Key Points
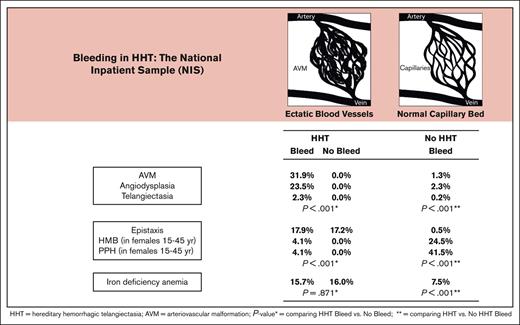
Bleeding from angiodysplasia and AVM is common in inpatient HHT, more so than heavy menstrual bleeding and postpartum hemorrhage in women.
IDA is common in HHT whether or not they bleed, suggesting need for universal iron screening.
Abstract
Hereditary hemorrhagic telangiectasia (HHT) is a common bleeding disorder, but little is known regarding prevalence and risk factors for bleeding. Adult discharges with HHT and bleeding were identified by International Classification of Disease, 10th edition (ICD-10) codes in the National Inpatient Sample (NIS), 2016-2018. Prevalence estimates were weighted using NIS discharge-level weights to reflect national estimates. Risk factors for bleeding were determined by weighted multivariable logistic regression. Among 18 170 849 discharges, 2528 (0.01%) had HHT, of whom 648 (25.6%) had bleeding. Arteriovenous malformation (AVM) (31.9% vs 1.3%), angiodysplasia (23.5% vs 2.3%), telangiectasia (2.3% vs 0.2%), and epistaxis (17.9% vs 0.6%) were more common in HHT than in non-HHT patients (non-HHT), each P < .001. In contrast, menstrual (HMB) and postpartum bleeding (PPH) were less common in reproductive-age HHT than non-HHT, each P < .001. Anemia associated with iron deficiency (IDA), was equally common in HHT with or without bleeding (15.7% vs 16.0%), but more common than in non-HHT (7.5%), P < .001. Comorbidities, including gastroesophageal reflux (25.9% vs 20.0%) and cirrhosis (10.0% vs 3.6%) were greater in HHT than non-HHT, each P < .001. In multivariable logistic regression, peptic ulcer disease (OR, 8.86; P < .001), portal vein thrombosis (OR, 3.68; P = .006), and hepatitis C, (OR, 2.13; P = .017) were significantly associated with bleeding in HHT. In conclusion, AVM and angiodysplasia are more common and HMB and PPH less common in patients in those with HHT than non-HHT. IDA deficiency is as common in HHT with and without bleeding, suggesting ongoing blood loss and need for universal iron screening.
Introduction
Hereditary hemorrhagic telangiectasia (Osler Weber-Rendu disease; HHT) is an autosomal dominant multisystem bleeding disorder, with a prevalence of 1 in 5000 people, characterized by disordered angiogenesis from small diffuse telangiectasias to large discrete arteriovenous malformations (AVMs).1 When these vascular lesions occur in visceral organs, eg, central nervous system, lungs, gastrointestinal (GI) tract, and liver,2-4 there may be bleeding, anemia associated with iron deficiency, high-output heart failure, hypoxia, strokes, or neurologic deficits.5,6 Epistaxis is typically the earliest symptom and may be recurrent, leading to anemia associated with iron deficiency and poor quality of life4,7 but is responsive to noninvasive approaches, including antifibrinolytics or antiangiogenics, or, if more severe, cautery, arterial ligature, or embolotherapy.8-14 Genetic variants, including ENG, ACVRL1/ALK1, and SMAD4, may be detected in up to ≥80% of affected families.9 The severity of HHT disease varies widely, increases with age,15 and in severe cases, may reduce life expectancy.10 In contrast to women with other mucocutaneous bleeding disorders, heavy menstrual bleeding (HMB) in women with HHT has not been described in the peer-reviewed literature, and bleeding complications of delivery appear to arise primarily in association with pulmonary or brain AVMs.16-19 Because studies of the prevalence of and risk factors for bleeding in HHT are limited, we analyzed a large national discharge database for prevalence and risk factors for bleeding in HHT.
Materials and methods
Study design and data source
This was a retrospective study using data from the US National Inpatient Sample (NIS) spanning the time period from 1 January 2016 to 31 December 2018. The NIS database is the largest publicly available all-payer inpatient database in the United States developed by Healthcare Cost and Utilization Project. It contains a stratified sample of 20% of all discharges from American community hospitals, excluding rehabilitation and long-term acute care hospitals and including patients who are covered under Medicare, Medicaid, and private insurance as well as those who are uninsured. It includes clinical and nonclinical elements, such as diagnoses, procedures, demographics, payment, and hospital characteristics of ∼7 million inpatient stays. Each observation is assigned a discharge weight which is projected to a nationally representative population, and it represents a single hospital stay, including 1 primary discharge diagnosis, up to 24 secondary diagnoses, patient demographics, procedures performed, in-hospital mortality status, insurance status, and length of stay.20 Diagnoses were identified based on the the International Classification of Disease, 10th edition, Clinical Modification codes.21
Populations
Patients discharged with and without HHT and with and without bleeding were identified. Those with HHT were identified based on the International Classification of Disease, 10th edition, Clinical Modification code I78.0. Bleeding was further dichotomized into different subsets to further investigate type, source, and prevalence. The following diagnostic codes were used: telangiectasia (I78.0), epistaxis (R04.0), mucosal bleeding (R04.89, R04.1, K06.8, L76.01, and R23.3), and AVMs (Q27.3, Q27.33, 747.32, Q28.2, Q27.39, and Q87.2); GI bleeding separated into upper (K25.09, I85.0, K21.0, K29.0, K29.81, K31.811, K31.82, C26.9, K63.6, K62.1, K31.7, and K22.6) and lower (K57.1, K57.3, K57.01, K57.21, K55.052, K64.9, K60.5, K62.6, C26.9, K63.5, K62.1, K31.7, K55.2, K51.9, K91.84, and K52); pulmonary bleeding (P26.8, R04.89), liver bleeding (K76.2), and bleeding in central nervous system (I62.9). Other bleeding included HMB/menorrhagia (N92.04), postpartum bleeding (O72.1), surgical or procedural bleeding (L76.22 and T81.31), postoperative bleeding (L76.22), traumatic bleeding (S06.36), hemarthrosis (M25), muscle hematoma (M79.81), bleeding from minor wounds (R58 and S81.812A), and dental bleeding (K06.8).
Risk factors and comorbidities
Potential risk factors for bleeding were identified, and their frequency in HHT was evaluated with the following diagnosis codes: risks for bleeding, including use of aspirin (Z79.82), anticoagulants (Z79.01), nonsteroidal anti-inflammatory drugs (Z79.1), steroids (Z79.5); treatments for bleeding, including transfusion of blood or blood products (Z51.3), transfusion of platelets (30233R1), transfusion of coagulation factors (99.06), and desmopressin administration (Z79.818); and comorbidities, including smoking (Z87.891, F17.200, and F17.219), excessive alcohol consumption (F10.129), obesity (E66), age >65 years (R54), hypertension (HTN) (I10-16), hyperlipidemia (E78.5), diabetes with and without complications (E101-105, 109, 111-115, 119, 131-135, 139, 141-145, and 149), iron deficiency anemia (D50.9), gastroesophageal reflux disease (K21.9), Helicobacter pylori infection (B96.81), peptic ulcer disease (PUD) (K26-28), altered mental status (R41.82), HIV (B20-24), thrombocytopenia (D69.9), international normalized ratio > 1.5 (R79.1), systolic blood pressure ≤ 90 mmHg (I95.1), cardiac disease (I59.1), acute or chronic myocardial infarction (MI) (I20-22, I252), congestive heart disease (I50), liver disease (categorized into mild or moderate/severe) (K73, K76.9, K702, K703, K717, K721, K729, K740, K742 – K745, K766, and K767), cirrhosis (K74.6) categorized into alcoholic (K70.30 and K70.31), post necrotic or hepatitis (K74.69), biliary (K74.3), cardiac (K76.1), nonspecific metabolic cirrhosis or NASH (K75.81), portal HTN (K76.6), portal vein thrombosis (PVT) (I81), peripheral vascular disease (I71, I729, I790, R02, Z958, and Z959), cerebrovascular disease (I60-66, I669-688, 46, G450-452, G458, and G459), dementia (F00-02 and F051), chronic pulmonary disease (J40-47 and J60-65), rheumatologic disease (M32-34, M32-34, and M50-69), any malignancy (including leukemia and lymphoma) (C0-6, C40-49, C70-96, and C883-9430), hemiplegia (G41, G81, and G820-822), weight loss surgery (Z98.84), radiation therapy (Z92.3), hepatitis C virus infection (HCV) (B18.2), renal disease (N01, N03, N18, N19, N25, N52-56, and N72-74), and end-stage renal disease (N18.6). Deyo's modification of Charlson Comorbidity Index was used to define severity of illness among the groups.22 The Charlson Comorbidity Index is a scoring system, constructed from mortality rates of 16 diseases, that controls for confounding by assigning weights based on the strength of their association with mortality.
Statistical analysis
We compared bleeding prevalence between individuals with and without HHT and also compared the demographics (age, gender, and race), length of stay, inpatient mortality, payer, medical comorbidities, and potential causes of and risk factors for bleeding, stratified based on HHT and bleeding status. Categorical variables were compared using Rao-Scott χ2 test, and continuous variables, using weighted simple linear regression. Weighted multivariable logistic regression was performed to identify factors independently associated with bleeding and estimate odds ratios (ORs) stratified in discharged patients with HHT. Covariates included in the multivariable model were selected based on clinical and statistical significance. Analyses used discharge-level weights to reflect national estimates and were performed using Stata/SE 17.0 (StatCorp LLC, College Stations, TX). P < .05 was considered statistically significant.
Results
Admission characteristics
During the 2-year period between 1 January 2016 and 31 December 2018, there were 18 170 849 adult discharges, of which 2528 had a diagnosis of HHT (0.01%; Table 1). Among those with HHT, 648 (25.6%) were admitted with bleeding; they were of similar age (63.1 vs 62.9 years; P = .701) but more likely to be male (44.8% vs 39.1%; P = .015), and African American (15.9% vs 12.6%; P = .045) than those who were discharged without bleeding.
Characteristics in individuals with HHT with and without bleeding
| . | HHT with bleeding . | HHT without bleeding . | . |
|---|---|---|---|
| Variable . | Percent or mean (standard error) . | P value . | |
| n (%) | 648 (25.6%) | 1880 (74.4%) | |
| Age (y) | 63.1 (0.6) | 62.9 (0.4) | .701 |
| Race | .045 | ||
| Caucasian | 70.0 | 75.6 | |
| African American | 15.9 | 12.6 | |
| Asian | 0.8 | 1.0 | |
| Other | 13.4 | 10.9 | |
| Gender | .015 | ||
| Female | 55.2 | 60.9 | |
| Male | 44.8 | 39.1 | |
| HTN | 58.0 | 52.2 | .013 |
| Congestive heart failure | 26.7 | 27.7 | .631 |
| Chronic pulmonary disease | 25.8 | 30.0 | .050 |
| Liver disease | |||
| Cirrhosis | 10.0 | 5.5 | <.001 |
| Portal HTN | 7.3 | 3.0 | <.001 |
| HCV | 3.4 | 1.3 | .001 |
| Postnecrotic cirrhosis | 2.3 | 1.4 | .138 |
| PVT | 1.5 | 0.3 | .001 |
| Iron deficiency anemia | 15.7 | 16.0 | .871 |
| PUD | 9.0 | 1.1 | <.001 |
| Peripheral vascular disease | 7.1 | 4.9 | .032 |
| Malignancy | 4.0 | 6.9 | .009 |
| Charlson comorbidity score | 2.3 (0.1) | 2.1 (0.1) | .034 |
| Length of stay (d) | 5.5 (0.2) | 5.1 (0.2) | .176 |
| Inpatient mortality | 1.9 | 2.3 | .494 |
| . | HHT with bleeding . | HHT without bleeding . | . |
|---|---|---|---|
| Variable . | Percent or mean (standard error) . | P value . | |
| n (%) | 648 (25.6%) | 1880 (74.4%) | |
| Age (y) | 63.1 (0.6) | 62.9 (0.4) | .701 |
| Race | .045 | ||
| Caucasian | 70.0 | 75.6 | |
| African American | 15.9 | 12.6 | |
| Asian | 0.8 | 1.0 | |
| Other | 13.4 | 10.9 | |
| Gender | .015 | ||
| Female | 55.2 | 60.9 | |
| Male | 44.8 | 39.1 | |
| HTN | 58.0 | 52.2 | .013 |
| Congestive heart failure | 26.7 | 27.7 | .631 |
| Chronic pulmonary disease | 25.8 | 30.0 | .050 |
| Liver disease | |||
| Cirrhosis | 10.0 | 5.5 | <.001 |
| Portal HTN | 7.3 | 3.0 | <.001 |
| HCV | 3.4 | 1.3 | .001 |
| Postnecrotic cirrhosis | 2.3 | 1.4 | .138 |
| PVT | 1.5 | 0.3 | .001 |
| Iron deficiency anemia | 15.7 | 16.0 | .871 |
| PUD | 9.0 | 1.1 | <.001 |
| Peripheral vascular disease | 7.1 | 4.9 | .032 |
| Malignancy | 4.0 | 6.9 | .009 |
| Charlson comorbidity score | 2.3 (0.1) | 2.1 (0.1) | .034 |
| Length of stay (d) | 5.5 (0.2) | 5.1 (0.2) | .176 |
| Inpatient mortality | 1.9 | 2.3 | .494 |
Unadjusted analyses
In univariate analysis, discharged patients with HHT bleeding were more likely to have liver disease, including cirrhosis (10.0% vs 5.5%), portal HTN (7.3% vs 3.0%), HCV (3.4% vs 1.3%), and PVT (1.5% vs 0.3%), than those with HHT without bleeding, each with P < .001 (Table 1). High-output cardiac failure, considered a common symptom of liver involvement,3 was similar between patients with HHT with and without bleeding (26.7% vs 27.7%; P = .631), but liver decompensation, as defined by albumin < 3 g/dL, international normalized ratio >1.5, or thrombocytopenia, was rare, and the Charlson comorbidity score was low (mean, 2.3 [standard error of the mean, 0.1] vs 2.1 [standard error of the mean, 0.1]; P = .034). Unexpectedly in the discharged patients with HHT, iron deficiency was similar between those with and without bleeding (15.7% vs 16.0%; P = .871).
Overall, among the discharged patients with bleeding, the most common vascular lesion in HHT was AVM in 31.9%, followed by angiodysplasia in 23.5%, and telangiectasia in 2.3%, each significantly greater than in those without HHT (P < .001; Table 2). The primary site of bleeding was the GI tract, 70.4% in patients with HHT and 76.6% in those without HHT and more common in the upper GI tract than in the lower GI tract (36.1% vs 19.9% and 36.9% vs 30.1%, respectively; Table 2). In those with HHT, angiodysplasia was the most common cause of upper GI tract bleeding (18.1%) and the second most common cause of lower GI tract bleeding (5.4%), each significantly more common than in individuals without HHT (P < .001). By contrast, ulcer disease in the upper GI tract and diverticulosis in the lower GI tract were the most common causes of GI bleeding in the discharged patients without HHT.
Types of bleeding in individuals with and without HHT
| . | HHT with bleeding . | No HHT with bleeding . | . |
|---|---|---|---|
| Variable . | Percent or mean (standard error) . | P value . | |
| n (%) | 648 (0.06%) | 1 005 594 (99.94%) | |
| Age (y) | 63.1 (0.6) | 62.0 (0.1) | .060 |
| Gender (female) | 55.2 | 57.5 | .273 |
| GI bleeding | 70.4 | 76.6 | < .001 |
| Upper GI bleeding | 36.1 | 36.9 | .684 |
| Angiodysplasia | 18.1 | 1.6 | < .001 |
| Gastric and/or duodenal ulcers | 6.5 | 12.3 | < .001 |
| Esophagitis | 4.3 | 10.6 | < .001 |
| Gastritis/duodenitis | 1.4 | 3.7 | .002 |
| Lower GI bleeding | 19.9 | 30.1 | < .001 |
| Diverticulosis | 9.9 | 17.0 | < .001 |
| Angiodysplasia | 5.4 | 0.7 | < .001 |
| Rectal (hemorrhoid, fissure, or ulcer) | 3.5 | 6.3 | .004 |
| Inflammatory bowel disease | 1.4 | 5.2 | < .001 |
| AVM | 31.9 | 1.3 | .001 |
| GI AVM | 20.1 | 1.0 | < .001 |
| Spinal AVM | 9.6 | 0.1 | < .001 |
| Pulmonry AVM | 7.7 | 0.0 | < .001 |
| Cerebral AVM | 5.1 | 0.5 | < .001 |
| Epistaxis | 17.9 | 0.6 | < .001 |
| HMB∗ | 4.1 | 24.9 | < .001 |
| Postpartum hemorrhage∗ | 4.1 | 41.5 | < .001 |
| Telangiectasia | 2.3 | 0.2 | < .001 |
| Surgical/procedural bleeding | 0.8 | 4.1 | < .001 |
| . | HHT with bleeding . | No HHT with bleeding . | . |
|---|---|---|---|
| Variable . | Percent or mean (standard error) . | P value . | |
| n (%) | 648 (0.06%) | 1 005 594 (99.94%) | |
| Age (y) | 63.1 (0.6) | 62.0 (0.1) | .060 |
| Gender (female) | 55.2 | 57.5 | .273 |
| GI bleeding | 70.4 | 76.6 | < .001 |
| Upper GI bleeding | 36.1 | 36.9 | .684 |
| Angiodysplasia | 18.1 | 1.6 | < .001 |
| Gastric and/or duodenal ulcers | 6.5 | 12.3 | < .001 |
| Esophagitis | 4.3 | 10.6 | < .001 |
| Gastritis/duodenitis | 1.4 | 3.7 | .002 |
| Lower GI bleeding | 19.9 | 30.1 | < .001 |
| Diverticulosis | 9.9 | 17.0 | < .001 |
| Angiodysplasia | 5.4 | 0.7 | < .001 |
| Rectal (hemorrhoid, fissure, or ulcer) | 3.5 | 6.3 | .004 |
| Inflammatory bowel disease | 1.4 | 5.2 | < .001 |
| AVM | 31.9 | 1.3 | .001 |
| GI AVM | 20.1 | 1.0 | < .001 |
| Spinal AVM | 9.6 | 0.1 | < .001 |
| Pulmonry AVM | 7.7 | 0.0 | < .001 |
| Cerebral AVM | 5.1 | 0.5 | < .001 |
| Epistaxis | 17.9 | 0.6 | < .001 |
| HMB∗ | 4.1 | 24.9 | < .001 |
| Postpartum hemorrhage∗ | 4.1 | 41.5 | < .001 |
| Telangiectasia | 2.3 | 0.2 | < .001 |
| Surgical/procedural bleeding | 0.8 | 4.1 | < .001 |
Analysis limited to females of reproductive potential (15-45 years of age).
More women (55.2%) than men with HHT were hospitalized for bleeding, similar to women without HHT (57.5%), but in their reproductive years, women with HHT were significantly less likely to have HMB than women without HHT (4.1% vs 24.9%) or postpartum hemorrhage (4.1% vs 41.5%), each with P < .001 (Table 2). Among males and females, surgical or procedural bleeding was significantly less common in patients with HHT than those without HHT (0.8% vs 4.1%; P < .001). Platelet transfusions were rarely administered to those with HHT (1.2% vs 1.4%) compared with those without HHT (P = .653), and neither group received blood or blood product transfusions (Table 3). Despite this, iron deficiency anemia was twice as common in discharged patients with HHT than in those without HHT (15.7% vs 7.5%; P < .001) despite lower rates of anticoagulation (5.2% vs 10.7%) or aspirin use (4.6% vs 13.5%), each with P < .001 (Table 3).
Comorbidities associated with bleeding in individuals with and without HHT
| Variable . | HHT with bleeding . | No HHT with bleeding . | P value . |
|---|---|---|---|
| Percent or mean (standard error) . | |||
| n (%) | 648 (0.06%) | 1 005 594 (99.94%) | |
| Congestive heart failure | 26.7 | 18.3 | < .001 |
| GERD | 25.9 | 20.0 | .001 |
| Hyperlipidemia | 21.9 | 30.0 | < .001 |
| Renal disease | 17.6 | 20.9 | .048 |
| Iron deficiency anemia | 15.7 | 7.5 | < .001 |
| Obesity | 10.0 | 15.5 | < .001 |
| Liver disease | |||
| Cirrhosis | 10.0 | 3.6 | < .001 |
| Portal HTN | 7.3 | 3.9 | < .001 |
| HCV | 3.4 | 1.3 | < .001 |
| Postnecrotic cirrhosis | 2.3 | 0.7 | < .001 |
| Diabetes with complications | 8.8 | 11.7 | .023 |
| Anticoagulation | 5.2 | 10.7 | < .001 |
| Aspirin use | 4.6 | 13.5 | < .001 |
| Malignancy | 4.0 | 10.1 | < .001 |
| Inpatient mortality | 1.9 | 3.6 | .017 |
| Platelet transfusion | 1.2 | 1.4 | .653 |
| Blood or blood product transfusion | 0.0 | 0.0 | NA |
| Length of stay (d) | 5.5 (0.2) | 6.0 (0.0) | .043 |
| Charlson comorbidity score | 2.3 (0.1) | 2.6 (0.0) | .005 |
| Variable . | HHT with bleeding . | No HHT with bleeding . | P value . |
|---|---|---|---|
| Percent or mean (standard error) . | |||
| n (%) | 648 (0.06%) | 1 005 594 (99.94%) | |
| Congestive heart failure | 26.7 | 18.3 | < .001 |
| GERD | 25.9 | 20.0 | .001 |
| Hyperlipidemia | 21.9 | 30.0 | < .001 |
| Renal disease | 17.6 | 20.9 | .048 |
| Iron deficiency anemia | 15.7 | 7.5 | < .001 |
| Obesity | 10.0 | 15.5 | < .001 |
| Liver disease | |||
| Cirrhosis | 10.0 | 3.6 | < .001 |
| Portal HTN | 7.3 | 3.9 | < .001 |
| HCV | 3.4 | 1.3 | < .001 |
| Postnecrotic cirrhosis | 2.3 | 0.7 | < .001 |
| Diabetes with complications | 8.8 | 11.7 | .023 |
| Anticoagulation | 5.2 | 10.7 | < .001 |
| Aspirin use | 4.6 | 13.5 | < .001 |
| Malignancy | 4.0 | 10.1 | < .001 |
| Inpatient mortality | 1.9 | 3.6 | .017 |
| Platelet transfusion | 1.2 | 1.4 | .653 |
| Blood or blood product transfusion | 0.0 | 0.0 | NA |
| Length of stay (d) | 5.5 (0.2) | 6.0 (0.0) | .043 |
| Charlson comorbidity score | 2.3 (0.1) | 2.6 (0.0) | .005 |
GERD, gastroesophageal reflux; NA, not available.
Comorbidities associated with bleeding in patients with HHT included congestive heart failure (26.7% vs 18.3%), gastroesophageal reflux disease (25.9% vs 20.0%), cirrhosis (10.0% vs 3.6%), portal HTN (7.3% vs 3.9%), and HCV (3.4% vs 1.3%), each more common than in those without HHT (P < .001; Table 3). Despite this, the mean length of stay (5.5 vs 6.0 days; P = .043), inpatient mortality (1.9% vs 3.6%; P = .017), and the mean Charlson comorbidity score (2.3 vs 2.6; P = .005) were lower in the HHT group than in the non-HHT group (Table 3).
Multivariable analysis
After fitting a multivariable logistic regression model, variables significantly associated with bleeding in HHT were PUD (adjusted OR, 8.86; 95% confidence interval [CI], 5.11-15.38), PVT (OR, 3.68; CI, 1.44-9.36), and HCV (OR, 2.13; CI, 1.14-3.98; Table 4). PUD was also the variable most significantly associated with bleeding in discharged individuals without HHT (adjusted OR, 25.44; CI, 25.11-25.76), followed by portal HTN (OR, 2.59; CI, 2.53-2.64), and liver disease, including mild liver disease (OR, 2.38; CI, 2.33-2.43), moderate or severe liver disease (OR, 2.32; CI, 2.27-2.37), and cirrhosis (OR, 2.10; CI, 2.06-2.13; Table 5).
Adjusted ORs for bleeding in HHT by multivariable logistic regression
| Variable . | OR . | 95% CIs . | P value . |
|---|---|---|---|
| PUD | 8.86 | 5.11-15.38 | < .001 |
| PVT | 3.68 | 1.44-9.36 | .006 |
| HCV | 2.13 | 1.14-3.98 | .017 |
| Liver disease | Omnibus P = .116 | ||
| No liver disease | Reference | ||
| Cirrhosis | 1.54 | 1.01-2.34 | .044 |
| Moderate or severe liver disease | 2.01 | 0.97-4.17 | .061 |
| Mild liver disease | 0.81 | 0.36-1.80 | .603 |
| Peripheral vascular disease | 1.31 | 0.88-1.96 | .190 |
| Race | Omnibus P = .222 | ||
| Caucasian | Reference | ||
| African American | 1.31 | 0.99-1.74 | .060 |
| Asian | 0.82 | 0.28-2.43 | .727 |
| Other | 1.19 | 0.87-1.63 | .268 |
| Portal HTN | 1.28 | 0.68-2.41 | .436 |
| HTN | 1.26 | 1.02-1.56 | .030 |
| Charlson comorbidity score | 1.00 | 0.94-1.06 | .986 |
| Age per 10 y (continuous) | 0.98 | 0.92-1.05 | .634 |
| Female (ref = male) | 0.82 | 0.67-1.00 | .048 |
| Chronic pulmonary disease | 0.79 | 0.62-1.00 | .046 |
| Malignancy | 0.60 | 0.37-0.96 | .035 |
| Variable . | OR . | 95% CIs . | P value . |
|---|---|---|---|
| PUD | 8.86 | 5.11-15.38 | < .001 |
| PVT | 3.68 | 1.44-9.36 | .006 |
| HCV | 2.13 | 1.14-3.98 | .017 |
| Liver disease | Omnibus P = .116 | ||
| No liver disease | Reference | ||
| Cirrhosis | 1.54 | 1.01-2.34 | .044 |
| Moderate or severe liver disease | 2.01 | 0.97-4.17 | .061 |
| Mild liver disease | 0.81 | 0.36-1.80 | .603 |
| Peripheral vascular disease | 1.31 | 0.88-1.96 | .190 |
| Race | Omnibus P = .222 | ||
| Caucasian | Reference | ||
| African American | 1.31 | 0.99-1.74 | .060 |
| Asian | 0.82 | 0.28-2.43 | .727 |
| Other | 1.19 | 0.87-1.63 | .268 |
| Portal HTN | 1.28 | 0.68-2.41 | .436 |
| HTN | 1.26 | 1.02-1.56 | .030 |
| Charlson comorbidity score | 1.00 | 0.94-1.06 | .986 |
| Age per 10 y (continuous) | 0.98 | 0.92-1.05 | .634 |
| Female (ref = male) | 0.82 | 0.67-1.00 | .048 |
| Chronic pulmonary disease | 0.79 | 0.62-1.00 | .046 |
| Malignancy | 0.60 | 0.37-0.96 | .035 |
Adjusted ORs for bleeding in non-HHT by multivariable logistic regression
| Variable . | OR . | 95% CIs . | P value . |
|---|---|---|---|
| PUD | 25.44 | 25.11-25.76 | < .001 |
| Portal HTN | 2.59 | 2.53-2.64 | < .001 |
| Liver disease | Omnibus P < .001 | ||
| No liver disease | Reference | ||
| Mild liver disease | 2.38 | 2.33-2.43 | < .001 |
| Moderate or severe liver disease | 2.32 | 2.27- 2.37 | < .001 |
| Cirrhosis | 2.10 | 2.06-2.13 | < .001 |
| PVT | 1.54 | 1.48-1.60 | < .001 |
| Female (ref = male) | 1.15 | 1.14-1.16 | < .001 |
| Race | Omnibus P < .001 | ||
| Caucasian | Reference | ||
| Asian | 1.13 | 1.11-1.16 | < .001 |
| African American | 1.12 | 1.11-1.13 | < .001 |
| Other | 1.08 | 1.07-1.09 | < .001 |
| Malignancy | 1.11 | 1.10- 1.13 | < .001 |
| Peripheral vascular disease | 1.11 | 1.10-1.12 | < .001 |
| Age per 10 y (continuous) | 1.09 | 1.09-1.10 | < .001 |
| Charlson comorbidity score | 1.02 | 1.02-1.02 | < .001 |
| HCV | 1.00 | 0.98-1.03 | .778 |
| HTN | 1.00 | 0.99-1.00 | .373 |
| Chronic pulmonary disease | 0.92 | 0.91-0.92 | < .001 |
| Variable . | OR . | 95% CIs . | P value . |
|---|---|---|---|
| PUD | 25.44 | 25.11-25.76 | < .001 |
| Portal HTN | 2.59 | 2.53-2.64 | < .001 |
| Liver disease | Omnibus P < .001 | ||
| No liver disease | Reference | ||
| Mild liver disease | 2.38 | 2.33-2.43 | < .001 |
| Moderate or severe liver disease | 2.32 | 2.27- 2.37 | < .001 |
| Cirrhosis | 2.10 | 2.06-2.13 | < .001 |
| PVT | 1.54 | 1.48-1.60 | < .001 |
| Female (ref = male) | 1.15 | 1.14-1.16 | < .001 |
| Race | Omnibus P < .001 | ||
| Caucasian | Reference | ||
| Asian | 1.13 | 1.11-1.16 | < .001 |
| African American | 1.12 | 1.11-1.13 | < .001 |
| Other | 1.08 | 1.07-1.09 | < .001 |
| Malignancy | 1.11 | 1.10- 1.13 | < .001 |
| Peripheral vascular disease | 1.11 | 1.10-1.12 | < .001 |
| Age per 10 y (continuous) | 1.09 | 1.09-1.10 | < .001 |
| Charlson comorbidity score | 1.02 | 1.02-1.02 | < .001 |
| HCV | 1.00 | 0.98-1.03 | .778 |
| HTN | 1.00 | 0.99-1.00 | .373 |
| Chronic pulmonary disease | 0.92 | 0.91-0.92 | < .001 |
In a multivariable model with HHT as the primary variable and adjusting for age and other covariates, the adjusted ORs for bleeding among all discharged patients (HHT and non-HHT) were PUD (OR, 25.43; CI, 25.11-25.75), HHT (OR, 5.47; CI, 4.93-6.06), and portal HTN (OR, 2.59; CI, 2.53-2.64; Table 6). Finally, variables significantly associated with HHT in a multivariable model among all individuals with bleeding, adjusting for age and other covariates, included liver disease, including cirrhosis (OR, 4.03; CI, 2.74-5.93), and moderate or severe liver disease (OR, 2.71; CI, 1.47-4.98); congestive heart failure (OR, 2.43; CI, 1.89-3.13); and anemia associated with iron deficiency (OR, 2.27; CI, 1.84-2.81; Table 7). Hyperlipidemia (OR, 0.70; CI, 0.56-0.86), obesity (OR, 0.62; CI, 0.47-0.82), and malignancy (OR, 0.51; CI, 0.33-0.79) appeared to be protective.
Adjusted ORs for bleeding in all (HHT and non-HHT), using multivariable logistic regression∗
| Variable . | OR . | 95% CIs . | P value . |
|---|---|---|---|
| PUD | 25.43 | 25.11-25.75 | < .001 |
| HHT | 5.47 | 4.93-6.06 | < .001 |
| Portal HTN | 2.59 | 2.53-2.64 | < .001 |
| Liver disease | Omnibus P < .001 | ||
| No liver disease | Reference | ||
| Mild liver disease | 2.38 | 2.33-2.43 | < .001 |
| Moderate or severe liver disease | 2.32 | 2.27-2.37 | < .001 |
| Cirrhosis | 2.10 | 2.06-2.13 | < .001 |
| PVT | 1.54 | 1.48-1.60 | < .001 |
| Female (ref = male) | 1.15 | 1.14-1.16 | < .001 |
| Race | Omnibus P < .001 | ||
| Caucasian | Reference | ||
| Asian | 1.13 | 1.11-1.16 | < .001 |
| African American | 1.12 | 1.11-1.13 | < .001 |
| Other | 1.08 | 1.07-1.09 | < .001 |
| Malignancy | 1.11 | 1.10-1.13 | < .000 |
| Peripheral vascular disease | 1.11 | 1.10-1.12 | < .001 |
| Age per 10 y (continuous) | 1.09 | 1.09-1.10 | < .001 |
| Charlson comorbidity score | 1.02 | 1.02-1.02 | < .001 |
| HCV | 1.00 | 0.98-1.03 | .709 |
| HTN | 1.00 | 0.99-1.00 | < .001 |
| Chronic pulmonary disease | 0.92 | 0.91-0.92 | < .001 |
| Variable . | OR . | 95% CIs . | P value . |
|---|---|---|---|
| PUD | 25.43 | 25.11-25.75 | < .001 |
| HHT | 5.47 | 4.93-6.06 | < .001 |
| Portal HTN | 2.59 | 2.53-2.64 | < .001 |
| Liver disease | Omnibus P < .001 | ||
| No liver disease | Reference | ||
| Mild liver disease | 2.38 | 2.33-2.43 | < .001 |
| Moderate or severe liver disease | 2.32 | 2.27-2.37 | < .001 |
| Cirrhosis | 2.10 | 2.06-2.13 | < .001 |
| PVT | 1.54 | 1.48-1.60 | < .001 |
| Female (ref = male) | 1.15 | 1.14-1.16 | < .001 |
| Race | Omnibus P < .001 | ||
| Caucasian | Reference | ||
| Asian | 1.13 | 1.11-1.16 | < .001 |
| African American | 1.12 | 1.11-1.13 | < .001 |
| Other | 1.08 | 1.07-1.09 | < .001 |
| Malignancy | 1.11 | 1.10-1.13 | < .000 |
| Peripheral vascular disease | 1.11 | 1.10-1.12 | < .001 |
| Age per 10 y (continuous) | 1.09 | 1.09-1.10 | < .001 |
| Charlson comorbidity score | 1.02 | 1.02-1.02 | < .001 |
| HCV | 1.00 | 0.98-1.03 | .709 |
| HTN | 1.00 | 0.99-1.00 | < .001 |
| Chronic pulmonary disease | 0.92 | 0.91-0.92 | < .001 |
Adjusted for age and other covariates.
Adjusted ORs for HHT in individuals with bleeding, using multivariable logistic regression
| Variable . | OR . | 95% CIs . | P value . |
|---|---|---|---|
| Liver disease | Omnibus P < .001 | ||
| No liver disease | Reference | ||
| Cirrhosis | 4.03 | 2.74-5.93 | < .001 |
| Moderate or severe liver disease | 2.71 | 1.47-4.98 | .001 |
| Mild liver disease | 0.91 | 0.41-2.04 | .819 |
| Congestive heart failure | 2.43 | 1.89-3.13 | < .001 |
| Iron deficiency anemia | 2.27 | 1.84-2.81 | < .001 |
| HCV | 1.78 | 1.11-2.86 | .017 |
| Metastatic cancer | 1.54 | 0.67-3.54 | .305 |
| GERD | 1.46 | 1.21-1.76 | < .001 |
| Age per 10 y (continuous) | 1.11 | 1.06-1.16 | < .001 |
| Diabetes with complications | 1.11 | 0.79-1.57 | .533 |
| Renal disease | 1.04 | 0.78-1.39 | .787 |
| Portal HTN | 0.99 | 0.61-1.61 | .975 |
| Charlson score | 0.85 | 0.79-0.92 | < .001 |
| Hyperlipidemia | 0.70 | 0.56-0.86 | .001 |
| Obesity | 0.62 | 0.47, 0.82 | .001 |
| End-stage renal disease | 0.59 | 0.35-1.00 | .051 |
| Malignancy | 0.51 | 0.33-0.79 | .002 |
| Dementia | 0.44 | 0.20- 0.99 | .046 |
| Anticoagulation | 0.44 | 0.30-0.62 | < .001 |
| Aspirin use | 0.32 | 0.22-0.47 | < .001 |
| INR > 1.5 | 0.32 | 0.10-1.00 | .051 |
| Bariatric surgery | 0.30 | 0.10-0.95 | .041 |
| NSAIDs use | 0.24 | 0.06-0.98 | .047 |
| Variable . | OR . | 95% CIs . | P value . |
|---|---|---|---|
| Liver disease | Omnibus P < .001 | ||
| No liver disease | Reference | ||
| Cirrhosis | 4.03 | 2.74-5.93 | < .001 |
| Moderate or severe liver disease | 2.71 | 1.47-4.98 | .001 |
| Mild liver disease | 0.91 | 0.41-2.04 | .819 |
| Congestive heart failure | 2.43 | 1.89-3.13 | < .001 |
| Iron deficiency anemia | 2.27 | 1.84-2.81 | < .001 |
| HCV | 1.78 | 1.11-2.86 | .017 |
| Metastatic cancer | 1.54 | 0.67-3.54 | .305 |
| GERD | 1.46 | 1.21-1.76 | < .001 |
| Age per 10 y (continuous) | 1.11 | 1.06-1.16 | < .001 |
| Diabetes with complications | 1.11 | 0.79-1.57 | .533 |
| Renal disease | 1.04 | 0.78-1.39 | .787 |
| Portal HTN | 0.99 | 0.61-1.61 | .975 |
| Charlson score | 0.85 | 0.79-0.92 | < .001 |
| Hyperlipidemia | 0.70 | 0.56-0.86 | .001 |
| Obesity | 0.62 | 0.47, 0.82 | .001 |
| End-stage renal disease | 0.59 | 0.35-1.00 | .051 |
| Malignancy | 0.51 | 0.33-0.79 | .002 |
| Dementia | 0.44 | 0.20- 0.99 | .046 |
| Anticoagulation | 0.44 | 0.30-0.62 | < .001 |
| Aspirin use | 0.32 | 0.22-0.47 | < .001 |
| INR > 1.5 | 0.32 | 0.10-1.00 | .051 |
| Bariatric surgery | 0.30 | 0.10-0.95 | .041 |
| NSAIDs use | 0.24 | 0.06-0.98 | .047 |
GERD, gastroesophageal reflux; INR, international normalized ratio; NSAID, nonsteroidal anti-inflammatory agent.
Discussion
This retrospective cross-sectional analysis of discharges from the NIS registry confirms the high frequency of vascular lesions associated with bleeding and the high frequency of iron deficiency anemia in patients with HHT. Bleeding in patients with HHT is associated with AVM, in 32.1%, which is ∼25-fold greater than in those without HHT; angiodysplasia, in 22.9%, which is 10-fold greater than in those without HHT; and telangiectasia, in 2.3%, which is 11-fold greater than in those without HHT. In fact, the high frequency of these vascular lesions in a patient with bleeding strongly suggests a diagnosis of HHT. It is noted that these inpatient figures grossly underestimate the majority of patients with HHT who are managed as outpatients. With the growing recognition of HHT-associated genetic mutations and availability of whole-genome sequencing, individuals in whom AVM, angiodysplasia, or telangiectasia are identified should undergo genetic screening as well as family screening to confirm the diagnosis in order to receive early treatment and management of bleeding because up to 80% of the individuals may be affected.9
Based on the anatomic site, bleeding in HHT was observed most commonly in the GI tract, in 70.4%, as in discharged patients without HHT, but the preponderance of vascular lesions appeared to be significantly greater. The second most common site of bleeding in patients with HHT, the nasopharynx, with epistaxis occurring in 17.9%, also appeared to be significantly more common than in those without HHT. Although bleeding in discharged patients with HHT is common, it did not appear to require red blood cell transfusion but was sufficiently significant to result in iron deficiency in 15.7% of the patients. Although the iron deficiency prevalence in our study was lower than the 50% prevalence reported in a selected HHT population, all of whom had bleeding, anemia, and/or transfusion history,6 it is consistent with the 13% prevalence of iron deficiency reported from a large retrospective Danish HHT cohort receiving standardized routine screening for iron deficiency and vascular lesions.23 Furthermore, the similar prevalence of iron deficiency in our discharged patients with HHT but without bleeding, 16.0%, suggests that bleeding in HHT may be occult and/or unrecognized. Thus, it is appropriate that regardless of symptoms, adult patients with HHT should be screened for iron deficiency (including iron, total iron binding capacity, ferritin, and hemoglobin) at least annually, or as clinically indicated, and offered iron supplementation and posttreatment monitoring, an approach supported by the HHT International Guidelines,10 European Reference Network for Rare Vascular Diseases,23 and HHT Foundation International.24 Although iron replacement by the oral or IV route may be equally effective, vascular lesions in the GI tract of patients with HHT may interfere with absorption of oral iron, for which the IV route may be more effective. More research is needed to assess this question and advise on optimal strategies to avert anemia associated with iron deficiency, given its high frequency and adverse consequence on cognition, psychosocial health, and quality of life.25
Liver disease, including cirrhosis, portal HTN, postnecrotic cirrhosis, and HCV were identified in this study as important comorbidities in individuals with HHT who bleed. As well-recognized and evident in this study, liver disease involvement in HHT was found to be associated with high-output cardiac failure and portal HTN, rather than thrombocytopenia or the coagulopathy of liver disease.2-4 It is noteworthy that discharged patients with HHT without bleeding appeared to have a similar frequency of high-output cardiac disease as those with bleeding but a lower frequency of hepatocellular liver disease and portal HTN. It is possible that discharged patients with HHT without bleeding had vascular lesions outside the liver or different genetic mutations, but it is not possible to determine individual level data in this inpatient sample.
In contrast to women with other bleeding disorders, such as von Willebrand disease, in whom HMB and postpartum hemorrhage are common,26,27 menorrhagia and postpartum hemorrhage appear to be significantly uncommon in hospitalized women with HHT (0.9% and 0.3%, respectively). This suggests that uterine vascular lesions are uncommon, but this has not been studied. It is noteworthy that preliminary estimates of HMB in 74% of outpatient women with HHT28 are higher than in inpatient women with HHT in this study, but these findings will require peer review. Despite this, women with HHT may still suffer from iron deficiency from monthly blood loss, even in the absence of HHT-related bleeding; thus, should be carefully screened.
We acknowledge several limitations. First, discharge diagnosis codes were used for patient inclusion, and this may be limited by coding inaccuracy and misclassification bias. Although the accuracy of data collected based on discharge codes has been shown to be accurate,29-31 it is not possible to confirm HHT diagnosis. A second limitation is that neither the severity and specific types of HHT nor the methods used to determine specific causes of bleeding or treatment to control bleeding are provided in the NIS database. Third, miscoding of HHT cases is possible, because the NIS database includes admission level data only. Fourth, selection bias is another potential limitation, because this is an inpatient database with preferential inclusion of patient who are more ill or have more severe disease than the rest. Similarly, estimates for common bleeding problems, such as epistaxis or HMB, in an inpatient database may reflect the small number of patients who require hospitalization, rather than the larger number managed as outpatients. Fifth, multiple admissions for the same patient are possible, because this database reports hospital discharges rather than specific patient encounters. Sixth, this study of hospitalized patients may reflect the well-known impact of age on impact disease severity and hospitalization.15 Seventh, it is not possible to prove causality in a retrospective study. Finally, because the NIS database of 20% of all US hospital admissions may not be representative, discharge-level-weights were used to limit this effect.
In summary, we found that the GI tract was the most common site of bleeding in patients with HHT and that the most common site of bleeding in inpatients with HHT is the GI tract, and the most common lesions are AVM, angiodysplasia, and telangiectasia, but HMB and PPH are uncommon. The most important predictors of bleeding in this inpatient sample study of HHT were found to be PUD, PVT, and HCV. The latter predictors likely reflect common sites of vascular lesions (GI tract and great vessels,) and transfusion-related HCV. Those with HHT who bleed were noted to be significantly more likely to have comorbid liver disease, suggesting the need for early screening for liver disease and early treatment of HCV.
Unexpectedly, anemia associated with iron deficiency appeared to be as common in HHT who bleed as in HHT who do not bleed. Whether this is related to ongoing, unrecognized occult blood loss, reduced GI iron absorption, poor response to iron replacement, or poor compliance is unknown. Finally, our findings suggest screening for iron deficiency annually or more frequently for cause, is warranted in all patients with HHT whether or not they bleed.
Acknowledgments
The study was funded in part by State Support of Hemophilia Center of Western Pennsylvania, SAP #41000079797, and HRSA Federal Hemophilia Treatment Centers grant HC30MC24050-04-00.
Authorship
Contribution: J.Z. and M.V.R. contributed to the study design, data acquisition, interpretation of the data, and writing of the manuscript; K.J. contributed to the data acquisition, performance of the data analysis, and critical review of the manuscript; J.G.Y. contributed to the study design, data acquisition, performance of the data analysis, and critical review of the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: M.V.R. receives research funding from BioMarin, Sanofi, SPARK Therapeutics, and Takeda, and has served on advisory boards for Alnylam, Be Biopharma, BioMarin, Hemab Therapeutics, Sanofi, SPARK Therapeutics, Takeda, and Institute for Clinical and Economic Review. The remaining authors declare no competing financial interests.
Correspondence: Margaret V. Ragni, Division of Hematology/Oncology, Department of Medicine, University of Pittsburgh Medical Center, Hemophilia Center of Western Pennsylvania, 3636 Blvd of the Allies, Pittsburgh, PA 15213-4306; e-mail: ragni@pitt.edu.
References
Author notes
The data set and analysis of the selected year from the NIS are available on request from the corresponding author, Margaret V. Ragni (ragni@pitt.edu).