Key Points
In recipients of HCT who experience CMV reactivation, NKG2C+ NK cells emerge only after CD8+ T cells are restored.
The majority of NKG2C+ NK cells are CD57+FcεR1γ+, which are more responsive than the adaptive NKG2C+CD57+FcεR1γ− NK cell population.
Abstract
Cytomegalovirus (CMV) infection is associated with the expansion of a mature NKG2C+FcεR1γ− natural killer (NK) cell population. The exact mechanism underlying the emergence of NKG2C+ NK cells, however, remains unknown. Allogeneic hematopoietic cell transplantation (HCT) provides an opportunity to longitudinally study lymphocyte recovery in the setting of CMV reactivation, particularly in patients receiving T-cell−depleted (TCD) allografts. We analyzed peripheral blood lymphocytes from 119 patients at serial time points after infusion of their TCD allograft and compared immune recovery with that in samples obtained from recipients of T-cell−replete (T-replete) (n = 96) or double umbilical cord blood (DUCB) (n = 52) allografts. NKG2C+ NK cells were detected in 92% (45 of 49) of recipients of TCD HCT who experienced CMV reactivation. Although NKG2A+ cells were routinely identifiable early after HCT, NKG2C+ NK cells were identified only after T cells could be detected. T-cell reconstitution occurred at variable times after HCT among patients and predominantly comprised CD8+ T cells. In patients with CMV reactivation, recipients of TCD HCT expressed significantly higher frequencies of NKG2C+ and CD56neg NK cells compared with patients who received T-replete HCT or DUCB transplantation. NKG2C+ NK cells after TCD HCT were CD57+FcεR1γ+ and degranulated significantly more in response to target cells compared with the adaptive the NKG2C+CD57+FcεR1γ− NK cell population. We conclude that the presence of circulating T cells is associated with the expansion of a CMV-induced NKG2C+ NK cell population, a potentially novel example of developmental cooperation between lymphocyte populations in response to viral infection.
Introduction
Cytomegalovirus (CMV) infection alters the natural killer (NK) cell repertoire by driving the expansion of a population of adaptive NKG2C-expressing NK cells that exhibit enhanced cytokine response to antibody-dependent cellular cytotoxicity (ADCC).1-8 Expressed on the cell surface as a heterodimer with CD94, NKG2C is an activating receptor that signals via DAP12 after binding to HLA-E.9,10 The inhibitory counterpart to NKG2C is NKG2A, which also recognizes HLA-E but with higher affinity.9,10 The adaptive NK cell population expresses NKG2C, but not NKG2A, and is biased toward the expression of self-HLA–specific killer immunoglobulin-like receptors (KIRs). Adaptive NK cells express CD56dim and CD57 but lack the signaling proteins FcεR1γ, SYK, and EAT-2 and the transcription factors PLZF and IKZF2 because of epigenetic modification.11-16 In mice, murine CMV infection induces the expansion of NK cells bearing the Ly49H receptor, which recognizes the viral protein m157.17-19 In humans, it has been described that NKG2C binds to HLA-E loaded with CMV-derived peptides.20 Although the development of the adaptive NKG2C+ population after CMV reactivation is presumed to be part of an innate immune response to the viral pathogen,20 the underlying mechanisms required to drive the emergence of NKG2C+ NK cells during CMV infection remain unclear.
After allogeneic hematopoietic cell transplantation (HCT), NK cells are the first lymphocytes to reconstitute in the peripheral blood (PB) and are believed to be derived from the donor allograft, regardless of graft source.21-26 NK cell subsets can be defined by cell surface expression of CD56 and CD16, with CD56brightCD16– (hereafter referred to as CD56bright) cells comprising ∼5% to 10% of the PB NK cell repertoire and considered an intermediate NK cell maturation state, exhibiting strong cytokine production capability. In contrast, CD56dimCD16+ (referred to as CD56dim) cells comprise the majority of the PB NK cell repertoire, are considered mature, and exhibit strong cytotoxic function.27 A third CD56–CD16+ (referred to as CD56–) NK cell population has been observed in those with chronic HIV-1 infection, hepatitis C virus infection, and malaria,28-31 and in patients who have received HCT who experience CMV reactivation.3,32 After allogeneic HCT with unmanipulated allografts, early reconstituting NK cells express high levels of NKG2A and exhibit CD56bright expression.24,33-39 At later time points after HCT, the repertoire shifts to a predominantly CD56dim population with progressively fewer NKG2A+ and more CD57+ NK cells.34 In patients experiencing CMV reactivation, CD57+NKG2C+ NK cells rapidly emerge and express CD57,2,4,5,11,32,40 with some studies suggesting that NKG2C+CD57+ NK cells may differentiate from NKG2A+CD57− NK cells.34,41 Donor NKG2C copy number variation may further influence NKG2C+ NK cell reconstitution in a dose-dependent manner, affecting CMV clearance.42,43 Compellingly, development of the CD56dimCD57+NKG2C+ population may also provide protection from leukemia relapse.44,45
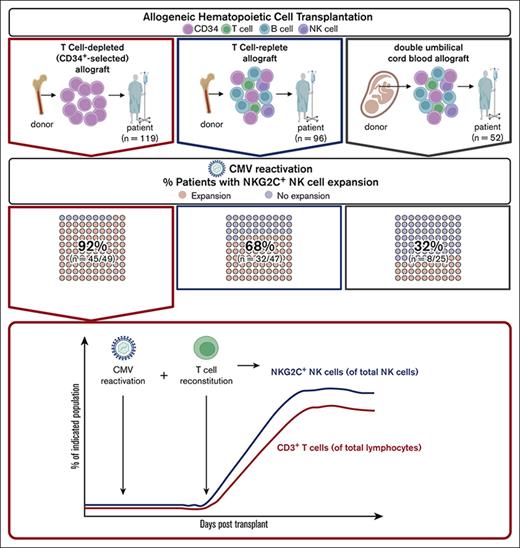
CD34+-selected (T-cell–depleted [TCD]) allografts, designed to minimize the risk of graft-versus-host disease (GVHD) after allogeneic HCT, are severely depleted of T-cell content.46,47 A consequence of TCD HCT is a higher incidence of CMV reactivation and CMV disease after transplantation when compared with transplants with unmanipulated (T-cell–replete) allografts.48-52 Studying the kinetics of NK cell development after TCD HCT provides a unique opportunity to analyze changes in the NK cell repertoire in the absence of T cells and during early T-cell reconstitution.
We examined NK cell and T-cell reconstitution in patients who underwent TCD HCT, comparing their kinetics with that of patients who received unmanipulated T-cell–replete or double umbilical cord (DUCB) allograft infusions. After TCD HCT, we found that CMV reactivation alone is not sufficient for the NK cell repertoire to expand an NKG2C+ NK cell population. Instead, the NKG2C+ NK cell population emerges after CMV reactivation only after T cells become detectable in the PB. Among all patients who underwent HCT and experienced CMV reactivation, patients who received TCD HCT have the highest frequency of NKG2C+ and CD56– NK cells compared with patients who received T-replete HCT and DUCB transplantation (DUCB-T). In contrast to the adaptive NKG2C+CD57+FcεR1γ− NK cell population found in healthy donors, the NKG2C+ NK cell population emerging after TCD HCT expresses both CD57 and FcεR1γ and degranulates more to ADCC and missing self-activation. Using different HCT platforms to evaluate NK cell development, we identified major differences in NK cell reconstitution between transplant types, revealing a novel association between T cells and the development of functionally enhanced NKG2C+ NK cells after CMV reactivation.
Materials and methods
Patients and transplant procedures
A total of 267 adult patients who underwent allogenic HCT at the Memorial Sloan Kettering Cancer Center (MSKCC) between 2006 and 2017 were included in the study. Patients received either a TCD infusion (n = 119), T-cell–replete (n = 96), or DUCB allografts (n = 52). Because they lacked NKG2C expression, 8 patients were excluded.53 CD34+ selection was performed using CliniMACS CD34+ Reagent System (Miltenyi Biotec) or Isolex 300I (Baxter Healthcare). Patient characteristics are shown in Table 1. Patients and donors provided informed written consent for research, and studies were approved by the MSKCC institutional review board.
Patient characteristics
| Characteristic . | Entire cohort . | TCD HCT . | T-replete–T . | DUCB-T . |
|---|---|---|---|---|
| N (%) . | n (%) . | n (%) . | n (%) . | |
| Total N = 267 . | Total n = 119 . | Total n = 96 . | Total n = 52 . | |
| Age | ||||
| Median (range), y | 53.6 (21.1-73.9) | 55.7 (21.4-70.8) | 54.3 (24-73.9) | 50.8 (21.1-69.9) |
| Gender | ||||
| Female | 103 (38.6%) | 47 (39.5%) | 31 (32.3%) | 25 (48.1%) |
| Male | 164 (61.4%) | 72 (60.5%) | 65 (67.7%) | 27 (51.9%) |
| CMV serostatus (recipient [R]/donor [D]) | ||||
| R+/D+ | 73 (27.3%) | 32 (26.9%) | 41 (42.7%) | 0 (0%) |
| R+/D− | 93 (34.8%) | 38 (31.9%) | 20 (20.8%) | 34 (65.4%) |
| R−/D+ | 25 (9.4%) | 14 (11.8%) | 12 (12.5%) | 0 (0%) |
| R−/D− | 76 (28.5%) | 35 (29.4%) | 23 (24%) | 18 (34.6%) |
| CMV reactivation | ||||
| Yes | 129 (48.3%) | 57 (47.9%) | 47 (49%) | 25 (48.1%) |
| No | 138 (51.7%) | 62 (52.1%) | 49 (51%) | 27 (51.9%) |
| Underlying disease | ||||
| Acute leukemia | 123 (46.1%) | 58 (48.7%) | 33 (34.4%) | 32 (61.5%) |
| Chronic leukemia | 20 (7.5%) | 5 (4.2%) | 9 (9.4%) | 5 (9.6%) |
| Myelodysplastic syndrome | 34 (12.7%) | 26 (21.8%) | 7 (7.3%) | 1 (1.9%) |
| Multiple myeloma | 24 (9%) | 23 (19.3%) | 1 (1%) | 0 (0%) |
| Hodgkin disease | 7 (2.6%) | 0 (0%) | 5 (5.2%) | 2 (3.8%) |
| Non-Hodgkin lymphoma | 44 (16.5%) | 1 (0.8%) | 32 (33.3%) | 11 (21.2%) |
| Other∗ | 15 (5.6%) | 6 (5%) | 9 (9.4%) | 1 (1.9%) |
| Conditioning regimen | ||||
| Busulfan based | 107 (40.1%) | 86 (72.3%) | 21 (21.9%) | 0 (0%) |
| Carmustine/etoposide/cytarabine/melphalan | 1 (0.4%) | 0 (0%) | 1 (1%) | 0 (0%) |
| Clofarabine/thio-TEPA/melphalan | 10 (3.7%) | 6 (5%) | 4 (4.2%) | 0 (0%) |
| Cyclophosphamide/fludarabine/TBI | 46 (17.2%) | 0 (0%) | 30 (31.3%) | 16 (30.8%) |
| Cyclophosphamide/fludarabine/thio-TEPA/TBI | 40 (15%) | 0 (0%) | 5 (5.2%) | 35 (67.3%) |
| Fludarabine/melphalan | 27 (10.1%) | 0 (0%) | 26 (27.1%) | 1 (1.9%) |
| Fludarabine/TBI | 1 (0.4%) | 0 (0%) | 1 (1%) | 0 (0%) |
| High-dose TBI based | 35 (13.1%) | 27 (22.7%) | 8 (8.3%) | 0 (0%) |
| GVHD prophylaxis | ||||
| Calcineurin inhibitor/methotrexate with/without sirolimus | 96 (35.9%) | 0 | 96 (100%) | 0 |
| CSA/MMF | 52 (19.5%) | 0 | 0 | 52 (100%) |
| Donor type | ||||
| Matched related | 79 (29.6%) | 44 (37%) | 35 (36.5%) | 0 (0%) |
| Matched unrelated† | 99 (37.1%) | 45 (37.8%) | 54 (56.3%) | 0 (0%) |
| Mismatched | 89 (33.3%) | 30 (25.2%) | 7 (7.3%) | 52 (100%) |
| Characteristic . | Entire cohort . | TCD HCT . | T-replete–T . | DUCB-T . |
|---|---|---|---|---|
| N (%) . | n (%) . | n (%) . | n (%) . | |
| Total N = 267 . | Total n = 119 . | Total n = 96 . | Total n = 52 . | |
| Age | ||||
| Median (range), y | 53.6 (21.1-73.9) | 55.7 (21.4-70.8) | 54.3 (24-73.9) | 50.8 (21.1-69.9) |
| Gender | ||||
| Female | 103 (38.6%) | 47 (39.5%) | 31 (32.3%) | 25 (48.1%) |
| Male | 164 (61.4%) | 72 (60.5%) | 65 (67.7%) | 27 (51.9%) |
| CMV serostatus (recipient [R]/donor [D]) | ||||
| R+/D+ | 73 (27.3%) | 32 (26.9%) | 41 (42.7%) | 0 (0%) |
| R+/D− | 93 (34.8%) | 38 (31.9%) | 20 (20.8%) | 34 (65.4%) |
| R−/D+ | 25 (9.4%) | 14 (11.8%) | 12 (12.5%) | 0 (0%) |
| R−/D− | 76 (28.5%) | 35 (29.4%) | 23 (24%) | 18 (34.6%) |
| CMV reactivation | ||||
| Yes | 129 (48.3%) | 57 (47.9%) | 47 (49%) | 25 (48.1%) |
| No | 138 (51.7%) | 62 (52.1%) | 49 (51%) | 27 (51.9%) |
| Underlying disease | ||||
| Acute leukemia | 123 (46.1%) | 58 (48.7%) | 33 (34.4%) | 32 (61.5%) |
| Chronic leukemia | 20 (7.5%) | 5 (4.2%) | 9 (9.4%) | 5 (9.6%) |
| Myelodysplastic syndrome | 34 (12.7%) | 26 (21.8%) | 7 (7.3%) | 1 (1.9%) |
| Multiple myeloma | 24 (9%) | 23 (19.3%) | 1 (1%) | 0 (0%) |
| Hodgkin disease | 7 (2.6%) | 0 (0%) | 5 (5.2%) | 2 (3.8%) |
| Non-Hodgkin lymphoma | 44 (16.5%) | 1 (0.8%) | 32 (33.3%) | 11 (21.2%) |
| Other∗ | 15 (5.6%) | 6 (5%) | 9 (9.4%) | 1 (1.9%) |
| Conditioning regimen | ||||
| Busulfan based | 107 (40.1%) | 86 (72.3%) | 21 (21.9%) | 0 (0%) |
| Carmustine/etoposide/cytarabine/melphalan | 1 (0.4%) | 0 (0%) | 1 (1%) | 0 (0%) |
| Clofarabine/thio-TEPA/melphalan | 10 (3.7%) | 6 (5%) | 4 (4.2%) | 0 (0%) |
| Cyclophosphamide/fludarabine/TBI | 46 (17.2%) | 0 (0%) | 30 (31.3%) | 16 (30.8%) |
| Cyclophosphamide/fludarabine/thio-TEPA/TBI | 40 (15%) | 0 (0%) | 5 (5.2%) | 35 (67.3%) |
| Fludarabine/melphalan | 27 (10.1%) | 0 (0%) | 26 (27.1%) | 1 (1.9%) |
| Fludarabine/TBI | 1 (0.4%) | 0 (0%) | 1 (1%) | 0 (0%) |
| High-dose TBI based | 35 (13.1%) | 27 (22.7%) | 8 (8.3%) | 0 (0%) |
| GVHD prophylaxis | ||||
| Calcineurin inhibitor/methotrexate with/without sirolimus | 96 (35.9%) | 0 | 96 (100%) | 0 |
| CSA/MMF | 52 (19.5%) | 0 | 0 | 52 (100%) |
| Donor type | ||||
| Matched related | 79 (29.6%) | 44 (37%) | 35 (36.5%) | 0 (0%) |
| Matched unrelated† | 99 (37.1%) | 45 (37.8%) | 54 (56.3%) | 0 (0%) |
| Mismatched | 89 (33.3%) | 30 (25.2%) | 7 (7.3%) | 52 (100%) |
CSA, cyclosporine A; MMF, mycophenolate mofetil; TBI, total body irradiation; thio-TEPA, N,N’N’-triethylenethiophosphoramide.
Nonmalignant hematologic disorder, myeloproliferative disorder, Waldenström macroglobulinemia, or aplastic anemia.
9/10 or 10/10 HLA–matched unrelated donor.
Patients with acute leukemia in first complete remission and patients with myelodysplastic syndrome preferentially received CD34+-selected TCD HCT if they were deemed able to receive myeloablative cytoreductive conditioning and had a ≥8/10 HLA–matched donor. Patients with leukemia or myelodysplastic syndrome not eligible for TCD and patients with lymphoma, chronic leukemia, myeloproliferative syndromes, or nonmalignant indications (eg, aplastic anemia) received unmodified HCT with reduced intensity conditioning.54,55
Patients receiving TCD allografts did not receive additional pharmacologic GVHD prophylaxis. Recipients of conventional allografts received a calcineurin inhibitor (in combination with sirolimus, in some cases) and methotrexate as a GVHD prophylaxis. Recipients of DUCB allografts received cyclosporine and mycophenolate mofetil treatment as GVHD prophylaxis.
All patients received acyclovir prophylaxis starting at admission for HCT and continued for at least 12 months. Recipients of HCT who were tested to be CMV seropositive (R+) or seronegative with a seropositive donor (R−/D+) were routinely monitored, at least weekly, starting on day 14 after HCT. For recipients treated before 2010, pp65 antigenemia assays (number of pp65+ cells per 50 PB leukocytes) were used for postengraftment CMV monitoring, and values >1 positive cell per slide were considered positive. From 2010 to 2013, CMV quantitative polymerase chain reaction (PCR) of the whole blood was performed by using the Roche Molecular Diagnostics Assay (Roche Diagnostics), with a positive quantitation range from 500 to 1 000 000 copies per mL. After 2013, CMV viral load was determined in plasma samples using the Cobas Ampliprep/Cobas Taqman CMV assay (Roche Diagnostics) with a quantitation positive range from 137 to 9 100 000 IU/mL. Per institutional guidelines, the initiation of preemptive therapy was indicated when ≥2 consecutive PCRs showed >500 copies per mL in whole blood or >300 IU/mL in plasma for patients at low risk of CMV infection/reactivation (unmodified HCT from HLA–matched donor) and ≥2 consecutive PCRs at any value for patients at high risk of CMV infection/reactivation (TCD or HLA-mismatched donor HCT).56 Preemptive therapy was initiated with induction dose of valganciclovir (VGCV) or ganciclovir (oral VGCV, 900 mg, every 12 hours; or IV ganciclovir, 5 mg/kg every 12 hours) or IV foscarnet (90 mg/kg every 12 hours) when VGCV was contraindicated. Induction was continued for 10 or 14 days or until CMV viral load became nonquantifiable for 2 consecutive measurements, whichever lasted longer. Maintenance doses of the same agent (VGCV 900 mg every 24 hours, ganciclovir 5 mg/kg every 24 hours, or foscarnet 90 mg/kg every 24 hours) were typically continued for 4 or 6 weeks based on the patient’s risk of CMV infection/reactivation.57
Sample preparation, culture conditions, and phenotypic analysis
PB mononuclear cells (PBMCs) from recipients of HCT were isolated via Ficoll centrifugation, cryopreserved, and subsequently analyzed on an LSR Fortessa flow cytometer instrument (BD Biosciences). PBMCs from recipients were collected before transplantation and at multiple time points after transplantation. PBMCs (0.5 × 106) were stained with Live Dead Aqua (Invitrogen) for 20 minutes at room temperature and subsequently surface stained with indicated antibodies (Supplemental Table 1) in phosphate-buffered saline with 0.5% bovine serum albumin and 2 mM EDTA for 30 minutes. Cells were thereafter fixed and permeabilized using FIX & PERM Kit (Invitrogen) and stained intracellularly for 20 minutes. Flow cytometry data were analyzed using FlowJo software (version 10.6.1, BD). NK cells tested positive for both NKG2C and NKG2A were excluded from the analysis.
Cytokine quantification
Plasma was prepared via the centrifugation of whole blood at 700 g for 7 minutes and stored in aliquots at −80°C. For analyses, plasma samples were thawed, centrifuged to remove debris, and assayed for multiplex cytokine quantification per manufacturer instructions using MILLIPLEX MAP Th17 Magnetic Bead and MILLIPLEX MAP TGFβ 1, 2, and 3 Magnetic Bead kits (Millipore). The samples were acquired on a Luminex FLEXMAP 3D (Luminex).
NK cell activation analysis
CD107a mobilization and interferon γ (IFN-γ) production were used to determine NK cell activation. In V-bottom 96-well plates, PBMCs (2 × 105 cells per well) were incubated with the HLA class I–negative K562 (ATCC) or BE(2)N cell lines at a 1:4 ratio in the presence of anti-CD107a antibody (BD Biosciences) for 5 hours. After 1 hour of coculture, 55.5 μg/mL of brefeldin A (MP Biomedicals) was added. Cells were stained as described earlier, using antibodies shown in Supplemental Table 1. For ADCC assays, anti-GD2 (3F8) antibody (10 μg/mL) (kindly provided by Nai-Kong Cheung, MSKCC) was added to PBMCs before BE(2)N target cells were added.
Statistical analysis
Unpaired/paired/nonparametric t test, one-way analysis of variance, and two-way analysis of variance with multiple comparisons were used for statistical analysis, as indicated in the figure legends. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ∗∗∗∗P ≤ .0001 were used as significant P values. The analysis was performed using Prism 7 software (GraphPad) and R (figures created using the package ggplot2).
Results
NKG2A and NKG2C expression on NK cells are inversely related after CMV reactivation in recipients of TCD HCT
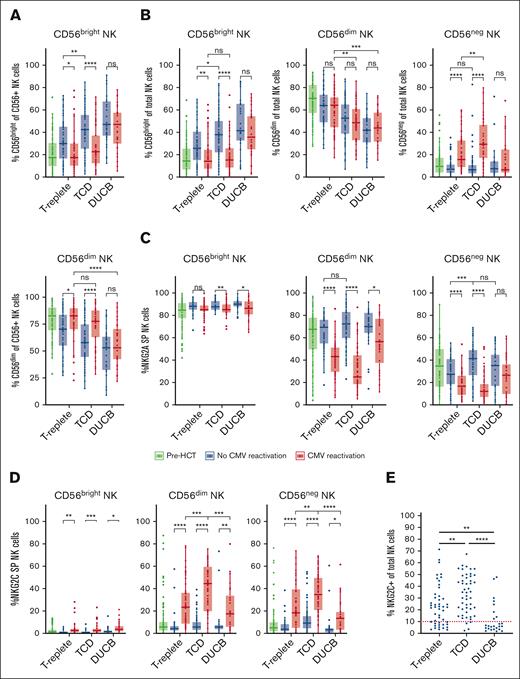
Prior studies examining NK cells after T-replete HCT and DUCB-T reported that the majority of NK cells found early in reconstitution are NKG2A+.24,33-39 Over time after HCT, NK cells expressing NKG2C emerge after CMV reactivation.2,4,5,11,32,40 We examined whether there were differences in NK cell reconstitution after TCD HCT. We investigated the development of NKG2C+NKG2A− (henceforth referred to as NKG2C+) and NKG2C−NKG2A+ (referred to as NKG2A+) NK cells in 29 patients at sequential time points after TCD HCT via multiparameter flow cytometry, stratifying patients based on whether they experienced post-HCT CMV reactivation (Figure 1A-C). At early time points after HCT, all patients had a NKG2C+ cell frequency <2.5%; however, the NKG2C+ cell population increased in those who developed a CMV reactivation (n = 16) compared with those who did not (n = 13; day 365, P < .0001; Figure 1A-B). Only absolute counts of CD56+CD16+ NK cells were measured, and among this population, NKG2C+ NK cell counts were significantly higher in patients with CMV reactivation than in those without (day 200, P < .0001; Figure 1C). Although the frequency of NKG2A+ NK cells as a percentage of total NK cells decreased significantly over time in patients with CMV reactivation compared with patients without (day 270, P = .0007; Figure 1B), there was no difference in absolute counts of NKG2A+ NK cells among CD56+CD16+ NK cells between patients with and without CMV reactivation (Figure 1C). In addition, recipients of TCD HCT with persistent CMV infection, and higher viral load over time (Supplemental Figure 1), had significantly higher percentages of NKG2C+ NK cells compared with patients without persistent CMV infection (P = .01; Figure 1D). Together, these findings indicate that the duration of CMV exposure and higher viral load induce higher NKG2C+ NK cell expansion, leaving the NKG2A+ NK cell population intact.
CMV reactivation influences relative frequencies and absolute counts of NKG2C+ and NKG2A+ NK cells in recipients of TCD HCT. (A) Dot plots of NKG2A vs NKG2C NK cells from representative recipients of TCD HCT with and without CMV reactivation. (B) Percentages of NKG2C+ (left) and NKG2A+ (right) NK cells of total NK cells and (C) absolute counts of NKG2C+ (left) and NKG2A+ (right) CD56+CD16+ NK cells of recipients of TCD HCT are shown at sequential time points (mean ± standard error of the mean [SEM]). Two-way analysis of variance (ANOVA) with multiple comparisons was used for statistical analysis, with ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001. (D) Percentages of NKG2C+ NK cells of total NK cells at late time points (≥ 180 days after HCT) in recipients of TCD HCT with (n = 16) or without (n = 32) persistent CMV infection. Unpaired/nonparametric t test was used for statistical analysis, with ∗P ≤ .05. (E) Percentage of NKG2C+ NK cells (left) and patients with first detection of NKG2C+ NK cells (percentages >4% were considered positive for NKG2C expression) at indicated time points relative to CMV detection (T0) (right) (n = 16). Data from each recipient of TCD HCT with CMV reactivation are plotted individually over time (left). T0 is the first immune-monitoring time point after CMV detection. Immune-monitoring time points before CMV detection were, therefore, denoted reverse chronologically as T−1 and T−2, and immune-monitoring time points after CMV detection were denoted from T1 to T4.
CMV reactivation influences relative frequencies and absolute counts of NKG2C+ and NKG2A+ NK cells in recipients of TCD HCT. (A) Dot plots of NKG2A vs NKG2C NK cells from representative recipients of TCD HCT with and without CMV reactivation. (B) Percentages of NKG2C+ (left) and NKG2A+ (right) NK cells of total NK cells and (C) absolute counts of NKG2C+ (left) and NKG2A+ (right) CD56+CD16+ NK cells of recipients of TCD HCT are shown at sequential time points (mean ± standard error of the mean [SEM]). Two-way analysis of variance (ANOVA) with multiple comparisons was used for statistical analysis, with ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001. (D) Percentages of NKG2C+ NK cells of total NK cells at late time points (≥ 180 days after HCT) in recipients of TCD HCT with (n = 16) or without (n = 32) persistent CMV infection. Unpaired/nonparametric t test was used for statistical analysis, with ∗P ≤ .05. (E) Percentage of NKG2C+ NK cells (left) and patients with first detection of NKG2C+ NK cells (percentages >4% were considered positive for NKG2C expression) at indicated time points relative to CMV detection (T0) (right) (n = 16). Data from each recipient of TCD HCT with CMV reactivation are plotted individually over time (left). T0 is the first immune-monitoring time point after CMV detection. Immune-monitoring time points before CMV detection were, therefore, denoted reverse chronologically as T−1 and T−2, and immune-monitoring time points after CMV detection were denoted from T1 to T4.
To further explore the impact of CMV reactivation on the NKG2C+ NK cell population, we mapped the percentage of NKG2C+ NK cells to immune-monitoring time points relative to first detection of CMV reactivation, denoting the first time point after CMV detection as T0 (Figure 1E). In all patients, CMV reactivation occurred between day 20 and day 60 after HCT. Surprisingly, only 4 of 16 (25%) recipients of TCD HCT exhibited NKG2C+ NK cells at T0 despite prior CMV detection, whereas the remaining patients developed NKG2C+ NK cells at later time points after CMV detection (Figure 1E), suggesting that the expansion of NKG2C+ NK cells in recipients of TCD HCT experiencing CMV reactivation may not be driven by CMV reactivation alone.
After CMV reactivation, higher frequencies of CD56– and NKG2C+ NK cells are generated in recipients of TCD HCT than in recipients of T-replete HCT or DUCB-T
It has been reported that the graft source in combination with CMV reactivation affects the reconstitution of mature NK cells58 and, more specifically, that the absence or reduced number of T cells in allografts results in more immature NK cells.36,38,59,60 We, therefore, examined whether and in what manner the presence of T cells in graft sources affects NK cell phenotype in patients undergoing TCD HCT vs in those undergoing T-replete HCT or DUCB-T with or without CMV reactivation. Via flow cytometry, we analyzed patient PBMC samples obtained ≥60 days after infusion and at least 30 days after CMV reactivation of T-replete HCT (n = 96) or DUCB-T allografts (n = 52), comparing the NK cell repertoires/reconstitution from these patients with the NK cell repertoires of recipients of TCD HCT. Because T-cell recovery after TCD HCT is significantly delayed compared with after transplants using other allograft sources, we analyzed PBMC samples obtained ≥180 days after TCD HCT (n = 119), the time point at which T-cell reconstitution had commenced. Distributions of the sample time points among the different cohorts are shown in Supplemental Figure 2. Using these time points, we followed the NK cell recovery timeline of patients with or without CMV reactivation.
We observed a decreased frequency of CD56bright NK cells in recipients of CMV-reactivated T-replete HCT (P < .05) and TCD HCT (P < .001) when compared with patients without CMV reactivation in the same HCT settings (Figure 2A). Interestingly, recipients of DUCB-T exhibited high frequencies of CD56bright NK cells, ∼50% of all CD56pos NK cells, regardless of CMV reactivation. The inverse observations were made in the CD56dim NK cell population, with increased frequency of CD56dim of CMV-reactivated T-replete HCT (P < .05) and TCD HCT (P < .001; Figure 2A).
CMV reactivation differentially affects CD56, NKG2C, and NKG2A NK cell expression among patients receiving TCD, T-replete, and DUCB HCT. Data gathered at late time points after different allograft infusions are shown. (A) CD56bright (top) and CD56dim (bottom) population frequencies within CD56+ NK cells in patients stratified based on the CMV reactivation status. (B) CD56dim (left), CD56bright (middle), and CD56– (right) population frequencies within total NK cells in patients stratified according to CMV reactivation status. (C) NKG2A+ frequencies within CD56bright (left), CD56dim (middle), and CD56– (right) NK cell subpopulations in patients, stratified based on the CMV reactivation status. (D) NKG2C+ frequencies within CD56bright (left), CD56dim (middle), and CD56neg (right) NK cell subpopulations in patients, stratified based on the CMV reactivation status. (E) NKG2C+ frequencies in total NK cells including CD56bright, CD56dim, and CD56– NK cells, from patients experiencing CMV reactivation. A threshold of 10%, separating individuals with or without expansion, was determined based on patients without CMV reactivation. Unpaired t tests were used for statistical analysis, with ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001.
CMV reactivation differentially affects CD56, NKG2C, and NKG2A NK cell expression among patients receiving TCD, T-replete, and DUCB HCT. Data gathered at late time points after different allograft infusions are shown. (A) CD56bright (top) and CD56dim (bottom) population frequencies within CD56+ NK cells in patients stratified based on the CMV reactivation status. (B) CD56dim (left), CD56bright (middle), and CD56– (right) population frequencies within total NK cells in patients stratified according to CMV reactivation status. (C) NKG2A+ frequencies within CD56bright (left), CD56dim (middle), and CD56– (right) NK cell subpopulations in patients, stratified based on the CMV reactivation status. (D) NKG2C+ frequencies within CD56bright (left), CD56dim (middle), and CD56neg (right) NK cell subpopulations in patients, stratified based on the CMV reactivation status. (E) NKG2C+ frequencies in total NK cells including CD56bright, CD56dim, and CD56– NK cells, from patients experiencing CMV reactivation. A threshold of 10%, separating individuals with or without expansion, was determined based on patients without CMV reactivation. Unpaired t tests were used for statistical analysis, with ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001.
The CD3–CD56–CD16+ population is frequently overlooked as an NK cell population but represents a substantial fraction of NK cells after viral infection and is presumed to represent an aberrant dysfunctional NK cell subset.28 When the total NK cell population including CD56–CD16+ is considered (Supplemental Figure 3), we observed similar patterns in the CD56bright NK cell subset, compared with the analysis excluding CD56– NK cells (Figure 2B); however, no commensurate increase was observed in the CD56dim NK cell numbers, and no differences were observed between patients with and without CMV reactivation across all transplant settings. Instead, we found a significant increase of CD56– NK cells after CMV reactivation in T-replete (P < .0001) and TCD (P < .0001) HCT, suggesting that CMV reactivation accelerates maturation out of the CD56bright stage to a CD56dim population, eventually leading to accumulation of a significant subset of CD56neg dysfunctional cells. Moreover, the frequency of CD56– NK cells was more significant in TCD HCT than in T-replete HCT, in the context of CMV reactivation (P < .001), suggesting that the lack of T cells exacerbates virally induced NK cell dysfunction. Interestingly, even with consideration of the CD56– population, the CD56bright population remains high in DUCB-T with or without CMV reactivation (Figure 2B).
We observed a decrease of NKG2A+ and an increase of NKG2C+ NK cells in patients with CMV reactivation across all transplant groups (Figure 2C-D). In the CD56bright NK cell subset, we observed an overall dominance of NKG2A expression, with an average of >80% of NK cells expressing NKG2A and <5% expressing NKG2C. Among CD56dim NK cells, significantly lower percentages of NKG2C+ NK cells were found in patients after T-replete HCT and DUCB-T compared with in patients after TCD HCT (P < .001 and P < .0001; Figure 2D). The frequency of NKG2A+ NK cells was correspondingly higher in recipients of T-replete HCT and DUCB-T compared with in recipients of TCD HCT (P < .05 and P < .0001, respectively; Figure 2C). Among CD56– NK cells, the frequencies of NKG2C+ cells were comparable with those observed in CD56dim cells (Figure 2C-D), suggesting that prolonged CMV exposure to NK cells in the absence of T cells does not require expression of NKG2C to become dysfunctional. Overall, among patients experiencing CMV reactivation, 45 of 49 (92%) recipients of TCD HCT exhibited an expansion of the NKG2C+ NK cell population, compared with 32 of 47 (68%) patients who received T-replete HCT and only 8 of 25 (32%) who received DUCB-T (Figure 2E).
To ensure that our results were not influenced by differences in sample time point distribution between transplant settings (Supplemental Figure 2), we performed a complementary analysis, subgrouping data based on early time points (days 60-200) and late time points (days 270-365). The results were consistent between early and late time points (data not shown). Taken together, the data indicate that absence of T cells in the setting of CMV reactivation leads to greater expansion of the NKG2C+ population and greater accumulation of the CD56neg phenotype after HCT.
NKG2C+ NK cells are detected only after evidence of T-cell reconstitution in recipients of TCD HCT experiencing CMV reactivation
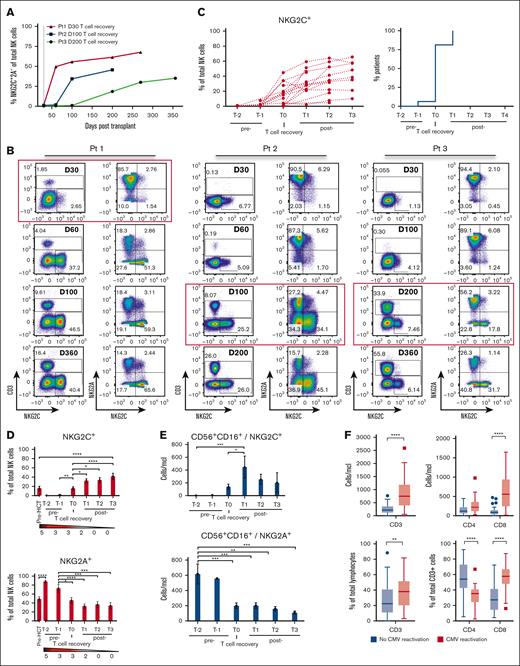
We examined whether there was a relationship between NKG2C+ NK cell frequency in recipients of TCD HCT experiencing CMV reactivation and T-cell reconstitution. Although CMV reactivation generally occurs by 30 days after TCD HCT, T-cell reconstitution occurs at vastly varying times, becoming detectable anywhere from day 30 to beyond day 180. We observed that in patients with CMV reactivation, NKG2C+ NK cells were detectable only after T cells were detected in the PB and that their codetection varied depending on the timing of T-cell recovery (Figure 3A-B). We evaluated the frequency of NKG2C+ NK cells at time points relative to T-cell detection in the PB, denoting T0 as the time at which PB T cells were initially detected via flow cytometry (Figure 3C-E). In 15 of 16 (94%) patients, we observed that NKG2C+ NK cells could be found only after T cells were detected (Figure 3C). Upon detection of T cells, the percentage of NKG2C+ NK cells increased significantly compared with measurements before T-cell recovery (T0 vs T−1; P = .0095; Figure 3D), a finding matched by absolute counts of NKG2C+ NK cells (Figure 3E), with a commensurate decrease in NKG2A+ NK cell percentages and absolute counts (Figure 3D-E). There was a similar impact of T-cell reconstitution on the evolution of CD56bright NK cells in TCD HCT, with a synchronized decrease of CD56bright NK frequency after T-cell detection (Supplemental Figure 4A).
Early T-cell reconstitution is associated with the emergence of CMV-induced NKG2C+ NK cells in recipients of TCD HCT. (A) NKG2C+ cell reconstitution in 3 recipients of TCD HCT who all experienced CMV reactivation by day 30 (gray dotted line) after HCT but exhibited different times of T-cell recovery. (B) Recovery of CD3+ T cells and expansion of NKG2C+ NK cells at sequential time points after HCT in 3 individuals. Red squares highlight the time point at which T cells were first detected. (C) Percentage of NKG2C+ cells among all NK cells (left) and the percentage of patients with NKG2C+ NK cell expansions (percentages >4% were considered positive for NKG2C expansion based on non–CMV-reactivated data) at indicated time points relative to T-cell recovery (T0) (right) (n = 16). (D) Percentage of NKG2C+ (top) and NKG2A+ (bottom) of total NK cells and (E) absolute counts of NKG2C+ (top) and NKG2A+ (bottom) of CD56+CD16+ NK cells, relative to time of first T-cell detection (T0) are shown (mean ± SEM) (T1, 447.4 ± 139.6 cells per μL vs T−2, 2.9 ± 0.7 cell per μL; P = .0004). The gradient bar indicates the number of patients with CMV reactivation at the indicated time point. (C-E) T0 is denoted as the time at which T cells were initially detected via flow cytometry in the PB, with T−2 and T−1 as time points before T-cell recovery, and T1 to T3 are time points after T-cell recovery. (D-E) Two-way ANOVA with multiple comparisons was used for statistical analysis. (F) CD3+, CD4+, and CD8+ T-cell absolute counts (CMV reactivation, n = 38; no CMV reactivation, n = 35) (top) and percentages (CMV reactivation, n = 50; no CMV reactivation, n = 60) (bottom) are measured at late time point samples. Interquartile range is depicted by a box with the middle line plotted as the median, and Tukey values are represented by whiskers and outliers. Unpaired/nonparametric t test (CD3) and two-way ANOVA with multiple comparisons (CD4 and CD8) were used for statistical analysis. One-way ANOVA with multiple comparisons was used for statistical analysis. ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001.
Early T-cell reconstitution is associated with the emergence of CMV-induced NKG2C+ NK cells in recipients of TCD HCT. (A) NKG2C+ cell reconstitution in 3 recipients of TCD HCT who all experienced CMV reactivation by day 30 (gray dotted line) after HCT but exhibited different times of T-cell recovery. (B) Recovery of CD3+ T cells and expansion of NKG2C+ NK cells at sequential time points after HCT in 3 individuals. Red squares highlight the time point at which T cells were first detected. (C) Percentage of NKG2C+ cells among all NK cells (left) and the percentage of patients with NKG2C+ NK cell expansions (percentages >4% were considered positive for NKG2C expansion based on non–CMV-reactivated data) at indicated time points relative to T-cell recovery (T0) (right) (n = 16). (D) Percentage of NKG2C+ (top) and NKG2A+ (bottom) of total NK cells and (E) absolute counts of NKG2C+ (top) and NKG2A+ (bottom) of CD56+CD16+ NK cells, relative to time of first T-cell detection (T0) are shown (mean ± SEM) (T1, 447.4 ± 139.6 cells per μL vs T−2, 2.9 ± 0.7 cell per μL; P = .0004). The gradient bar indicates the number of patients with CMV reactivation at the indicated time point. (C-E) T0 is denoted as the time at which T cells were initially detected via flow cytometry in the PB, with T−2 and T−1 as time points before T-cell recovery, and T1 to T3 are time points after T-cell recovery. (D-E) Two-way ANOVA with multiple comparisons was used for statistical analysis. (F) CD3+, CD4+, and CD8+ T-cell absolute counts (CMV reactivation, n = 38; no CMV reactivation, n = 35) (top) and percentages (CMV reactivation, n = 50; no CMV reactivation, n = 60) (bottom) are measured at late time point samples. Interquartile range is depicted by a box with the middle line plotted as the median, and Tukey values are represented by whiskers and outliers. Unpaired/nonparametric t test (CD3) and two-way ANOVA with multiple comparisons (CD4 and CD8) were used for statistical analysis. One-way ANOVA with multiple comparisons was used for statistical analysis. ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001.
To investigate which T-cell subset is associated with NKG2C expansion, we evaluated the different T-cell subsets among the reconstituted T cells in recipients of TCD HCT with and without CMV reactivation. By analyzing longitudinal data (Supplemental Figure 4B) and data collected at ≥180 days after HCT (Figure 3F), we observed significantly higher counts and percentages of total CD3+ T cells, in particular CD8+ T cells, in recipients of TCD HCT with CMV reactivation than in those without CMV reactivation (P < .0001).
We further observed that the majority of early reconstituted NK cells in recipients of TCD HCT have a NKG2A+CD56brightCD16lowKIRlow phenotype, changing only after CMV reactivation and early T-cell recovery. Thereafter, the majority of NK cells were more differentiated with a NKG2C+CD56dimCD16+self-KIR+ phenotype (Supplemental Figures 5 and 6; Supplemental Table 2). We analyzed cytokines (interleukin-2 [IL-2], IL-15, IL-12P70, IL-21, IFN-γ, tumor necrosis factor α, transforming growth factor β-1 [TGFβ-1], and TGFβ-2) concentration in plasma by Luminex analysis of some recipients of TCD HCT (Supplemental Figure 7). Because our goal was to determine whether T-cell reconstitution corresponded to an increase of cytokines that could be necessary for NK cell differentiation, we synchronized the analysis based on the time of T-cell reconstitution. Surprisingly, we found that T-cell reconstitution was synchronized with a momentary decrease of almost all measured cytokines/chemokines, including IL-21, which has been described to be specifically produced by NK T cells and CD4+ T cells.61 Together, these results indicate that although CMV reactivation induces rapid NK cell maturation and expansion of the NKG2C+ NK cell population in recipients of TCD HCT, NKG2C+ NK expansion occurs only after CD8 T-cell reconstitution is detected without a clear commensurate rise in cytokines related to T-cell production, proliferation, or activation.
NKG2C+ NK cells are predominantly CD57+FcεR1γ+
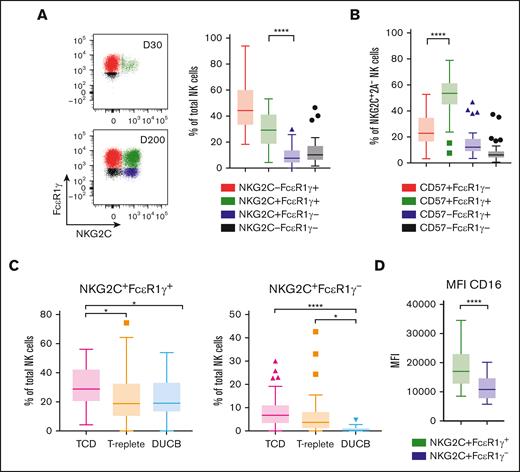
Prior studies have examined the so-called adaptive NKG2C+CD57+ or NKG2C+FcεR1γ− NK cell populations in recipients of HCT receiving T-replete2-5,32 or DUCB allograft infusions.40,62 We found that in recipients of TCD HCT with CMV reactivation, the overwhelming majority of NKG2C+ NK cells strikingly express FcεR1γ and CD57, with only a small population demonstrating the lack of FcεR1γ expression characteristic of adaptive NK cells found in healthy individuals tested CMV-seropositive 14-16 and after CMV reactivation in recipients of T-replete and DUCB allografts2-5,32,40,62 (P < .0001; Figure 4A-B).
The majority of NKG2C+ NK cells express FcεR1γ and CD57 in recipients of TCD HCT with CMV reactivation. (A) FcεR1γ vs NKG2C dot plots gated on total NK cells of day 30 and day 200 after HCT from a representative recipient of TCD HCT with CMV reactivation (left). (A) Percentages of NKG2C and FcεR1γ subsets of total NK cells (right) and (B) percentages of CD57 vs FcεR1γ subsets gated on NKG2C+ NK cells lacking NKG2A from recipients of TCD HCT with CMV reactivation (n = 50). (C) Percentages of NKG2C+FcεR1γ+ (left) and NKG2C+FcεR1γ− (right) NK cells after HCT of patients receiving TCD (n = 50), T-replete (n = 47), or DUCB (n = 25) allograft infusions. All patients shown experienced CMV reactivation. (D) The mean fluorescence intensity (MFI) of CD16 on NKG2C+FcεR1γ+ and NKG2C+FcεR1γ− NK cells are depicted. Data gathered at late time points are shown. Interquartile range is depicted by a box with the middle line plotted as the median, and Tukey values are represented by whiskers and outliers. One-way ANOVA with multiple comparisons test (A-C) and paired/nonparametric t test (D) were used for statistical analysis, with ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001.
The majority of NKG2C+ NK cells express FcεR1γ and CD57 in recipients of TCD HCT with CMV reactivation. (A) FcεR1γ vs NKG2C dot plots gated on total NK cells of day 30 and day 200 after HCT from a representative recipient of TCD HCT with CMV reactivation (left). (A) Percentages of NKG2C and FcεR1γ subsets of total NK cells (right) and (B) percentages of CD57 vs FcεR1γ subsets gated on NKG2C+ NK cells lacking NKG2A from recipients of TCD HCT with CMV reactivation (n = 50). (C) Percentages of NKG2C+FcεR1γ+ (left) and NKG2C+FcεR1γ− (right) NK cells after HCT of patients receiving TCD (n = 50), T-replete (n = 47), or DUCB (n = 25) allograft infusions. All patients shown experienced CMV reactivation. (D) The mean fluorescence intensity (MFI) of CD16 on NKG2C+FcεR1γ+ and NKG2C+FcεR1γ− NK cells are depicted. Data gathered at late time points are shown. Interquartile range is depicted by a box with the middle line plotted as the median, and Tukey values are represented by whiskers and outliers. One-way ANOVA with multiple comparisons test (A-C) and paired/nonparametric t test (D) were used for statistical analysis, with ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001.
Accordingly, recipients of TCD HCT had a significantly higher percentage of NKG2C+FcεR1γ+ NK cells compared with those who received T-replete HCT or DUCB-T (P = .041 vs P = .037, respectively; Figure 4C). Of note, recipients of DUCB-T did not develop NKG2C+FcεR1γ− NK cells at all (Figure 4C). This, together with the observation that no difference was found in total CD3+ T-cell counts in recipients of DUCB-T with or without CMV reactivation, whereas significant differences were observed in the other transplant settings (Supplemental Figure 8), may further indicate an association between CMV-reactive T cells and development or expansion of adaptive NKG2C+ NK cells.
Finally, the NKG2C+FcεR1γ+ NK cells found after CMV reactivation in TCD HCT had a significantly higher density of CD16 expression compared with the previously described adaptive NKG2C+FcεR1γ− NK cells (P < .0001; Figure 4D). Taken together, these findings establish that in patients experiencing CMV reactivation, NKG2C+FcεR1γ+ NK cells are predominantly expressed in comparison with NKG2C+FcεR1γ− NK cells in all transplant settings.
NKG2C+CD57+FcεR1γ+ are more responsive than the adaptive NKG2C+CD57+FcεR1γ− NK cell population
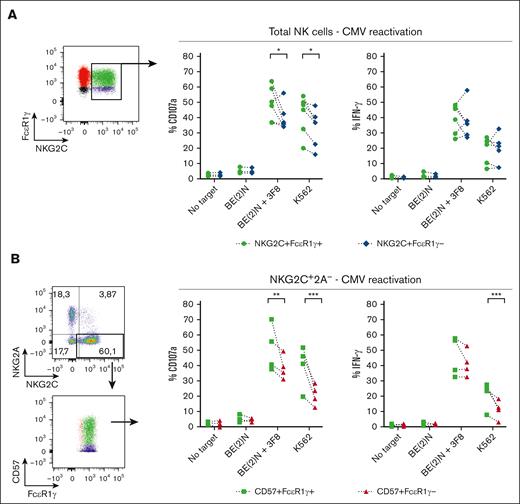
Expression of the signaling protein FcεR1γ combined with higher expression of CD16 suggests that the NKG2C+ population that expands after CMV reactivation in TCD HCT may have enhanced effector function compared with adaptive NKG2C+FcεR1γ− NK cells. We, therefore, examined missing self-NK activation by coculturing cells with K562 target cells and ADCC activation by coculturing cells with the GD2-expressing BE(2)N target cells in the presence of anti-GD2 antibody. In recipients of TCD HCT with CMV reactivation, NKG2C+FcεR1γ+ NK cells degranulated and produced more IFN-γ compared with NKG2C+FcεR1γ− NK cells against both target cells (Figure 5A). Among the more mature NKG2C+CD57+ NK population, FcεR1γ+ cells also degranulated better and produced more IFN-γ against both target cells compared with FcεR1γ− cells (Figure 5B). We conclude that in recipients of TCD HCT with CMV reactivation, the expanded NKG2C+FcεR1γ+ NK cell population exhibits higher cytotoxic and cytokine response compared with the adaptive CD57+FcεR1γ− NK cells found in recipients of T-replete and DUCB-T.
NKG2C+CD57+FcεR1γ+ NK cells from recipients of TCD HCT with CMV reactivation exhibit greater effector response compared with NKG2C+CD57+FcεR1γ− NK cells. Comparing the effector function of (A) NKG2C+FcεR1γ+ vs NKG2C+FcεR1γ− of total NK cells, and (B) CD57+FcεR1γ+ vs CD57+FcεR1γ− of NKG2C+2A− NK cells derived from recipients of TCD HCT with CMV reactivation (n = 6). The gates in the dot plots represent NK cell populations that were analyzed for effector function. Percentages of CD107a (left) and IFN-γ (right) of indicated NK cell subpopulations are depicted. Two-way ANOVA with multiple comparisons were used for statistical analysis, with ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001.
NKG2C+CD57+FcεR1γ+ NK cells from recipients of TCD HCT with CMV reactivation exhibit greater effector response compared with NKG2C+CD57+FcεR1γ− NK cells. Comparing the effector function of (A) NKG2C+FcεR1γ+ vs NKG2C+FcεR1γ− of total NK cells, and (B) CD57+FcεR1γ+ vs CD57+FcεR1γ− of NKG2C+2A− NK cells derived from recipients of TCD HCT with CMV reactivation (n = 6). The gates in the dot plots represent NK cell populations that were analyzed for effector function. Percentages of CD107a (left) and IFN-γ (right) of indicated NK cell subpopulations are depicted. Two-way ANOVA with multiple comparisons were used for statistical analysis, with ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001.
Discussion
We demonstrate that although CMV reactivation is necessary for the rapid emergence of NKG2C+ NK cells after TCD HCT, the NK population is only detected after evidence of PB T-cell recovery. Furthermore, the NKG2C+ cells are predominantly CD57+FcεR1γ+, which exhibit enhanced degranulation after ADCC and missing self-activation compared with the canonical adaptive NKG2C+CD57+FcεR1γ− population.
Previous studies have described the relationship between CMV reactivation and the expansion of NKG2C+ NK cells after T-replete or DUCB HCT.2-5,32,40,62 On assessing the expansion of the NKG2C+ population after TCD HCT, we were surprised to find that in addition to CMV reactivation, PB T cells appear to be necessary for the emergence of NKG2C+ NK cells. Although others have reported that the absence or reduced numbers of T cells in HCT results in more immature and less functional NK cells,37,38,59,60 none of the studies were performed in the context of CMV reactivation or with assessment of the NKG2C+ NK population. In further support of a T-cell influence on NK cell development, investigators examining rhesus macaques that received transplantation using barcoded autologous hematopoietic stem and progenitor cells to track clonal hematopoiesis found that rhesus CMV infection is not the only driver of mature NK clonal dynamics and suggested that T cells might be involved.63 In this study, NK cells in recipients of TCD HCT continued having a NKG2A+CD56brightCD16lowKIRlow NK cell phenotype well after CMV reactivation, giving way to an NK cell repertoire dominated by a more differentiated NKG2C+CD56dimCD16+self-KIR+ phenotype only after T cells began to reconstitute the PB. Thus, although CMV reactivation is necessary, it is not sufficient for the development of the NKG2C+ NK cell population. These findings strongly suggest that expansion of NKG2C+ NK cells rely on a contribution of T cells, whose numbers are higher in patients with CMV reactivation and predominantly comprise CD8+ T cells. CMV infection/reactivation leaves a potent imprint on the immune repertoire, leading to clonal expansion and high percentages of CMV-specific CD8+ T cells after HCT.58,64-67 We propose that T cells, most likely CMV-specific clones, drive rapid NK cell maturation, leading to the expansion of NKG2C+FcεR1γ+ NK cells and eventually the emergence of adaptive NKG2C+FcεR1γ− NK cells.
To identify a mechanism in support of these findings, we examined plasma cytokine concentrations, anticipating that elevations in levels of cytokines such as IL-2, IL-15, and IL-21 might help the emergence of NKG2C+ NK cells, whereas others such as TGFβ maintain NK cells in an immature state.68,69 Surprisingly, we found that T-cell reconstitution was synchronized with a decrease of almost all measured cytokines, including TGFβ. One potential hypothesis linking the data is that T-cell consumption of TGFβ could unleash NK cell differentiation. An alternative hypothesis is that CD8+ T-cell reconstitution and NK cell differentiation are not causal but synchronized. Adaptive NK cell development may not be dependent on T-cell reconstitution but, rather, synchronized with lymphoid organ recovery vital to T-cell reconstitution. Additional studies are still needed to investigate how CMV infection coordinates the reconstitution of these 2 very separate lymphocyte populations.
Longitudinal evaluation of NK reconstitution revealed additional differences between transplant types, specifically the persistence of a significant CD56bright population in recipients of DUCB-T despite CMV reactivation and the accumulation of CD56neg NK cells after CMV reactivation in T-replete HCT and TCD HCT but not in DUCB-T. Although CMV reactivation accelerates maturation of the NK population from a CD56bright to CD56dim phenotype in recipients of TCD HCT and T-replete allografts, it is unclear why the same does not occur in recipients of DUCB-T but may be related to primary CMV infection vs reactivation.4,5 Regardless, because CD56bright NK cells with known enhanced cytokine capacity are reportedly associated with better leukemia control and lower relapse in patients with CMV reactivation,70 the persistence of a CD56bright NK cell population after DUCB-T may contribute to the lower risk for acute myeloid leukemia relapse previously reported with this graft source.71
The CD56– NK cell population in recipients of transplantation is less functional compared with CD56dim NK cells,3,32,72 although IL-2 treatment in vitro can rescue some CD56 expression.29 It could therefore be surmised that rescue of these dysfunctional NK cells may contribute to the reported decreased relapse and disease-free survival benefit of post-HCT IL-2 treatment73,74 because it was reported that accumulation of CD56– NK cells in patients with acute myeloid leukemia at diagnosis was associated with adverse clinical outcomes.75 The frequency of CD56– NK cells was greater in recipients of TCD HCT than in recipients of T-replete HCT, suggesting that the presence of CMV-reactive T cells may prevent accumulation of CD56neg NK cells. The frequency of NKG2C+ cells was very similar among the CD56– and CD56dim NK cells in all transplant settings, whereas NKG2A expression was lower in CD56neg compared with CD56dim NK cells. These data are consistent with the likelihood that CD56neg NK cells are issued from the CD56dim compartment32,76 and likely represent altered NK cells responding to CMV infection.
Comparing NKG2C+ NK cell frequencies among the different transplantation settings, we detect higher percentages of total NKG2C+, NKG2C+FcεR1γ+, and NKG2C+FcεR1γ− NK cells at late time points after TCD HCT compared with after T-replete HCT and DUCB-T among those experiencing CMV reactivation. Notably, recipients of TCD HCT with persistent CMV infection have even higher percentages of NKG2C+ NK cells. Based on these observations, we surmise that in contrast to the T-replete transplant or DUCB-T settings, complete absence of T cells in the months after TCD HCT results in longer NK cell exposure to CMV, resulting in either a greater likelihood of epigenetic priming of NK cells to adopt an NKG2C+ fate14,15 or substantial expansion of a tiny pre-existing population of NKG2C+ NK cells. It should be noted that differences in the pharmacological management between HCT settings, including GVHD prophylaxis, could have an impact on our observations. More investigation is required to understand the mechanism by which T cells facilitate CMV-induced NKG2C+ NK cell expansion and differentiation.
We found significantly enhanced effector function among NKG2C+CD57+FcεR1γ+ NK cells compared with the adaptive NKG2C+CD57+FcεR1γ− NK cell population in recipients of TCD HCT, in contrast to what has been observed in DUCB-T, in which the ADCC response of both NK cell populations is similar.62 Higher CD16 mean fluorescence intensity likely explains the enhanced ADCC potential of these NKG2C+CD57+FcεR1γ+ NK cells, but presence of the FcεR1γ+ signaling molecule itself most likely enhances the leukemotoxic potential of the NKG2C+ cell, credited with protection against relapse in patients who experience CMV reactivation after HCT.44,45 Understanding the factors, including the prophylactic use of the antiviral letermovir, that may promote or prevent the generation of a functionally enhanced NKG2C+ population is important for developing new therapeutic approaches to enhance the graft-versus-leukemia effect and to lower the risk of relapse after HCT.
Acknowledgments
The authors thank Douwe Kiela for the extensive data processing support and Dr. M. Kazim Panjwani for his helpful comments.
This study was supported by the National Institutes of Health (NIH), National Cancer Institute (grants P01 CA23766) and National Institute of Allergy and Infectious Diseases (R01 AI125651). Support was also provided by Leukemia & Lymphoma Society, and an NIH, National Cancer Institute core grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center.
Authorship
Contribution: J-B.L.L. and K.C.H. conceived the study; K.v.d.P., R.S., J-B.L.L., and K.C.H. designed the study; K.v.d.P., R.S. T.K., B.C.S., G.A.P., M.A.M., C.C., M.A.P., and J-B.L.L. collected and assembled the data; K.v.d.P., R.S., J-B.L.L, and K.C.H. analyzed and interpreted the data; K.v.d.P., J-B.L.L., and K.C.H. wrote the manuscript; and all authors reviewed and revised the manuscript, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for K.v.d.P. is Department of Medicine, Stanford University School of Medicine, Stanford, CA.
The current affiliation for R.S. is Humanitas Clinical and Research Center, 20090, Pieve Emanuele, Italy.
Correspondence: Jean-Benoît Le Luduec, Human Oncology and Pathogenesis Program, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: leluduej@mskcc.org; and Katharine C. Hsu, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: hsuk@mskcc.org.
References
Author notes
∗J.-B.L.L. and K.C.H. are joint senior authors.
Data are available on request from the corresponding author, Katharine C. Hsu (hsuk@mskcc.org).
The full-text version of this article contains a data supplement.

![CMV reactivation influences relative frequencies and absolute counts of NKG2C+ and NKG2A+ NK cells in recipients of TCD HCT. (A) Dot plots of NKG2A vs NKG2C NK cells from representative recipients of TCD HCT with and without CMV reactivation. (B) Percentages of NKG2C+ (left) and NKG2A+ (right) NK cells of total NK cells and (C) absolute counts of NKG2C+ (left) and NKG2A+ (right) CD56+CD16+ NK cells of recipients of TCD HCT are shown at sequential time points (mean ± standard error of the mean [SEM]). Two-way analysis of variance (ANOVA) with multiple comparisons was used for statistical analysis, with ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001. (D) Percentages of NKG2C+ NK cells of total NK cells at late time points (≥ 180 days after HCT) in recipients of TCD HCT with (n = 16) or without (n = 32) persistent CMV infection. Unpaired/nonparametric t test was used for statistical analysis, with ∗P ≤ .05. (E) Percentage of NKG2C+ NK cells (left) and patients with first detection of NKG2C+ NK cells (percentages >4% were considered positive for NKG2C expression) at indicated time points relative to CMV detection (T0) (right) (n = 16). Data from each recipient of TCD HCT with CMV reactivation are plotted individually over time (left). T0 is the first immune-monitoring time point after CMV detection. Immune-monitoring time points before CMV detection were, therefore, denoted reverse chronologically as T−1 and T−2, and immune-monitoring time points after CMV detection were denoted from T1 to T4.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/19/10.1182_bloodadvances.2022008952/2/m_blooda_adv-2022-008952-gr1.jpeg?Expires=1765884039&Signature=VVAmPG5najZtYD9bNIxZ7v5enAoCFIEtsmJiP4peoFldtKxP2Ec4sNOASMjLXR1-B0OZfDoMKcvth1CRi81iM9z4WAxnC~~XrMm8AtrKcgKU0vKoblFo9KMtAYDjKCZIznzXi6gqVrYKIh4ZN2j3eXNSa2MNFXLBWQt0V2Byq-Z-mvNcEMsAmMViIy1GEEZF2DaaBm7BNjATHgkPp9pwO8G-ieKNRghpXM4VanNOzJum3CJY56Xx5mZ1~~XcCKeuKqHKsaKFUulM968JcNt48BBrZLtI-1DvH9hlFp7I8w8l-WSN2FYhTzPOI-aipstWLIGAh-sSl28Hf1-YN5iWYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)