2-52.8)% | .518 | ||
Clinical evidence of relapse only.
Graft-cell dose, hematopoietic engraftment, graft failure, donor receipt of a second HCT or DLI, and donor chimerism
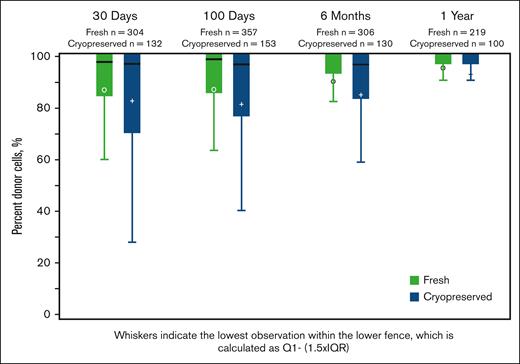
The graft-cell doses were reported at the time of infusion (after thawing, for cryopreserved products). Recipients of cryopreserved grafts received lower median PBSC CD34+ cell doses (5.7 × 106/kg; range, 0.01-3478.4 × 106/kg; vs 6.3 × 106/kg; range, 0.02-83 855 × 106/kg; P < .01) and lower median BM total nucleated cell doses (2.50 × 108/kg; range, 0.04-13.1 vs 3.1 × 108/kg; range, 0.01-767.5×108/kg; P < .01). Kinetic neutrophil and platelet engraftment was slightly delayed among recipients of cryopreserved PBSC grafts. For those with cryopreserved PBSC grafts, the median time to ANC recovery was day 16+ (0-104) vs day 15+ (0-72) for those with fresh products (P < .01). For those with cryopreserved PBSC grafts, the median time to platelet recovery was day 20+ (1-224) vs day 18+ (1-202) for those with fresh products (P < .01). The cumulative incidence of platelet engraftment by day 100+ was lower in recipients of cryopreserved grafts (92.8% [91.4-94] vs 94.8% [93.9-95.7]; P = .011) than in those with fresh grafts (Table 2). The risks of primary graft failure (6% vs 4%; OR, 1.35; 95% CI, 1.02-1.79), secondary graft failure (6% vs 4%; OR, 1.63; 95% CI, 1.14-2.32), and subsequent need for a second HCT or DLI (4% vs 3%; OR, 1.62; 95% CI, 1.14-2.31) were higher with cryopreserved products than with fresh products. Complete donor T-cell chimerism was similar on days 30+ and 100+ and 1 year after HCT but was lower in the group with cryopreserved grafts on day 180+ (fresh, 72% vs cryopreserved, 58%; P = .02; Figure 1).
Complete donor chimerism in recipients of fresh or frozen allografts assessed at days 30+ and 100+, 6 months, and 1 year after HCT in a subset of cohorts. The differences are statistically significant (P = .02) at 6 months only.
Complete donor chimerism in recipients of fresh or frozen allografts assessed at days 30+ and 100+, 6 months, and 1 year after HCT in a subset of cohorts. The differences are statistically significant (P = .02) at 6 months only.
aGVHD, cGVHD, GRFS, and relapse
The cumulative incidence of aGVHD grades from 2 to 4 (36.0%; 95% CI, 33.5-38.5 with cryopreserved vs 32.7%; 95% CI, 30.8-34.6 with fresh; P = .042) was higher among those with cryopreserved products than those with fresh products, but the incidence of aGVHD grades from 3 to 4 (9.8%; 95% CI, 8.3-11.4 with cryopreserved vs 10.4%; 95% CI, 9.2-11.7 with fresh; P = .504) 100 days after HCT was not different. The cumulative incidence of moderate-to-severe cGVHD 365 days after HCT was lower with cryopreserved products (16.9%; 95% CI, 15-18.9) relative to that with fresh products (19.8%; 95% CI, 18.2-21.4; P = .023). The cumulative incidence of relapse 1 year after HCT was higher in the group with cryopreserved grafts (22.2%; 95% CI, 19.9-24.5) compared with the group with fresh grafts (19.2%; 95% CI, 17.6-20.9; P = .042). As a result, GRFS at 1 year was not different (50.0%; 95% CI, 47.2-52.8 in the roup with cryopreserved grafts vs 51.1%; 95% CI, 49-53.2 in the group with fresh grafts [P = .518]).
Multivariable regression analysis
The results of the multivariable analyses are described below and presented in Table 3, as well as in Figures 2 and 3.
Multivariable analysis results summary
| Outcome . | HR (95% CI) . | P value . |
|---|---|---|
| OS | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.123 (0.984-1.283) | .0857 |
| Relapse∗ | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.211 (1.040-1.410) | .0135 |
| NRM∗ | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.014 (0.840-1.223) | .8863 |
| DFS∗ | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.183 (1.051-1.332) | .0055 |
| Grade II-IV aGVHD | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.110 (0.994-1.240) | .0637 |
| Grade III-IV aGVHD | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 0.925 (0.758-1.130) | .4456 |
| Moderate-to-severe cGVHD, domestic donor center | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 0.647 (0.496-0.843) | .0013 |
| Moderate-to-severe cGVHD, international donor center | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 0.813 (0.633-1.045) | .1063 |
| Moderate-to-severe cGVHD, related donor/no donor center | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.171 (0.860-1.594) | .3161 |
| Moderate-to-severe cGVHD, missing donor center | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 0.245 (0.015-4.030) | .3246 |
| GRFS∗ | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.029 (0.930-1.139) | .5793 |
| Outcome . | HR (95% CI) . | P value . |
|---|---|---|
| OS | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.123 (0.984-1.283) | .0857 |
| Relapse∗ | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.211 (1.040-1.410) | .0135 |
| NRM∗ | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.014 (0.840-1.223) | .8863 |
| DFS∗ | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.183 (1.051-1.332) | .0055 |
| Grade II-IV aGVHD | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.110 (0.994-1.240) | .0637 |
| Grade III-IV aGVHD | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 0.925 (0.758-1.130) | .4456 |
| Moderate-to-severe cGVHD, domestic donor center | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 0.647 (0.496-0.843) | .0013 |
| Moderate-to-severe cGVHD, international donor center | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 0.813 (0.633-1.045) | .1063 |
| Moderate-to-severe cGVHD, related donor/no donor center | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.171 (0.860-1.594) | .3161 |
| Moderate-to-severe cGVHD, missing donor center | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 0.245 (0.015-4.030) | .3246 |
| GRFS∗ | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.029 (0.930-1.139) | .5793 |
| Outcome . | OR (95% CI) . | P value . |
|---|---|---|
| Primary graft failure | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.481 (1.100-1.995) | .0097 |
| Platelet recovery | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 0.665 (0.518-0.853) | .0013 |
| Outcome . | OR (95% CI) . | P value . |
|---|---|---|
| Primary graft failure | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 1.481 (1.100-1.995) | .0097 |
| Platelet recovery | ||
| Fresh product (ref) | 1.00 | |
| Cryopreserved product | 0.665 (0.518-0.853) | .0013 |
ref., reference group.
OS: age, Karnofsky/Lansky score, HCT-CI, and donor type, and refined DRI. Variables adjusted for within each model.
Relapse: conditioning intensity and refined DRI. Variables adjusted for within each model.
NRM: age, Karnofsky/Lansky score, HCT-CI, and refined DRI. Variables adjusted for within each model.
DFS: age, Karnofsky/Lansky score, HCT-CI, and refined DRI. Variables adjusted for within each model.
Grade II-IV aGVHD: conditioning intensity, cell count, refined DRI, donor type, and GVHD prophylaxis. Variables adjusted for within each model.
Grade III-IV aGVHD: refined DRI and GVHD prophylaxis. Variables adjusted for within each model.
Moderate-to-severe cGVHD: conditioning intensity, donor center location, graft type, GVHD prophylaxis, and the use of in vivo T-cell depletion. Variables adjusted for within each model.
GRFS: Karnofsky/Lansky score, HCT-CI, graft type, refined DRI, and GVHD prophylaxis. Variables adjusted for within each model.
Primary graft failure: HCT-CI, graft type, and GVHD prophylaxis. Variables adjusted for within each model.
Platelet recovery: Karnofsky/Lansky score, HCT-CI, refined DRI, and donor type. Variables adjusted for within each model.
Clinical evidence of relapse only.
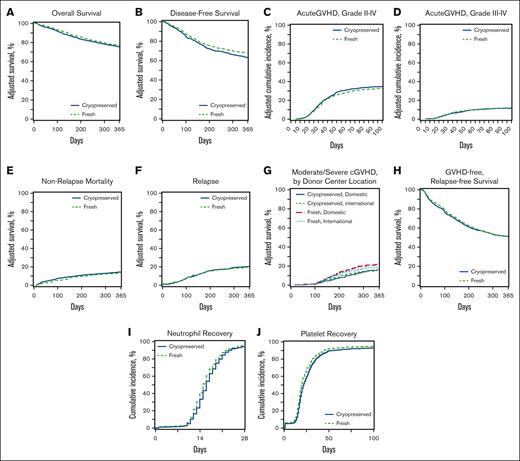
Clinical endpoints. Adjusted curves comparing fresh (green dotted lines) and cryopreserved (blue lines) allografts for clinical outcomes including OS (A), DFS (B), aGVHD grade II-IV (C), aGVHD grades III-IV (D), NRM (E), relapse (F), moderate-to-severe cGVHD (G), GRFS (H), neutrophil recovery (I), and platelet recovery (J).
Clinical endpoints. Adjusted curves comparing fresh (green dotted lines) and cryopreserved (blue lines) allografts for clinical outcomes including OS (A), DFS (B), aGVHD grade II-IV (C), aGVHD grades III-IV (D), NRM (E), relapse (F), moderate-to-severe cGVHD (G), GRFS (H), neutrophil recovery (I), and platelet recovery (J).
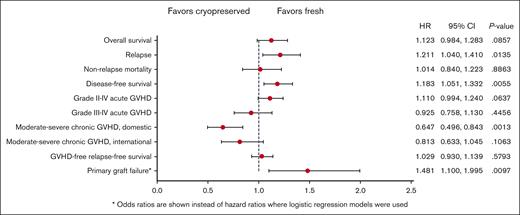
Forest plot comparing HRs for clinical outcomes in multivariable analysis comparing fresh and cryopreserved allografts. The OR is presented for primary graft failure.
Forest plot comparing HRs for clinical outcomes in multivariable analysis comparing fresh and cryopreserved allografts. The OR is presented for primary graft failure.
OS, DFS, and NRM
There was no difference in the 1-year OS after HCT between the groups (hazard ratio [HR], 1.12; 95% CI, 0.98-1.28; P = .09). The OS results did not change when the analysis was limited to recipients of URD HCT (data not shown). Other factors affecting OS included older age, low Karnofsky/Lansky performance status during HCT, high HCT-comorbidity index (HCT-CI), and the donor type. One-year DFS was lower in the group with cryopreserved grafts (HR, 1.18; 95% CI, 1.05-1.33; P = .006) than that in the group with fresh grafts. Other factors affecting DFS included older age, low Karnofsky/Lansky performance status at HCT, and high HCT-CI. NRM 1 year after HCT was not different (HR, 1.01; 95% CI, 0.84-1.22; P = .89) between the groups. Other factors affect the NRM included older age, low Karnofsky/Lansky performance status at HCT, high HCT-CI and high refined disease risk index (DRI).
Graft failure and platelet recovery
The risk of primary graft failure was higher in the group with cryopreserved grafts than in those with fresh ones (OR, 1.48; 95% CI, 1.19-2.00; P = .01), and the odds of platelet recovery by day 100+ after HCT was lower with cryopreserved products than with fresh products (OR, 0.67; 95% CI, 0.52-0.85; P = .001). Other factors negatively affecting hematopoietic engraftment included a low Karnofsky/Lansky performance status at HCT, high HCT-CI, GVHD prophylaxis regimen, high refined DRI, and receipt of a BM graft.
aGVHD, cGVHD, relapse, and GRFS
There was no difference in the risk of aGVHD grades from 2 to 4 or from 3 to 4 between the 2 groups. The risk of moderate-to-severe cGVHD at 1 year was lower in the group with cryopreserved grafts using products from domestic donor centers than in the group with fresh products (HR, 0.65; 95% CI, 0.50-0.84; P = .001). The risk of relapse at 1 year after HCT was higher in the group with cryopreserved grafts than in the group with fresh grafts (HR, 1.21; 95% CI, 1.04-1.41; P = .01). There was no difference in GRFS at 1 year after HCT between the 2 groups (HR for Cryopreservation, 1.03; 95% CI, 0.93-1.14; P = .58). Other factors negatively affecting the GRFS included a low Karnofsky performance status, high HCT-CI, and recieipt of a PB graft.
Subgroup analysis
Impact of the POC and product transit time
We analyzed the data of 1 189 recipients of domestic (N = 555) and international (N = 634) URD products planned for cryopreservation. NMDP operations data demonstrated longer median transit times for international vs domestic collections (international median time, 39 hours vs domestic median time, 17 hours). There was no difference in the infused cell dose between domestic and international cryopreserved BM products (P = .4619) or cryopreserved PBSC products (P = .4670). In the univariate analysis, the cumulative incidence of ANC recovery by day 28+ was lower with international products that were cryopreserved at the transplantation centers than with fresh international, fresh domestic, or cryopreserved domestic products (P = .040). However, platelet recovery by day 100+ did not differ based on the POC. Neither the POC nor product type (cryopreserved vs fresh) affected the 1-year OS (data not shown).
Discussion
The onset of the COVID-19 pandemic in March 2020 necessitated a rapid switch to the cryopreservation of allogeneic hematopoietic cell grafts from both related and URD in order to ensure that patients had a viable graft available on the day of HCT.9 This study found that although cryopreservation resulted in the infusion of ∼10% to 20% lower cell doses relative to fresh grafts, the subsequent short delay in neutrophil and platelet engraftment kinetics and incomplete platelet recovery by day 100 did not adversely affect the OS within a year of HCT. We observed a negative impact of cryopreservation on the risk of primary graft failure and risk of relapse, which translated into a low 1-year DFS; however, the absolute difference in DFS between the 2 groups was small (63.2% for cryopreservation and 66.9% for fresh). Considering the widespread impact of the Omicron variant surge as well as the possibility of future COVID-19 waves, these data provide some reassurance to HCT clinicians and patients that cryopreservation poses no risk of major clinical compromise in outcomes when necessary to safeguard the provision of donor allografts. However, based on these data, the NMDP recommends fresh grafts now that pandemic-related logistical hurdles have mostly been resolved. Cryopreservation should be reserved as an option when fresh grafts are not feasible for various reasons.
Cryopreservation is a standard procedure for autologous and umbilical cord blood transplantation and is well supported by a large body of clinical and technical literature.16 Yet at the onset of the COVID-19 pandemic, there was a surprising paucity of data beyond single center or small multicenter reports with which to ensure clinicians that cryopreservation would not adversely affect HCT outcomes. The CIBMTR quickly contributed 2 important retrospective analyses suggesting that cryopreservation should be avoided in patients with severe aplastic anemia because of high rates of graft failure but, otherwise, was not associated with adverse consequences in other settings, such as those that incorporate posttransplantation cyclophosphamide to prevent GVHD.3,17 A large single center study limited to recipients of cryopreserved PBSC allografts from related, unrelated and haploidentical donors for patients with a variety of hematological malignancies reported in 2020 found no difference between fresh vs cryopreserved grafts in neutrophil engraftment, platelet engraftment, graft failure, grade II-IV aGVHD, or OS.1 This study was limited to recipients of PBSC grafts obtained before the pandemic, and the vast majority of donors were HLA-matched relatives. Few patients receiving URD products were reported, and there was no information about the product transit time. Whether these results can be extrapolated to other centers using different standards of care remains unclear.
Another report from the CIBMTR in a large cohort of patients receiving conventional GVHD prophylaxis followed later and showed mixed results.5 Cryopreservation of BM grafts was not associated with delays in engraftment or inferior survival, but cryopreservation of related PBSC grafts was associated with delayed platelet engraftment, increased aGVHD, and inferior OS. In the same study, cryopreservation of URD PBSC grafts was associated with delayed engraftment, increased NRM and relapse rates, and decreased OS. Again, patients in this study underwent HCT before the pandemic, and the reasons for providing cryopreserved grafts to URD recipients were not routinely collected. However, a small subset analysis in this study suggested that most of the observed differences were due to the need to cryopreserve for patient related health reasons.
Recently, small single center studies of patients who underwent transplantation during the COVID-19 pandemic have been reported by Dana-Farber and Stanford groups.18,19 Both studies observed delayed hematopoietic engraftment in recipients of cryopreserved grafts. Although the Stanford study observed inferior OS with cryopreservation, a larger study by Dana-Farber did not. The Dana-Farber study did report delays in donor T-cell reconstitution after HCT. Given the potential deleterious impact of lower donor chimerism on the rates of secondary graft failure or disease relapse, this might have affected the results of this analysis, given the higher rates of relapse, secondary graft failure, and the need for subsequent HCT or DLI. Our study found lower rates of T-cell chimerism at day 180+ but not at other time points (up to 1 year after HCT). However, data were available for only ∼15% of the recipients of cryopreserved grafts so should be interpreted with caution. An Australian registry study failed to demonstrate evidence of delayed engraftment in recipients of grafts cryopreserved and transplanted during the pandemic but did find cell losses that were significant, particularly for grafts that experienced long transit times before the initiation of cryopreservation.20 Our study supports the potential negative impact of cryopreservation on infused cell doses observed by the Australian and Dana-Farber analyses. The extent to which this might have affected the higher risk of graft failure and the need for a second HCT remains speculative.
We observed a small but significant increase in the cumulative incidence of relapse 1 year after HCT in cryopreserved graft recipients. This is similar to a previous CIBMTR analysis, but contrasts with other reports.1,5,6,8,19,21,22 Based on the observational nature of the database used in our study, it is difficult to discern whether this is due to the immunosuppressive effects of cryopreservation on graft lymphocyte function or a higher intrinsic risk of relapse in cryopreserved graft recipients (compared with the fresh graft recipients) that could not be accounted for in our statistical models. It is theoretically possible that at the outset of the pandemic, only the patients considered at the highest risk for relapse (eg, evidence of measurable residual disease) were subjected to the risks of HCT imposed by the pandemic. However, we did not find differences in the proportion of patients with a high or very high CIBMTR DRI between the groups. Interestingly, we also observed a slightly decreased risk of cGVHD at 1 year, which could also represent the inverse consequence of cryopreservation on immune function. This may also be borne out by any differences observed in T-cell chimerism at day 180+ after HCT. Our findings are similar to those of a recent report from Dana Farber Cancer Instiitute.23 Of note, the impact of cryopreservation on cGVHD was observed only in grafts collected at domestic centers. However, the reason for this remains unclear. Interestingly, the opposing effects of increased relapse and decreased cGVHD resulted in, essentially, the same GRFS at 1 year in recipients of cryopreserved and fresh allografts.
Concerns have been raised by some donor registries that planned cryopreservation creates a scenario whereby a small proportion of the products collected ahead of time from URDs may not be used.24,25 The NMDP has been monitoring the rate of unused donor products since March 2020. To date, this represents <3% of the products NMDP delivered since the start of the pandemic.26 Efforts are made ahead of collection to inform donors about the potential risk that the product collected may never be used. Donors are given the autonomy to make their own decisions, and the NMDP has encountered very few refusals to move forward with donation, particularly under crisis conditions that necessitated cryopreservation during the pandemic.
This study has several limitations. The data were retrospective and because the study relied on centers reporting follow-up data to the CIBMTR, not every patient who received a cryopreserved graft during the pandemic was known. However, the data appear representative, given that they were gathered from 77 US centers reporting to the CIBMTR at a time when more than 90% of URD products were planned for cryopreservation. Data of recipients of fresh and cryopreserved grafts were collected a year apart so could be confounded by a time trend effect. We analyzed a heterogeneous group of patients with a variety of indications for HCT. Although we adjusted for several variables known to impact clinical outcomes, we were unable to perform propensity score matching. There were more missing data in the group with cryopreserved grafts than in the group with fresh grafts, but this appeared random, and we did not find evidence of transplantation center effects. We also did not collect granular data on cryopreservation methods, cell yields and postcryopreservation viability, or individual product transit times. Notwithstanding, to our knowledge, this is the largest study on the effects of cryopreservation on HCT outcomes at a time when there was a uniform reason for its use.
How should the results of this study be placed into context? They indicate that fresh grafts should be the first choice, given the negative impact of cryopreservation on hematopoietic engraftment, relapse, and DFS as well as the small risk that donor grafts may go unused. However, cryopreservation is a reasonable alternative to a fresh donor product when safety and logistical challenges may dictate the decision for 1 vs the other. Ultimately, each transplantation center will need to weigh the advantages and disadvantages of cryopreservation and discuss them with their patients before deciding on the option that is best for a particular individual. The advantages include receipt of a product with a known cell dose before starting conditioning, assuring greater patient safety, and often resulting in an easing of scheduling hurdles. These disadvantages are both real and theoretical. There are added costs, potential additional toxicity related to dimethyl sulfoxide, loss of cell viability, particularly with products enduring increased transit times, and clear resource strains on the transplantation center staff. There are additional challenges to cryopreserve BM compared with PBSC grafts, and many centers have chosen to avoid cryopreserving BM. Theoretical concerns that cryopreservation of an allogeneic product could result in functional immunological changes (eg, CD62 loss, T-cell suppression, etc) that may affect the immune reconstitution or result in relapse and risk of severe GVHD can be found in the scientific literature, although the magnitude of the clinical impact of this remains uncertain.4,7,27
In conclusion, although cryopreservation did not adversely affect OS within a year of HCT in patients with indications for HCT during the first 6 months of the COVID-19 pandemic, relapse and graft failure risks were higher and DFS was lower relative to fresh product HCTs. Longer follow-up will be necessary to understand any late effects on outcomes, including graft function, cGVHD, and relapse, beyond a year after HCT. Future studies that evaluate the impact of cryopreservation on immune reconstitution, graft vs tumor responses, and responses to vaccinations would also be highly desirable.
Acknowledgments
The authors acknowledge the courage of transplantation recipients and their families during the pandemic. The authors also acknowledge the resilient efforts of NMDP and CIBMTR staff and network colleagues, including volunteer couriers who continue to sacrifice for the benefit of patients in need. They thank the donors throughout the world; on behalf of our transplantation patients and practitioners, the NMDP and CIBMTR offers their sincerest gratitude for the altruism during one of humankind's most challenging crises.
The CIBMTR is supported primarily by Public Health Service grant U24CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases, HHSH250201700006C grant from the Health Resources and Services Administration, and grants N00014-21-1-2954 and N00014-23-1-2057 from the Office of Naval Research. Support is also provided by Be The Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie, Actinium Pharmaceuticals, Inc. Adaptimmune, Adaptive Biotechnologies Corporation, ADC Therapeutics, Adienne SA, Allogene, Allovir, Inc, Amgen, Inc, Angiocrine, Anthem, Astellas Pharma, AstraZeneca, Atara Biotherapeutics, BeiGene, bluebird bio, Bristol Myers Squibb, CareDx Inc, CRISPR, CSL Behring, CytoSen Therapeutics, Inc, Eurofins Viracor, DBA Eurofins Transplant Diagnostics, Gamida-Cell, Ltd, Gilead, GlaxoSmithKline, HistoGenetics, Incyte Corporation, Iovance, Janssen Research & Development, LLC, Janssen/Johnson & Johnson, Jasper Therapeutics, Jazz Pharmaceuticals, Inc, Kadmon, Karius, Kiadis Pharma, Kite, a Gilead Company, Kyowa Kirin, Legend Biotech, Magenta Therapeutics, Mallinckrodt Pharmaceuticals, Medexus Pharma, Merck & Co, Mesoblast, Millennium, the Takeda Oncology Co, Miltenyi Biotec, Inc, MorphoSys, Novartis Pharmaceuticals Corporation, Omeros Corporation, OptumHealth, Orca Biosystems, Inc, Ossium Health, Inc, Pfizer, Inc, Pharmacyclics, LLC, an AbbVie Company, Pluristem, PPD Development, LP, Sanofi, Sanofi-Aventis US Inc, Sobi, Inc, Stemcyte, Takeda Pharmaceuticals, Talaris Therapeutics, Terumo Blood and Cell Technologies, TG Therapeutics, Vertex Pharmaceuticals, Vor Biopharma Inc, and Xenikos BV.
Authorship
Contribution: S.M.D., B.R.L., and B.E.S. contributed to the study design, study supervision, and data interpretation, and wrote the manuscript; M.K., S.B.-S., C.B., and K.W.A. generated the statistical plan, performed the statistical analysis, and interpreted the data; and all authors contributed to manuscript writing and review and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict-of-interest disclosure: H.E.S. is on the advisory board and speakers bureau for Novartis. S.M.D. claims Orca Bio for consultancy. B.E.S. declares consultancy for Orca Bio and Mallinkrodt. The remaining authors declare no competing financial interests.
Correspondence: Steven M. Devine, Center for International Blood and Marrow Transplant Research, National Marrow Donor Program/Be The Match, 500 N 5th St, Minneapolis, MN 55401; e-mail: sdevine2@nmdp.org.
References
Author notes
Data are available on request from the corresponding author, Steven M. Devine (sdevine2@nmdp.org).