Key Points
Bone marrow–derived donor cells were identified in the brain of HSCT patients using a novel IBA1 IHC and XY FISH co-stain assay.
Donor cells in the brain express IBA1 and represent 8% or up to 25% of microglia, respectively, after myeloablative or double HSCT.
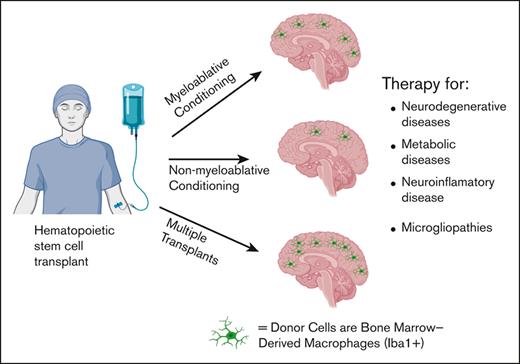
Visual Abstract
Donor cells engraft into the brain as Iba1+ bone marrow derived macrophages
Donor cells engraft into the brain as Iba1+ bone marrow derived macrophages
Abstract
Hematopoietic stem cell transplantation is a well-known treatment for hematologic malignancies, wherein nascent stem cells provide regenerating marrow and immunotherapy against the tumor. The progeny of hematopoietic stem cells also populate a wide spectrum of tissues, including the brain, as bone marrow–derived macrophages similar to microglial cells. We developed a sensitive and novel combined immunohistochemistry (IHC) and XY fluorescence in situ hybridization assay to detect, quantify, and characterize donor cells in the cerebral cortices of 19 female patients who underwent allogeneic stem cell transplantation. We showed that the number of male donor cells ranged from 0.14% to 3.0% of the total cells or from 1.2% to 25% of microglial cells. Using tyramide-based fluorescent IHC, we found that at least 80% of the donor cells expressed the microglial marker ionized calcium-binding adapter molecule-1, consistent with bone marrow–derived macrophages. The percentage of donor cells was related to pretransplantation conditioning; donor cells from radiation-based myeloablative cases averaged 8.1% of microglial cells, whereas those from nonmyeloablative cases averaged only 1.3%. The number of donor cells in patients conditioned with busulfan- or treosulfan-based myeloablation was similar to that in total body irradiation-based conditioning; donor cells averaged 6.8% of the microglial cells. Notably, patients who received multiple transplantations and those with the longest posttransplantation survival had the highest level of donor engraftment, with donor cells averaging 16.3% of the microglial cells. Our work represents the largest study characterizing bone marrow–derived macrophages in patients after transplantation. The efficiency of engraftment observed in our study warrants future research on microglial replacement as a therapeutic option for disorders of the central nervous system.
Introduction
Hematopoietic stem cell transplantation (HSCT), a form of cellular therapy used to treat hematologic malignancies and systemic genetic syndromes, provides both a nascent hematopoietic system and an immune system that includes donor-derived tissue-resident macrophages. These cells engraft into a variety of tissues, including the brain, where they are referred to as bone marrow–derived macrophages (BMDMs), and comprise a distinct population that shares many features of endogenous microglia.1,2
Microglial cells are unique among tissue-resident macrophages; they are exclusively derived from the yolk sac and are maintained via self-renewal.3-6 They function as immune sensors within the central nervous system (CNS) and serve essential homeostatic functions in synaptic remodeling, antigen presentation, cytokine-mediated immunity, neurogenesis, and dead cell and debris clearance.3,6 The functional capacity of BMDMs within the CNS and the extent of engraftment under normal conditions without injury or conditioning are active areas of investigation. Studies in mice have shown that BMDMs can functionally replace endogenous CNS microglia, treat genetic brain disease, and deliver therapeutic proteins into the CNS.7 Studies using animal models have shown that microglial replacement therapy with BMDMs is also an effective therapeutic strategy for a number of inherited metabolic diseases, microgliopathies, neuroinflammatory, and neurodegenerative diseases, including Alzheimer and Parkinson diseases.8-14 In fact, there are multiple ongoing clinical trials to evaluate the therapeutic effect of microglial replacement via bone marrow transplantation for these disorders in humans15-17 as listed on www.clinicaltrials.gov.
The ability of bone marrow–derived cells to stably engraft into the CNS was best demonstrated in animal models that received transplantation with green fluorescent protein (GFP)-labeled hematopoietic stem cells.18-20 The engrafted GFP+ donor cells expressed microglial marker ionized calcium-binding adapter molecule-1 (IBA1), indicating that they were BMDMs.18 CNS engraftment was shown to require cranial irradiation as a conditioning agent; however, subsequent studies have demonstrated that busulfan-based conditioning as well as immune or genetic ablation of endogenous microglial cells can also promote CNS engraftment.21-24 Recent studies have reported a near-complete microglial replacement using a combination of immune ablation and cranial irradiation in animal models.23,25
Donor-derived microglial replacement therapy (MRT) has been well studied in animal models; however, little is known regarding BMDM engraftment in humans. Few studies have identified a small number of donor-derived cells within the brains of a limited number of patients who underwent sex-mismatched stem cell transplantation.26-28 The dearth in human data regarding donor BMDM is attributed to difficulties in performing XY fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC) studies on poorly preserved postmortem tissues from patients who underwent stem cell transplantation. In this study, we developed a modified XY FISH assay and performed image analysis to enumerate and characterize donor-derived cells in the frontal cortex of 19 patients who underwent sex-mismatched HSCT, which, to our knowledge, is the largest human study performed thus far. We evaluated the effects of conditioning and other variables, including the time from transplantation, and showed that donor-derived cells comprise as much as 25% of the microglial population. Staining for the microglial marker IBA1 revealed that donor-derived cells express IBA1 consistent with being BMDMs. Our observation of the stable and robust engraftment of donor BMDM in the human brain provides a basis for future clinical studies using microglial replacement as a therapeutic modality.
Materials and methods
Clinical samples
Human cerebral cortex biopsies from female patients with a history of sex-mismatched HSCT were obtained from the Fred Hutchinson autopsy tissue repository. Cases were selected based on posttransplantation survival, conditioning regimens, and the number of transplantations. Sex-matched HSCT cases were included as controls. All specimen used in this study were obtained after obtaining written consent from the patients and approval from the Fred Hutchinson Cancer Center Institutional Review Board (protocol #1837). This study was conducted in accordance with the Declaration of Helsinki.
FISH
Brain tissue sections were stained using modified FISH techniques optimized for heavily fixed postmortem tissue, with increased proteolytic digestion to unmask the DNA, and overnight hybridization with Vysis CEP X (DXZ1) Spectrum Green Probe and Vysis CEP Y (DYZ1) Spectrum Orange Probe in Vysis IntelliFISH Hybridization Buffer (Abbott Molecular Diagnostics Des Plaines, IL) to visualize the X and Y chromosomes. After overnight hybridization, slides were washed and mounted in antifade mounting medium with 4′,6-diamidino-2-phenylindole (DAPI; Vectashield H-1200, Vector Labs; Burlingame, CA). FISH-labeled sections were imaged using a 40×/0.75 EC Plan-NEOFLUAR air objective on a Zeiss Axio Imager Z2 microscope as part of a TissueFAXS system (TissueGnostics; Vienna, Austria). Maximum projections of z-stacks (total size, 7.2 μm; interval, 0.8 μm) were used for image analysis.
Donor cell quantification via XY FISH analysis
Scanned images were analyzed using TissueQuest (TissueGnostics, Vienna, Austria). DAPI-stained nuclei were segmented based on size to create nuclear masks. Background thresholds were defined to detect the Y probe (red) and X probe (green) FISH signals within the nuclear mask. A small sample region (5 mm2; ∼1000 cells) was initially analyzed to ensure that automated detection using TissueQuest yielded accurate and comparable data to manual quantification. Following this validation, the number of donor and FISH-positive cells was quantified in a larger area (30-40 mm2 encompassing 10 000-15 000 cells). The identified donor cells were confirmed via blinded manual inspection. The percentage of donor cells was defined as the number of cells with a Y chromosome divided by the number of FISH-positive cells (XX, XY, X, or Y cells). Donor cell quantification was presumably underestimated because some donor cells were not identified, given that the tissue section did not include the Y chromosome (partial sampling of the nucleus). On average, ∼66% of the cells had a positive FISH signal (X or Y), 33% had a single X or Y signal, and 33% had 2 signals (XX or XY).
Combined IBA1 IHC and XY FISH staining
Selected cases were costained with IBA1 IHC and XY FISH to characterize the donor cells. Sections were first incubated with anti-IBA1 antibody (Wako Chemicals, Japan), then with poly horseradish peroxidase (HRP)-conjugated goat anti–rabbit immunoglobulin G secondary antibody (Invitrogen, Carlsbad, CA), and visualized using Alexa Fluor 488 tyramide reagent (Invitrogen) per manufacturer instructions. The tyramide reagent forms a stable, covalently ligated fluorochrome. The slides were imaged using a TissueFAXS. The same sections were then stained for XY FISH (as previously described) and reimaged using a TissueFAXS. Because some of the IHC signal was lost following FISH, digital scans of IBA1 IHC were overlaid onto FISH images using DAPI-stained nuclei for orientation (Adobe Photoshop).
IHC
Parallel sections of the brain tissue were stained with an anti-IBA1 antibody to detect and quantify microglia and BMDMs using standard conditions of citrate antigen retrieval and DAB detection (intelliPATH FLX, Biocare Medical). IBA1-positive microglia represented ∼12% of the nucleated cells in the area used for XY FISH analysis.
Results
The goal of our study was to detect and enumerate donor-derived cells in the brains of recipients who underwent stem cell transplantation. Brain tissue samples from female patients who had undergone sex-mismatched stem cell transplantation were obtained from the Fred Hutchinson tissue repository. Cases were selected based on posttransplantation survival, conditioning regimen, number of transplantations, and the absence of recent brain injury (Table 1). We focused on the frontal cortex because we observed reproducible staining and the highest number of donor cells in the cortical gray matter compared with that in sections from the pons and hippocampus.
Patient cohort and conditioning regimens
| Sample . | Type of BMT . | Conditioning . | Days P-TX . | Donor cells, % of total . | Donor cells, % of microglia . |
|---|---|---|---|---|---|
| B1 | Myeloablative TBI | CY, TBI (13.2 Gy) | 514 | 0.61 | 5.08 |
| B2 | Myeloablative TBI | CY, TBI (12 Gy) | 500 | 0.5 | 4.17 |
| B3 | Myeloablative TBI | CY, TBI (10 Gy) | 194 | 1.79 | 14.92 |
| B4 | Myeloablative TBI | CY, FLU, TBI (15.7 Gy) | 161 | 0.85 | 7.08 |
| B5 | Myeloablative TBI | CY, TBI (12 Gy) | 157 | 0.72 | 6.0 |
| B6 | Myeloablative TBI | CY, FLU, TBI (12 Gy) | 107 | 1.44 | 12.0 |
| B7 | Myeloablative TBI | CY, FLU, TBI (12 Gy) | 102 | 1.1 | 9.17 |
| B8 | Myeloablative TBI | TEPA, FLU, TBI (13.2 Gy) | 79 | 0.72 | 6.00 |
| B9 | Myeloablative Cy | CY (X4) | 208 | 0.92 | 7.67 |
| B10 | Myeloablative Bu | BU, CY, ATG | 262 | 0.79 | 6.58 |
| B11 | Myeloablative Treo | FLU, TREO | 85 | 0.53 | 4.42 |
| B12 | Myeloablative Bu | BU, FLU, ATG | 76 | 1.0 | 8.33 |
| B13 | Nonmyeloablative | FLU, TBI (3 Gy) | 178 | 0.16 | 1.33 |
| B14 | Nonmyeloablative | CY, FLU, TBI (2 Gy) | 143 | 0.14 | 1.17 |
| B15 | Nonmyeloablative | FLU, TBI (3 Gy) | 44 | 0.16 | 1.33 |
| Sample . | Type of BMT . | Conditioning . | Days P-TX . | Donor cells, % of total . | Donor cells, % of microglia . |
|---|---|---|---|---|---|
| B1 | Myeloablative TBI | CY, TBI (13.2 Gy) | 514 | 0.61 | 5.08 |
| B2 | Myeloablative TBI | CY, TBI (12 Gy) | 500 | 0.5 | 4.17 |
| B3 | Myeloablative TBI | CY, TBI (10 Gy) | 194 | 1.79 | 14.92 |
| B4 | Myeloablative TBI | CY, FLU, TBI (15.7 Gy) | 161 | 0.85 | 7.08 |
| B5 | Myeloablative TBI | CY, TBI (12 Gy) | 157 | 0.72 | 6.0 |
| B6 | Myeloablative TBI | CY, FLU, TBI (12 Gy) | 107 | 1.44 | 12.0 |
| B7 | Myeloablative TBI | CY, FLU, TBI (12 Gy) | 102 | 1.1 | 9.17 |
| B8 | Myeloablative TBI | TEPA, FLU, TBI (13.2 Gy) | 79 | 0.72 | 6.00 |
| B9 | Myeloablative Cy | CY (X4) | 208 | 0.92 | 7.67 |
| B10 | Myeloablative Bu | BU, CY, ATG | 262 | 0.79 | 6.58 |
| B11 | Myeloablative Treo | FLU, TREO | 85 | 0.53 | 4.42 |
| B12 | Myeloablative Bu | BU, FLU, ATG | 76 | 1.0 | 8.33 |
| B13 | Nonmyeloablative | FLU, TBI (3 Gy) | 178 | 0.16 | 1.33 |
| B14 | Nonmyeloablative | CY, FLU, TBI (2 Gy) | 143 | 0.14 | 1.17 |
| B15 | Nonmyeloablative | FLU, TBI (3 Gy) | 44 | 0.16 | 1.33 |
| Sample . | First HSCT . | Second HSCT . | Donor cells . | |||
|---|---|---|---|---|---|---|
| Conditioning . | Days-P-Tx . | Conditioning . | Days-P-Tx . | % Total . | % Microglia . | |
| B16 | CY, TBI (10 Gy) | 629 | No Conditioning | 42 | 1.59 | 13.25 |
| B17 | CY, TBI (12 Gy), MTX | 398 | BU, CY | 24 | 1.47 | 12.26 |
| B18 | CY, TBI | 5690 | TREO, FLU | 10 | 3.01 | 25.07 |
| B19 | BU, CY | 1716 | FLU, CY TBI | 61 | 1.76 | 14.66 |
| Sample . | First HSCT . | Second HSCT . | Donor cells . | |||
|---|---|---|---|---|---|---|
| Conditioning . | Days-P-Tx . | Conditioning . | Days-P-Tx . | % Total . | % Microglia . | |
| B16 | CY, TBI (10 Gy) | 629 | No Conditioning | 42 | 1.59 | 13.25 |
| B17 | CY, TBI (12 Gy), MTX | 398 | BU, CY | 24 | 1.47 | 12.26 |
| B18 | CY, TBI | 5690 | TREO, FLU | 10 | 3.01 | 25.07 |
| B19 | BU, CY | 1716 | FLU, CY TBI | 61 | 1.76 | 14.66 |
Cerebral cortical biopsies were obtained from 19 female patients who underwent sex-mismatched stem cell transplantation. Cases were selected based on availability, conditioning regimen, posttransplantation survival, and number of stem cell transplantations.
From B1 to B8, patients conditioned with TBI-based myeloablation (10-15.7 Gy); B9, patients treated with high-dose (4X) cytarabine myeloablation; from B10 to 12, patients conditioned with busulfan or treosulfan-based myeloablation; from B13 to 15, patients conditioned with TBI-based nonmyeloablation; and from B16 to 19, patients who received 2 separate stem cell transplantations.
Donor cells are reported as percent total cellularity (donor cells % of total) and percentage of microglia cells (donor cell % of microglia).
ATG, antithymocyte globulin; BU, busulfan; CY, cytarabine; Days PTx, posttransplantation survival listed as days after transplantation; FLU, fludarabine; MTX, methotrexate; TREO, treosulfan; TEPA, thiotepa.
Detection of donor cells via XY FISH
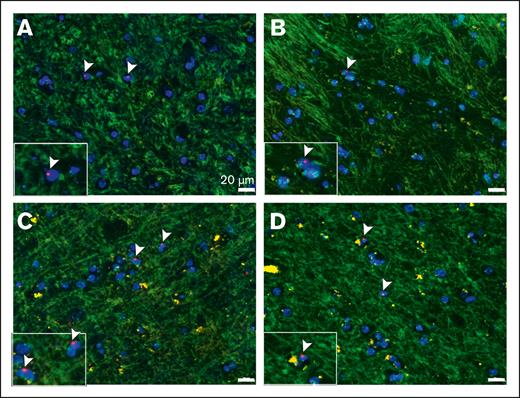
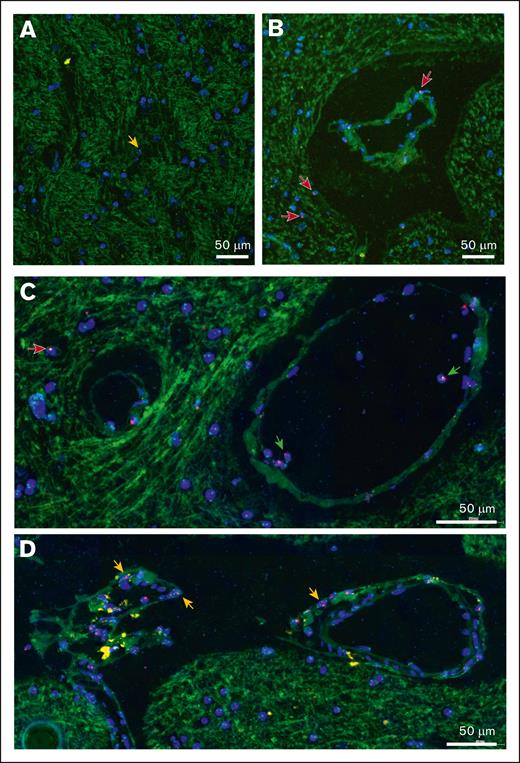
FISH studies are difficult to perform on postmortem brain tissues because of poor specimen preservation and background autofluorescence. To avoid these issues, previous studies have used chromogen-based detection but with limited sensitivity.26,27 We developed and optimized a more sensitive and robust XY FISH assay to detect male donor cells in cortex biopsies of female patients who underwent sex-mismatched stem cell transplantation. The assay uses high concentrations of pepsin protease to unmask the DNA and a commercial CEP Y spectrum orange probe and CEP X spectrum green Probe with IntelliFISH Hybridization Buffer to enhance the fluorescent signal. Using this assay, we detected male donor cells with XY staining within cortical brain tissue. Representative examples from 4 separate patients are presented in Figures 1A-D. X and Y chromosome detection was specific because no sex-mismatched cells were identified in tissues from female or male patients who underwent autologous transplantation (supplemental Figure 1). As is common with FISH-stained sections, only a single fluorescent signal (X or Y) was detected in many cells (50% of FISH-positive cells), which was attributed to partial nuclear sampling in thin tissue sections. Background autofluorescence has been used to visualize tissue architecture, including the blood vessels, ventricles, and arachnoid membranes. We observed that most intraparenchymal donor cells existed as isolated cells enriched in the perivascular regions (Figure 2A-B). Intravascular and peri-arachnoid donor cells were not included in the quantification but served as endogenous positive controls (Figure 2C-D). In addition, although brain sections with infection, inflammation, or adjacent hemorrhage had an increased number of donor cells, they were excluded from the analysis.
Detection of male donor cells via XY FISH. Representative XY FISH of frontal cortex biopsy specimen from 4 separate female patients with a history of sex-mismatched stem cell transplantation (A-D) Arrow-head, male donor cells. Light green, autofluorescence background highlighting the tissue architecture. Red probe, Y chromosome; green probe, X chromosome; blue, DAPI nuclei. Original magnification ×400; scale bar, 20μm.
Detection of male donor cells via XY FISH. Representative XY FISH of frontal cortex biopsy specimen from 4 separate female patients with a history of sex-mismatched stem cell transplantation (A-D) Arrow-head, male donor cells. Light green, autofluorescence background highlighting the tissue architecture. Red probe, Y chromosome; green probe, X chromosome; blue, DAPI nuclei. Original magnification ×400; scale bar, 20μm.
Distribution of male donor cells in the cerebral cortex, detected via XY FISH. Representative cortical biopsy sections from female patients with a history of sex-mismatched stem cell transplantation, illustrating the distribution of male donor cells. (A) Most donor cells exist as individual cells within the cortical gray matter (orange arrows). (B-C) Male donor cells are enriched in perivascular location (red arrows). Background autofluorescence identifies small blood vessels and intravascular donor cells (green arrows). (D) Donor cells are enriched along the cortical surface adjacent to the arachnoid membrane (orange arrows). Red probe, Y chromosome; green probe, X chromosome; blue, DAPI-stained nuclei. Original magnification ×400; scale bar, 50μm.
Distribution of male donor cells in the cerebral cortex, detected via XY FISH. Representative cortical biopsy sections from female patients with a history of sex-mismatched stem cell transplantation, illustrating the distribution of male donor cells. (A) Most donor cells exist as individual cells within the cortical gray matter (orange arrows). (B-C) Male donor cells are enriched in perivascular location (red arrows). Background autofluorescence identifies small blood vessels and intravascular donor cells (green arrows). (D) Donor cells are enriched along the cortical surface adjacent to the arachnoid membrane (orange arrows). Red probe, Y chromosome; green probe, X chromosome; blue, DAPI-stained nuclei. Original magnification ×400; scale bar, 50μm.
Characterization of donor cells
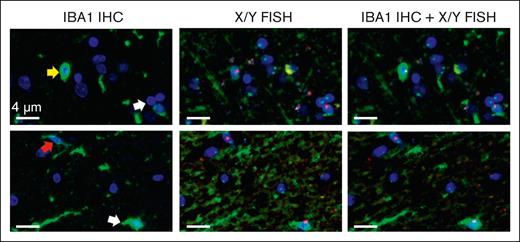
Most CNS-engrafted donor-derived cells in animal models are thought to be BMDMs based on the expression of IBA1, a macrophage/microglial protein specific to microglia and BMDMs in the CNS.18 To confirm that the donor cells we detected in our patient samples were BMDMs, we established a novel combined IBA1-IHC and XY FISH assay. Because the protease treatment required for FISH staining eliminates the IBA1 epitope and stable 3,3′-diaminobenzidine (DAB) IHC blocks the fluorescent FISH signal, we developed a tyramide-based fluorescent IHC assay in which a covalently ligated fluorochrome is formed and preserved during subsequent FISH staining. Tissue sections were first stained for IBA1, scanned, and then stained for XY FISH, followed by rescanning using TissueFAXS. Because some of the IHC fluorescent signal was still lost after FISH, scans of IBA1-IHC stained sections were overlaid onto XY FISH images using DAPI-stained nuclei for orientation (Figure 3). The overlapping images showed that most donor cells (>80%) expressed IBA1, consistent with their BMDM identity (Figure 3). In addition, similar to endogenous microglia, IBA1-positive donor cells were present in both ramified and amoeboid forms (Figure 3, arrows), with amoeboid cells (top) having higher levels of IBA1 than the ramified forms (bottom).
Tyramide IHC and XY FISH show that male donor cells express IBA1. Representative images from tyramide IBA1 IHC and XY FISH of 2 separate samples (top and bottom rows). (Column 1) tyramide-based IBA1 IHC (green); (column 2) same sections after XY FISH; (column 3) digital overlap of IHC and FISH images. Arrows indicate donor cells. Donor cells are present in both amoeboid (yellow arrow; top) and ramified (red arrow, bottom) forms, with a lower expression of IBA1 in ramified cells. Green, IBA1 IHC; red probe, Y chromosome; green probe, X chromosome; blue, DAPI-stained nuclei. Images taken at original magnification ×400; scale bar, 4 μm.
Tyramide IHC and XY FISH show that male donor cells express IBA1. Representative images from tyramide IBA1 IHC and XY FISH of 2 separate samples (top and bottom rows). (Column 1) tyramide-based IBA1 IHC (green); (column 2) same sections after XY FISH; (column 3) digital overlap of IHC and FISH images. Arrows indicate donor cells. Donor cells are present in both amoeboid (yellow arrow; top) and ramified (red arrow, bottom) forms, with a lower expression of IBA1 in ramified cells. Green, IBA1 IHC; red probe, Y chromosome; green probe, X chromosome; blue, DAPI-stained nuclei. Images taken at original magnification ×400; scale bar, 4 μm.
Quantification of donor cells
To quantify donor-derived cells, we digitally scanned the slides using a TissueFAXS and set up conditions to detect green X and red Y probes within the DAPI-stained nuclei. The automated detection system efficiently identified donor cells based on the presence of the Y chromosome (red probe). However, variable autofluorescence and background staining hindered automated detection of the X chromosome (green probe). Firstly, we calibrated the image analysis parameters in a small area, which was then compared with manual evaluation parameters. The established parameters were then applied to a larger region, encompassing ∼10 000 to 15 000 DAPI-positive cells. The identified donor cells were confirmed via blinded manual inspection. We defined the percentage of male donor cells as the number of cells stained with the Y probe compared with the total number of FISH-positive cells (cells stained with either X or Y probes). Cells without detectable FISH signals were excluded from our analysis. Presumably, a small number of cells with a single X chromosome were male donor cells that were not identified, because the section did not include the Y chromosome. Therefore, the calculated number of donor cells was probably underestimated.
Effect of conditioning regimens on donor cell engraftment
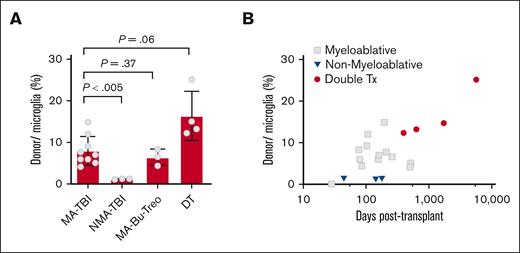
Preclinical studies have shown that host microglial depletion or pretransplantation conditioning disrupts the blood-brain barrier and facilitates donor cell engraftment into the brain.29,30 In agreement with this, we found that pretransplantation conditioning had a significant effect on donor cell engraftment into the CNS. Donor cells were quantified as described in “Materials and Methods.” Male donor cells comprised an average of 8.1% (4.2-14.9) of the microglial population after myeloablative conditioning (total body irradiation [TBI] >1000 cGy), whereas in nonmyeloablative cases (TBI <300 cGy), they averaged only 1.3% (Figure 4A; Table 1). The percentage of donor cells relative to the microglial population was calculated based on microglial cells representing 12% cellularity within the cortical gray matter, as determined from independent IBA1 IHC studies (supplemental Figure 2), in agreement with data from other studies.31 We also included cases with busulfan- or treosulfan-based myeloablative conditioning and found that both regimens supported BMDM engraftment, with donor cells averaging 6.8% (4.4-8.3) of microglia (Figure 4A; Table 1). Because donor cell engraftment may be limited by low numbers of circulating donor precursor cells (monocytes) in the early posttransplantation period, repeat stem cell transplantations could increase the number of donor cells in the CNS.32 Consistently, we observed that patients with 2 or more transplantations showed higher levels of donor cell engraftment than those with a single transplantation, averaging 16.3% (12.2-25.1) of microglial cells (Figure 4A; Table 1, samples B16-B19). Preclinical studies have reported that engrafted BMDMs are long-lived and capable of self-replication with clonal donor cell numbers increasing over time after transplantation.25,33 Our data agree with these reports. We observed that the donor cell number increased and correlated with posttransplantation time (Figure 4B). The patient who survived the longest (>15 years after transplantation) had the highest number of male donor cells (25% of the microglial population; Figure 4B; Table 1). However, because this patient also received multiple transplantations, the higher number of donor cells could be due to longer posttransplantation survival, repeated stem cell transplantations, or a combination of the 2.
Effect of pretransplantation conditioning on donor cell engraftment in the cerebral cortex. (A) Percentage of male donor cells identified in cerebral cortex biopsy specimen from patients conditioned with MA-TBI (TBI >1000 cGy), NMA-TBI, nonmyeloablative conditioning, (TBI <300 cGy), MA-Bu-Treo, or 2 separate stem cell transplantations (double Tx). Data are expressed as donor cell percentage relative to total number of microglial cells. Microglia cells represent ∼12% of the cellularity in the cerebral cortex, based on parallel IBA1 IHC studies (supplemental Figure 2). (B) Effect of posttransplantation survival on donor cell engraftment in the cerebral cortex. The data are expressed as the percentage of donor cells relative to the total number of microglial cells. Gray square, myeloablative transplantation; blue inverted triangle, nonmyeloablative transplantation; red circle, double transplantations. DTs, double transplantations; MA-Bu-Treo, busulfan- or treosulfan-based myeloablation; MA-TBI, TBI-based myeloablation; NMA-TBI, TBI-based nonmyeloablation.
Effect of pretransplantation conditioning on donor cell engraftment in the cerebral cortex. (A) Percentage of male donor cells identified in cerebral cortex biopsy specimen from patients conditioned with MA-TBI (TBI >1000 cGy), NMA-TBI, nonmyeloablative conditioning, (TBI <300 cGy), MA-Bu-Treo, or 2 separate stem cell transplantations (double Tx). Data are expressed as donor cell percentage relative to total number of microglial cells. Microglia cells represent ∼12% of the cellularity in the cerebral cortex, based on parallel IBA1 IHC studies (supplemental Figure 2). (B) Effect of posttransplantation survival on donor cell engraftment in the cerebral cortex. The data are expressed as the percentage of donor cells relative to the total number of microglial cells. Gray square, myeloablative transplantation; blue inverted triangle, nonmyeloablative transplantation; red circle, double transplantations. DTs, double transplantations; MA-Bu-Treo, busulfan- or treosulfan-based myeloablation; MA-TBI, TBI-based myeloablation; NMA-TBI, TBI-based nonmyeloablation.
Discussion
Preclinical studies have demonstrated the potential of microglial replacement therapy as a form of cellular therapy for the treatment of inherited brain diseases and the delivery of therapeutic proteins into the CNS.7,16 Although MRT has been well studied in murine models, very few studies have documented donor-derived cells in the brains of patients who underwent stem cell transplantation. Here, we present the largest and most comprehensive study using a cohort of 19 female patients with a history of sex-mismatched stem cell transplantation to identify and characterize donor-derived cells in the cerebral cortex. Using a sensitive XY FISH assay optimized for postmortem tissue, we showed that donor cells represent ∼1% of the total and 8.4% of the microglia population, respectively. Costaining with modified IHC and FISH showed that most donor cells expressed the microglial marker IBA1, suggesting that they are BMDMs akin to endogenous microglia. The percentage of donor cells was remarkably conserved among patients with similar pretransplantation conditioning. Pretransplantation conditioning strongly influences donor cell engraftment, with patients receiving myeloablative transplantations having a much higher number of donor cells than those receiving nonmyeloablative transplantations. The reduced donor cell engraftment detected in patients with nonmyeloablative conditioning may be related to less intense conditioning and/or lower donor chimerism. In agreement with animal studies, we show that busulfan- or treosulfan-based conditioning is sufficient to support bone marrow macrophage engraftment at levels similar to those of other myeloablative regimens. A preclinical study by Capotondo et al showed that treosulfan-based conditioning had inferior long-term BMDM engraftment than busulfan or irradiation, which was attributed to the reduced capacity of treosulfan to traverse the blood-brain barrier and deplete endogenous microglial cells.30 In our study, treosulfan-based myeloablative conditioning also resulted in the lowest number of donor cells among the myeloablative cases. However, we do not know whether low CNS engraftment is a characteristic of treosulfan because our cohort had only a single case of the treosulfan-based conditioning regimen. The engrafted cells were stable during the posttransplantation period and tended to increase with posttransplantation survival. Finally, patients who received 2 or more stem cell transplantations had the highest number of donor-derived cells, which may be related to repeated transplantations or a long posttransplantation survival period. Data from the double transplantation cases fit a linear regression model with an R2 value of 0.99, suggesting that the number of donor cells is expanded linearly over time, which is attributed to donor cell proliferation or ongoing engraftment. Larger studies are required to better define how donor cell populations in the brain expand in long-term survivors.
In agreement with preclinical studies in animals, we found that most donor-derived cells expressed IBA1, a specific marker of microglia and BMDM, in the CNS and peripheral tissues.34,35 Studies have shown that IBA1 is expressed by brain-invading monocytes only after their differentiation into macrophages in the CNS.36 Cases with a clinical history or morphologic evidence of recent brain injury, infection, or hemorrhage were excluded from the study because increased inflammation would increase the donor cell pool within the brain. Because the samples used in our study were from postmortem tissues, it is difficult to rule out the possibility that some of the IBA1-positive donor cells might have been derived from peripheral monocytes that infiltrated the CNS as a result of ischemia, acute brain injury, or changes in vascular permeability arising during the perimortem period.37,38 However, the positive correlation of donor cell number with conditioning regimens and posttransplantation survival argues against the significant contribution of infiltrating macrophages in the donor pool during the perimortem period.
In this study, we defined male donor cells based on the presence of a Y chromosome detected using X/Y FISH. This allowed the detection of a large number of donor cells and avoided inherent constraints related to background autofluorescence associated with postmortem tissue. The identified donor cells were all diploid because of FISH, and no heterokaryons were identified in the hematoxylin and eosin–stained sections. Prior stem cell transplantation studies in mice detected rare cell fusion events involving bone marrow–derived donor cells in the liver and brain. In neuronal cells, cell fusions (detected as heterokaryons) were reported to be limited to Purkinje cells in the cerebellum, where they comprised only 0.02% or 0.1% of all Purkinje cells. The identified cell fusions were heterokaryons containing 2 nuclei, 1 each from donor and host cells.39-41 Purkinje cell fusions may serve as cell-mediated neuroprotection to rescue damaged neurons.39 Based on these reports as well as the inability to detect heterokaryons in our samples, we do not believe that cell fusion is a significant contributor to the donor cell pool. If it does occur, it is below the detection limit of our assay.
MRT has shown great promise for the treatment of several CNS disorders.25 Preclinical studies have shown that wild-type or genetically modified cells can be used to treat a number of microgliopathies, including metabolic disorders such as adrenoleukodystrophy;42 lysosomal storage diseases, including Hurler syndrome;16,17,43 and neuroinflammatory and neurodegenerative diseases, including Amyotrophic lateral sclerosis (ALS),44 Alzheimer,10,11,45 and Parkinson diseases.9 In these disorders, donor BMDMs partially replace defective microglia, supplement normal microglial function, and provide the required wild-type proteins. Microglia replacement therapy can also be used as a therapeutic strategy to replace defective microglia in CSF1R-related leukoencephalopathy.16,46 Interestingly, we showed that patients with acute myeloid leukemia who express a polymorphism of CD33 are resistant to gemtuzamab (an antibody drug conjugate directed against CD33),47 and genetic studies have shown that this same polymorphism is protective against Alzheimer disease.48,49 This has led us and others to propose that autologous MRT with modified stem cells expressing the CD33 isoform may delay or prevent Alzheimer disease.45,50
There are several active clinical studies involving microglia replacement listed on www.clinicaltrials.gov. However, in many instances, significant neurologic improvement has been variable and limited.17,51 The lack of therapeutic efficacy in clinical trials involving MRT may be related to suboptimal donor cell engraftment and/or the treatment of patients with advanced disease. Furthermore, many CNS disorders are complex and require early therapeutic intervention before irreversible tissue and neuronal damage. In addition, cognitive and synaptic defects in mice with long-term colonization by BMDMs in the CNS have also been reported.52 Despite these limitations, the future of MRT appears promising, with recent advances in minimizing transplantation toxicity, enhancing the efficiency of cellular engraftment, and using genetically modified donor stem cells. For example, a combination of pretransplantation microglial depletion via CSF1R inhibition and conditioning yielded >92% donor cell engraftment in murine MRT models.23 Another study reported that after transplantation CSF1R inhibition resulted in near-complete BMDM engraftment with therapeutic efficacy in a mouse model of prosaposin deficiency.25 Although we reported engraftment rates as high as 25%, it will be interesting to see whether the above approaches can be used to further increase the efficiency of MRT to provide long-term therapy for intractable neurologic disorders in humans.
Acknowledgments
The authors thank Lena Schroeder and Dave McDonald, Cellular Imaging Shared Resource RRID:SCR_022609 of the Fred Hutch/University of Washington/Seattle Children’s Cancer Consortium (P30 CA015704). The authors thank Diana Lim for help with the imaging and figure preparation, Andy Larson, Brandon Seaton, Kristin Shrimp and histology laboratory, Fred Hutch Cancer Center, and Lena Glaskova, Fred Hutch Cytogenetics laboratory. The visual abstract was created using BioRender.com.
This study was supported by grants from the Core Center of Excellence in Hematology Grant (S.S.P. and K.R.L.), a Seattle Translational Tumor Research Data Generation and Bioinformatics grant (K.R.L.), and Rett Syndrome Research Trust (A.B.).
Authorship
Contribution: K.R.L. designed the experiments; A.M.L. and K.R.L. wrote the manuscript; A.M.L., S.S.P., and K.R.L. performed the experiments and analyzed the data; S.M., S.S.P., and A.B. provided general scientific guidance and designed the experiments; and all authors reviewed the manuscript before submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Keith R. Loeb, Fred Hutchinson Cancer Center, 1100 Fairview Ave E., Seattle, WA 98109; e-mail: kloeb@fredhutch.org.
References
Author notes
Data are available on request from the corresponding author, Keith R. Loeb, (kloeb@fredhutch.org).
The full-text version of this article contains a data supplement.