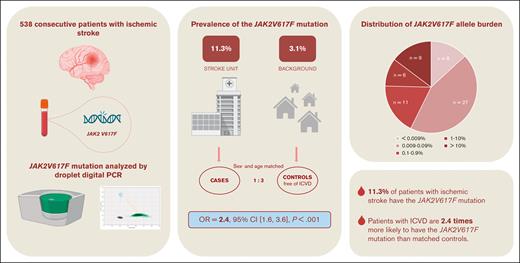
Key Points
The JAK2V617F mutation prevalence in patients with ischemic stroke is 11.3%.
Patients with ischemic stroke are 2.4 times more likely to have the JAK2V617F mutation than matched controls.
Abstract
Ischemic stroke has a high recurrence rate despite treatment. This underlines the significance of investigating new possible cerebrovascular risk factors, such as the acquired gene mutation JAK2V617F found in 3.1% of the general population. We aimed to investigate the prevalence of the JAK2V617F mutation in a population with ischemic stroke compared with that in matched controls. We enrolled 538 consecutive Danish patients with ischemic stroke (mean age, 69.5 ± 10.9 years; 39.2% female) within 7 days of symptom onset. Using multiple-adjusted conditional logistic regression analysis, we compared the prevalence of JAK2V617F with that in age- and sex-matched controls free of ischemic cerebrovascular disease (ICVD) from the Danish General Suburban Population Study. DNA was analyzed for JAK2V617F mutation using sensitive droplet digital polymerase chain reaction in patients and controls. Of the 538 patients with ischemic stroke, 61 (11.3%) had JAK2V617F mutation. There were no differences in patient demographics or cerebrovascular comorbidities between the patients with and without mutations. Patients with ischemic stroke were more likely to have the JAK2V617F mutation than matched controls, in whom the JAK2V617F prevalence was 4.4% (odds ratio, 2.37; 95% confidence interval, 1.57-3.58; P < .001). A subanalysis stratified by smoking history revealed that the association was strongest in current smokers (odds ratio, 4.78; 95% confidence interval, 2.22-10.28; P < .001). Patients with ischemic stroke were 2.4 times more likely to have the JAK2V617F mutation than matched controls without ICVD when adjusting for other cerebrovascular risk factors. This finding supports JAK2V617F mutation as a novel cerebrovascular risk factor.
Introduction
Stroke is a life-threatening and debilitating disease, being one of the most significant health burdens worldwide. In Europe, its prevalence is expected to increase by ∼30% over the next few decades, followed by an anticipated increase of 44% in health care costs.1,2 Despite decades of research on cerebrovascular risk factors and continuous optimization of preventive treatment, 1 in 4 stroke cases still has undetermined etiologies, and the risk of stroke recurrence is 4.3%.3,4 This underlines the need to investigate new possible cerebrovascular risk factors to relieve the heavy burden of stroke on individual patients and society.
One risk factor could be the acquired mutation JAK2V617F, a point mutation in the tyrosine kinase Janus kinase 2 (JAK2). JAK2 is involved in the intracellular signal transduction of cytokines and growth factor receptors and, hence, plays a crucial role in the inflammatory signaling pathway and the proliferation of hematopoietic cells.5,6 The JAK2V617F mutation alters JAK2 to a constitutively active state, consequently increasing proliferation, differentiation, and cytokine release.5,7 There is a link between the JAK2V617F mutation and inflammation; however, whether the JAK2V617F mutation precedes inflammation or vice versa, or most likely, in a self-perpetuating cycle, needs further attention.8-11
Inflammation is crucial in atherosclerosis and thrombus formation, and inflammatory conditions can increase the risk of cardiovascular events, such as stroke.12,13 From this perspective, the JAK2V617F mutation, as a promoter of inflammation, may play an important role in thrombus formation. Indeed, multiple studies have confirmed an association between JAK2V617F mutation and vascular diseases.14-17
Much of our understanding of the JAK2V617F mutation arises from studies of chronic blood cancers, such as Philadelphia-negative myeloproliferative neoplasms (MPN), in which the gene mutation was first described.18-20 MPNs are characterized by the hyperproliferation of myeloid cells, chronic inflammation, and high rates of cardiovascular complications, ranging from microvascular disturbances to debilitating major thrombotic events, such as acute myocardial infarction and stroke.8,21-26 The treatment of MPNs comprises a combination of antiplatelet, cytoreductive, and immunomodulatory therapies, accompanied by phlebotomies in patients with polycythemia vera, which effectively reduces thrombotic complications.27 It is evident that thrombotic events also occur in the very early stages of the disease because 20% to 25% of patients with MPNs suffer 1 or more thrombotic events before MPN diagnosis.28,29
In MPNs, the JAK2V617F mutation is an independent risk factor for thrombosis.30-34 Because the JAK2V617F mutation possesses both disease-driving and thrombosis-promoting abilities, we hypothesized that the incidence of thrombosis in patients prediagnosed with MPN is associated with the presence of the JAK2V617F mutation, even at low allele burdens.
Until recently, it was believed that the JAK2V617F mutation was predominantly related to orphan blood cancers and was only rarely present in otherwise healthy individuals.14,35-38 Yet, the assays for the detection of JAK2V617F have improved, and in 2019, we published a large general population study on the prevalence of JAK2V617F mutation detected by highly sensitive droplet digital polymerase chain reaction (ddPCR; detection limit = 0.009%) in 19 958 individuals and found a prevalence of 3.1%.39 Only a few (n = 14) of these had MPN; the remaining (n = 599) may be classified as clonal hematopoiesis of indeterminate potential (CHIP), which is defined as individuals who carry a somatic mutation >2% mutated alleles without a detectable hematological cancer.40 However, most individuals with the JAK2V617F mutation had allele burdens <2%. Thus, using a more sensitive assay, we identified many previously unknown individuals with the JAK2V617F mutation,39 highlighting the relevance of elucidating potential clinical consequences, including the risk of thrombosis, even in the absence of MPN.
In this study, we aimed to evaluate the prevalence of JAK2V617F mutation in an ischemic stroke population and compare it with that in a matched control group from the general population.
Methods
Participants
In a prospective observational study (JAK2Stroke), we consecutively enrolled Danish patients with ischemic stroke recruited at the Department of Neurology, Roskilde Hospital, Denmark, from September 2021 to February 2023. The inclusion criteria were age >18 years, symptom onset within 7 days, and a diagnosis of ischemic stroke (DI63). The exclusion criterion was inability to provide informed consent. A total of 989 patients were assessed for eligibility. We excluded 440 patients, 367 of whom were unable to provide informed consent, 73 of whom declined participation, and 11 patients in whom the ischemic stroke diagnosis was dropped during subsequent outpatient follow-ups. Finally, a total of 538 patients were included in this study.
The study was approved by the Regional Committee on Health Research Ethics (SJ-930) and the Danish Data Protection Agency (REG-098-2021) and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
Matched control group
From the Danish General Suburban Population Study (GESUS),39 we sampled an age- and sex-matched control group free of ischemic cerebrovascular disease (ICVD) (ICD10 diagnosis codes: DI63-64 and DG45) at a ratio of 1:3. Participants in the GESUS (N = 21 205) were recruited from 2010 to 2013 at the Department of Clinical Biochemistry, Naestved Hospital, Denmark. Thus, controls were sampled from a similar geographical area and time point as the patients in the JAK2Stroke cohort. The study was approved by the Regional Committee on Health Research Ethics (SJ-452 and SJ-114) and the Danish Data Protection Agency (REG-50-2015 and REG-27-2014) and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the participants.
Molecular analyses
At inclusion, a peripheral blood sample was immediately collected in EDTA tubes and stored at −20°C.
The ddPCR was performed in duplicate using 10 μL of 20 ng/μL DNA in each well, denatured at 95°C for 1 minute, and cooled to 37°C, followed by the addition of ddPCR Supermix for Probes (no deoxyuridine triphosphate) (Bio-Rad, Hercules, CA), and droplets were generated on a QX100 (Bio-Rad), according to the manufacturer's instructions. PCR was performed on a Veriti 96-Well Thermal Cycler (Thermo Fisher, Waltham, MA) with an initial stage at 95°C for 10 minutes, followed by 43 cycles at 94°C for 30 seconds and 57°C for 60 seconds, followed by a final stage at 98°C for 10 minutes. Droplets were counted on a QX100 Droplet Reader (Bio-Rad), and the results were analyzed using QuantaSoft Analysis Pro (Bio-Rad). A minimum of 5 copies of JAK2V617F were required before being considered a positive sample, and samples with 5 to 10 copies were rerun to confirm positivity. The same method was used in the GESUS population study, yet here the samples were initially analyzed in pooled samples with DNA from 4 participants, and samples that showed positive mutation results were rerun individually, similarly to the JAK2Stroke samples described earlier. The detection limit of the GESUS study was 0.009%.39 To reassure the reproducibility of the ddPCR assay, we ran 5 independent analyses of 8 individual patient samples in each of the following allele burden intervals: <0.0099, from 0.01 to 0.99, from 0.1 to 0.99, 1 to 9.9, and ≥10 and performed an intermediate precision analysis, assessing the relative standard deviation.
Covariates
The patient journals were assessed for the following data: age, sex, weight, height, smoking status, alcohol use, comorbidities, prior admission for COVID-19, COVID-19 at inclusion, medicine, laboratory results, admission time, time of symptom onset, diagnostic imaging findings, symptoms, National Institutes of Health Stroke Scale (NIHSS) score, infection during admission, discharge time, and final diagnosis. Hypertension, hyperlipidemia, and diabetes mellitus were registered if patients received the corresponding treatment or if diagnoses were reported in patient journals. Atrial fibrillation was registered if present on a 12-lead electrocardiogram or telemetry during admission or if reported in the journal. Ischemic heart disease was documented if reported in the patient's journal. Smoking status was reported as current, former, or never smoked. If no NIHSS score was reported, it was estimated based on reported symptoms. Similar lifestyle, comorbidities, and medical and laboratory data were available for the GESUS from self-reported questionnaires and access to the Danish National Patient Registry.
Laboratory results
As part of the standard admission procedure, routine blood samples were collected immediately after patients arrived at the hospital. EDTA anticoagulated whole blood was analyzed using Sysmex XE for comprehensive blood count. Elevated blood cell levels were defined as hemoglobin >16.5 g/dL (men) and >15.3 g/dL (women), hematocrit >49% (men) and >48% (women), platelet count >390 × 109/L, and white blood cells >8.8 × 109/L, according to local laboratory reference values. Similarly, the analyzed data are available for GESUS.
Statistical analysis
Statistical analyses were performed using the R statistical software (version 4.2.2, R Foundation for Statistical Computing, Vienna, Austria). The variables were assessed for normal distribution using histograms and Q-Q plots. Normally distributed continuous values are presented as mean ± standard deviation and were compared using the Welch 2 Sample t test. Nonparametric values are presented as median and interquartile range and were compared using the Mann-Whitney U test. Categorical variables were presented as n (%) and compared using the χ2 or Fisher exact test when the observations were ≤10. To compare the prevalence of JAK2V617F in the ischemic stroke population with that in the ICVD-free control group, we performed conditional logistic regression analysis adjusted for smoking status, hypertension, dyslipidemia, diabetes, atrial fibrillation, and ischemic heart disease. As the assay in the GESUS study had a detection limit of 0.009%, we labeled cases with a JAK2V617F allele burden below this threshold as negative to ensure adequate comparison with controls in the logistic regression analysis. Statistical significance was set at P < .05. To assess the prevalence of the JAK2V617F mutation in patients with ischemic stroke without MPN, we performed a similarly adjusted conditional regression subanalysis, excluding patients with MPN (CHIP only). To examine the impact of smoking, we conducted a new matching based on age, sex, and smoking status categorized as current, former, or never smoker. We subsequently performed conditional regression analyses according to smoking status. We adjusted all analyses for hypertension, dyslipidemia, diabetes, atrial fibrillation, and ischemic heart disease.
Results
JAK2V617F prevalence
Of the 538 patients, the JAK2V617F mutation was present in 61 (11.3%). Patient characteristics according to the JAK2V617F mutation status are presented in Table 1. There were no statistically significant differences in patient demographics, lifestyle factors, or cerebrovascular risk factors between patients with and without JAK2V617F. Patients with the JAK2V617F mutation had mean hemoglobin, hematocrit, and platelet counts significantly higher than those without the mutation; however, the values were still within the standard laboratory reference range. We found that significantly more patients with JAK2V617F mutation had platelet levels above the normal reference range; however, there was no difference in hemoglobin, hematocrit, or white blood cell levels above the reference range between the 2 groups (Table 1).
Patient characteristics for 538 patients with ischemic stroke according to JAK2V617F mutation status
| Patient characteristics . | All (N = 538) . | With JAK2V617F (n = 61) . | Without JAK2V617F (n = 477) . | P . |
|---|---|---|---|---|
| Age, mean ± SD | 69.5 ± 10.9 | 70.8 ± 10.7 | 69.3 ± 10.9 | .329 |
| Sex (female), n (%) | 211 (39.2) | 18 (29.5) | 193 (40.5) | .131 |
| BMI, mean ± SD | 26.9 ± 5.6 | 26.6 ± 5.8 | 26.9 ± 5.5 | .683 |
| Smoking active, n (%) | 163 (30.3) | 23 (37.7) | 140 (29.4) | .234 |
| Smoking former, n (%) | 190 (35.3) | 18 (29.5) | 172 (36.1) | .387 |
| Alcohol use above recommendation∗, n (%) | 65 (12.1) | 8 (13.1) | 57 (12) | .835 |
| Comorbidities | ||||
| Diabetes, n (%) | 109 (20.3) | 12 (19.7) | 97 (20.3) | >.99 |
| Hypertension, n (%) | 359 (66.7) | 37 (60.7) | 322 (67.5) | .355 |
| Hyperlipidemia, n (%) | 288 (53.5) | 32 (52.5) | 256 (53.7) | .967 |
| Atrial fibrillation, n (%) | 82 (15.2) | 10 (16.4) | 72 (15.1) | .850 |
| IHD, n (%) | 74 (13.8) | 10 (16.4) | 64 (13.4) | .553 |
| History of IS or TIA, n (%) | 106 (19.7) | 17 (27.9) | 89 (18.7) | .126 |
| History of VTE, n (%) | 12 (2.2) | 2 (3.3) | 10 (2.1) | .635 |
| History of cancer, n (%) | 64 (11.9) | 7 (11.5) | 57 (11.9) | >.99 |
| Admission | ||||
| NIHSS ≥ 6, n (%) | 125 (21.3) | 14 (23.0) | 111 (23.5) | >.99 |
| Infection at admission, n (%) | 61 (11.4) | 5 (8.2) | 56 (11.8) | .523 |
| CRP ≥ 3 mg/L, n (%) | 251 (47.2) | 23 (38.3) | 228 (48.3) | .187 |
| Hemoglobin, g/dL, mean ± SD | 14.1 ± 1.79 | 14.6 ± 1.61 | 14.1 ± 1.8 | .015 |
| Hematocrit, mean ± SD | 0.42 ± 0.05 | 0.44 ± 0.05 | 0.420 ± 0.05 | .006 |
| Platelet count, × 109/L, mean ± SD | 254 ± 88.6 | 294 ± 147 | 248 ± 77.1 | .021 |
| White blood cells, × 109/L, mean ± SD | 8.72 ± 3.17 | 9.18 ± 3.28 | 8.66 ± 3.16 | .246 |
| Hemoglobin above normal†, n (%) | 68 (12.7) | 11 (18.0) | 57 (12.0) | .262 |
| Hematocrit above normal‡, n (%) | 30 (6.1) | 5 (8.5) | 25 (5.8) | .386 |
| Platelet count above normal§, n (%) | 30 (5.7) | 8 (13.6) | 22 (4.7) | .012 |
| White blood cells above normal‖, n (%) | 214 (40.1) | 27 (44.3) | 187 (39.5) | .569 |
| Patient characteristics . | All (N = 538) . | With JAK2V617F (n = 61) . | Without JAK2V617F (n = 477) . | P . |
|---|---|---|---|---|
| Age, mean ± SD | 69.5 ± 10.9 | 70.8 ± 10.7 | 69.3 ± 10.9 | .329 |
| Sex (female), n (%) | 211 (39.2) | 18 (29.5) | 193 (40.5) | .131 |
| BMI, mean ± SD | 26.9 ± 5.6 | 26.6 ± 5.8 | 26.9 ± 5.5 | .683 |
| Smoking active, n (%) | 163 (30.3) | 23 (37.7) | 140 (29.4) | .234 |
| Smoking former, n (%) | 190 (35.3) | 18 (29.5) | 172 (36.1) | .387 |
| Alcohol use above recommendation∗, n (%) | 65 (12.1) | 8 (13.1) | 57 (12) | .835 |
| Comorbidities | ||||
| Diabetes, n (%) | 109 (20.3) | 12 (19.7) | 97 (20.3) | >.99 |
| Hypertension, n (%) | 359 (66.7) | 37 (60.7) | 322 (67.5) | .355 |
| Hyperlipidemia, n (%) | 288 (53.5) | 32 (52.5) | 256 (53.7) | .967 |
| Atrial fibrillation, n (%) | 82 (15.2) | 10 (16.4) | 72 (15.1) | .850 |
| IHD, n (%) | 74 (13.8) | 10 (16.4) | 64 (13.4) | .553 |
| History of IS or TIA, n (%) | 106 (19.7) | 17 (27.9) | 89 (18.7) | .126 |
| History of VTE, n (%) | 12 (2.2) | 2 (3.3) | 10 (2.1) | .635 |
| History of cancer, n (%) | 64 (11.9) | 7 (11.5) | 57 (11.9) | >.99 |
| Admission | ||||
| NIHSS ≥ 6, n (%) | 125 (21.3) | 14 (23.0) | 111 (23.5) | >.99 |
| Infection at admission, n (%) | 61 (11.4) | 5 (8.2) | 56 (11.8) | .523 |
| CRP ≥ 3 mg/L, n (%) | 251 (47.2) | 23 (38.3) | 228 (48.3) | .187 |
| Hemoglobin, g/dL, mean ± SD | 14.1 ± 1.79 | 14.6 ± 1.61 | 14.1 ± 1.8 | .015 |
| Hematocrit, mean ± SD | 0.42 ± 0.05 | 0.44 ± 0.05 | 0.420 ± 0.05 | .006 |
| Platelet count, × 109/L, mean ± SD | 254 ± 88.6 | 294 ± 147 | 248 ± 77.1 | .021 |
| White blood cells, × 109/L, mean ± SD | 8.72 ± 3.17 | 9.18 ± 3.28 | 8.66 ± 3.16 | .246 |
| Hemoglobin above normal†, n (%) | 68 (12.7) | 11 (18.0) | 57 (12.0) | .262 |
| Hematocrit above normal‡, n (%) | 30 (6.1) | 5 (8.5) | 25 (5.8) | .386 |
| Platelet count above normal§, n (%) | 30 (5.7) | 8 (13.6) | 22 (4.7) | .012 |
| White blood cells above normal‖, n (%) | 214 (40.1) | 27 (44.3) | 187 (39.5) | .569 |
AMI, acute myocardial infarction; BMI, body mass index; CRP, C-reactive protein; IHD, ischemic heart disease; IQR, interquartile range; IS, ischemic stroke; SD, standard deviation; TIA, transient ischemic attack; VTE, venous thromboembolism. P values < 0.05 are shown in bold.
>14 units per week, men; >7 units per week, women.
>16.5 g/dL, men; >15.3 g/dL, women.
> 49%, men; >48%, women.
>390 × 109/L.
> 8.8 × 109/L.
The average detection limit (median [range]) of the analysis was 0.0036% [0.002%-0.16%]. The intermediate precision analysis of the ddPCR assay showed good reproducibility with allele burdens >0.1% and satisfactory reproducibility with allele burdens <0.1% (supplemental Table 1; supplemental Figure 1). The distribution of allele burden is shown in Table 2. Of the 61 patients, 46 (75%) had an allele burden <1%. One patient were diagnosed with MPN before inclusion (primary myelofibrosis; JAK2V617F allele burden of 11%), 8 patients were diagnosed with MPN after inclusion (supplemental Table 2), 4 with polycythemia vera, 1 with essential thrombocythemia, 2 with prefibrotic primary myelofibrosis, and 1 with MPN-unclassifiable. All patients diagnosed with MPN after inclusion had JAK2V617F allele burdens >5% (5.4%, 12%, 14%, 14%, 23%, 28%, 33%, and 36%, respectively). All patients with JAK2V617F mutation were referred to the Department of Hematology for further assessment and follow-up. Table 3 presents the presence of elevated blood cell levels at inclusion according to JAK2V617F mutation status and MPN diagnosis.
Characteristics of the JAK2Stroke, GESUS cohort, and case-control populations
| . | JAK2Stroke . | GESUS . | JAK2Stroke AB ≥0.009 (cases) . | Control cohort (GESUS) free of ICVD (controls) . | P cases vs control . |
|---|---|---|---|---|---|
| Population, n | 538 | 19 958 | 538 | 1613 | |
| Age, mean ± SD | 69.5 ± 10.9 | 55.8 ± 13.5 | 69.5 ± 10.9 | 69.2 ± 10.9 | .581 |
| Individuals with JAK2V617F, n (%) | 61 (11.3) | 613 (3.1) | 53 (9.9) | 71 (4.4) | <.001 |
| MPN before inclusion, n (%) | 1 (0.2) | 14 (0.1) | 1 (0.2) | 1 (0.06) | .438 |
| JAK2V617F allele burden | |||||
| <0.009%, n (%) | 8 (13.1) | — | — | — | |
| 0.009%-0.09%, n (%) | 27 (44.3) | 255 (42) | 27 (50.9) | 27 (38.0) | .211 |
| 0.1%-0.9%, n (%) | 11 (18) | 253 (41) | 11 (20.8) | 31 (43.7) | .013 |
| 1%-10%, n (%) | 6 (9.8) | 75 (12) | 6 (11.3) | 9 (12.7) | >.99 |
| >10%, n (%) | 9 (14.8) | 30 (5) | 9 (17.0) | 4 (5.6) | .072 |
| Laboratory values | |||||
| Hemoglobin, g/dL, mean ± SD | 14.1 ± 1.79 | 14.0 ± 1.27 | 14.1 ± 1.79 | 14.08 ± 1.29 | .811 |
| Hematocrit, mean ± SD | 0.42 ± 0.05 | 0.43 ± 0.03 | 0.42 ± 0.05 | 0.43 ± 0.04 | <.001 |
| Platelet count, ×109/L, mean ± SD | 254 ± 88.6 | 251 ± 59 | 254 ± 88.6 | 241 ± 58 | .002 |
| White blood cells, ×109/L, mean ± SD | 8.72 ± 3.17 | 7.29 ± 1.96 | 8.72 ± 3.17 | 7.26 ± 1.95 | <.001 |
| . | JAK2Stroke . | GESUS . | JAK2Stroke AB ≥0.009 (cases) . | Control cohort (GESUS) free of ICVD (controls) . | P cases vs control . |
|---|---|---|---|---|---|
| Population, n | 538 | 19 958 | 538 | 1613 | |
| Age, mean ± SD | 69.5 ± 10.9 | 55.8 ± 13.5 | 69.5 ± 10.9 | 69.2 ± 10.9 | .581 |
| Individuals with JAK2V617F, n (%) | 61 (11.3) | 613 (3.1) | 53 (9.9) | 71 (4.4) | <.001 |
| MPN before inclusion, n (%) | 1 (0.2) | 14 (0.1) | 1 (0.2) | 1 (0.06) | .438 |
| JAK2V617F allele burden | |||||
| <0.009%, n (%) | 8 (13.1) | — | — | — | |
| 0.009%-0.09%, n (%) | 27 (44.3) | 255 (42) | 27 (50.9) | 27 (38.0) | .211 |
| 0.1%-0.9%, n (%) | 11 (18) | 253 (41) | 11 (20.8) | 31 (43.7) | .013 |
| 1%-10%, n (%) | 6 (9.8) | 75 (12) | 6 (11.3) | 9 (12.7) | >.99 |
| >10%, n (%) | 9 (14.8) | 30 (5) | 9 (17.0) | 4 (5.6) | .072 |
| Laboratory values | |||||
| Hemoglobin, g/dL, mean ± SD | 14.1 ± 1.79 | 14.0 ± 1.27 | 14.1 ± 1.79 | 14.08 ± 1.29 | .811 |
| Hematocrit, mean ± SD | 0.42 ± 0.05 | 0.43 ± 0.03 | 0.42 ± 0.05 | 0.43 ± 0.04 | <.001 |
| Platelet count, ×109/L, mean ± SD | 254 ± 88.6 | 251 ± 59 | 254 ± 88.6 | 241 ± 58 | .002 |
| White blood cells, ×109/L, mean ± SD | 8.72 ± 3.17 | 7.29 ± 1.96 | 8.72 ± 3.17 | 7.26 ± 1.95 | <.001 |
The JAK2V617F mutation prevalence, distribution of allele burden, and blood cell levels of the JAK2Stroke cohort, total GESUS population, JAK2Stroke cohort with an applied JAK2V617F detection limit of 0.009%, and age- and sex-matched control cohort from the GESUS population free of ICVD. P values < 0.05 are shown in bold.
AB, allele burden; SD, standard deviation.
Presence of cytosis according to JAK2V617F mutation status and MPN diagnosis
| . | Patients with JAK2V617F with MPN n = 9 . | Patients with JAK2V617F without MPN n = 52 . | Patients without JAK2V617F n = 477 . |
|---|---|---|---|
| Erythrocytosis∗, n (%) | 3 (33.3) | 8 (15.4) | 58 (13.2) |
| Thrombocytosis†, n (%) | 6 (66.7) | 2 (3.9) | 22 (4.7) |
| Leukocytosis‡, n (%) | 6 (66.7) | 21 (40.4) | 187 (39.5) |
| Any cytosis, n (%) | 8 (88.9) | 25 (48.1) | 223 (49.3) |
| . | Patients with JAK2V617F with MPN n = 9 . | Patients with JAK2V617F without MPN n = 52 . | Patients without JAK2V617F n = 477 . |
|---|---|---|---|
| Erythrocytosis∗, n (%) | 3 (33.3) | 8 (15.4) | 58 (13.2) |
| Thrombocytosis†, n (%) | 6 (66.7) | 2 (3.9) | 22 (4.7) |
| Leukocytosis‡, n (%) | 6 (66.7) | 21 (40.4) | 187 (39.5) |
| Any cytosis, n (%) | 8 (88.9) | 25 (48.1) | 223 (49.3) |
hb >16.5 g/dL, men; hb > 15.3 g/dL,women; hct >49%, men; hct >48%, women.
plt >390 × 109/L.
WBC >8.8 × 109/L.
Ten patients had a history of admission for COVID-19, none of whom had a JAK2V617F mutation. Eight patients had active COVID-19 at inclusion, 3 of whom had the JAK2V617F mutation. All the patients were asymptomatic or had mild symptoms. There was no correlation between JAK2V617F allele burden and stroke severity, as assessed by the NIHSS (supplemental Figure 2).
Age- and sex-matched controls
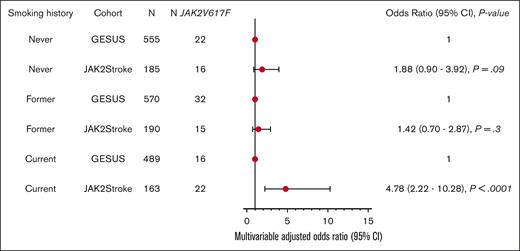
In the age- and sex-matched control group free of ICVD from the GESUS cohort (n = 1613), the prevalence of JAK2V617F mutation was 4.4% (n = 71; Table 2). Even when we applied a detection threshold of the JAK2Stroke cohort corresponding to the GESUS cohort, the prevalence in patients with ischemic stroke was markedly higher (9.9% vs 4.4%). The JAK2Stroke cohort presented with increased platelet levels and white blood cell counts; conversely, the control cohort presented with increased hematocrit, whereas there was no significant difference in hemoglobin levels between cases and controls. In a multiple-adjusted conditional logistic regression model, the odds ratio (OR) for the JAK2V617F mutation in patients with ischemic stroke vs controls free of ICVD was 2.37 (95% confidence interval [CI], 1.57-3.58; P < .001; Table 4). Excluding patients with MPN, the OR for the JAK2V617F mutation in patients with ischemic stroke vs controls was 1.90 (95% CI, 1.23-2.93; P = .004). Stratifying based on smoking history, the OR for the JAK2V617F mutation in patients with ischemic stroke was 4.78 (95% CI, 2.22-10.28; P < .001) compared with controls who were current smokers, whereas the association was not present among former or never smokers (Figure 1).
Conditional logistic regression analysis of JAK2V617F in patients with ischemic stroke vs controls
| Covariates . | OR . | 95% CI . | P . |
|---|---|---|---|
| Ischemic stroke | 2.37 | 1.57-3.58 | <.001 |
| Hypertension | 1.00 | 0.67-1.50 | .992 |
| Dyslipidemia | 1.00 | 0.64-1.56 | .997 |
| Diabetes | 0.83 | 0.44-1.55 | .553 |
| Ischemic heart disease | 1.25 | 0.74-2.11 | .394 |
| Atrial fibrillation | 1.26 | 0.72-2.21 | .413 |
| Smoking | 1.05 | 0.82-1.35 | .705 |
| Covariates . | OR . | 95% CI . | P . |
|---|---|---|---|
| Ischemic stroke | 2.37 | 1.57-3.58 | <.001 |
| Hypertension | 1.00 | 0.67-1.50 | .992 |
| Dyslipidemia | 1.00 | 0.64-1.56 | .997 |
| Diabetes | 0.83 | 0.44-1.55 | .553 |
| Ischemic heart disease | 1.25 | 0.74-2.11 | .394 |
| Atrial fibrillation | 1.26 | 0.72-2.21 | .413 |
| Smoking | 1.05 | 0.82-1.35 | .705 |
Covariates: hypertension, dyslipidemia, diabetes, ischemic heart disease, atrial fibrillation, and smoking as interdependent variables. P values < 0.05 are shown in bold.
Conditional logistic regression analysis of JAK2V617F in patients with ischemic stroke vs GESUS controls free of ischemic cerebrovascular disease (matched 1:3) according to their smoking history. The analyses are adjusted for hypertension, dyslipidemia, diabetes, ischemic heart disease, and atrial fibrillation.
Conditional logistic regression analysis of JAK2V617F in patients with ischemic stroke vs GESUS controls free of ischemic cerebrovascular disease (matched 1:3) according to their smoking history. The analyses are adjusted for hypertension, dyslipidemia, diabetes, ischemic heart disease, and atrial fibrillation.
Discussion
Our research is the first study, to our knowledge, on the JAK2V617F mutation using a highly sensitive ddPCR assay in a large cohort of consecutively enrolled patients with ischemic stroke, revealing a prevalence of the JAK2V617F mutation of 11.3%. The odds of having the JAK2V617F mutation were 2 times higher than that among matched controls overall and 4 times higher in current smokers than in matched controls.
This study presents a unique opportunity to compare the prevalence of ischemic stroke with the background prevalence, because the GESUS and JAK2Stroke populations were from the same geographical region of Denmark, and the studies used the same molecular analysis for JAK2V617F detection. In the total GESUS population study, the JAK2V617F mutation was found in 3.1% of the general population of all ages (mean age = 55.8). From the GESUS population, we created an age- and sex-matched control group free of ICVD for comparison with the JAK2Stroke cohort and found that the JAK2V617F mutation was associated with ischemic stroke, even after adjusting for other cerebrovascular risk factors. Notably, this association was present even when patients with MPN were excluded from the analysis.
Elevated blood cell counts
In extensive population studies, increased white blood cell count has been associated with the risk of stroke, coronary heart disease, and cardiovascular death.41,42 High hemoglobin levels are associated with the severity, prognosis, and risk of stroke.43-46 Likewise, in patients with MPNs, thrombosis is associated with elevated red and white blood cells22,23,47 and the JAK2V617F mutation.48 Thus, it is reasonable to suspect that elevated blood cell levels mediate increased susceptibility to thrombosis in individuals with the JAK2V617F mutation, and it has been proposed that JAK2V617F analysis should be restricted to patients with elevated blood cell levels.49 However, we found no difference in the proportion of patients who presented with hemoglobin, hematocrit, or white blood cell values above the normal range between the 2 groups, whereas significantly more patients had platelet counts above the normal range in the group with the JAK2V617F mutation. Within the reference range, patients with the JAK2V617F mutation had increased hemoglobin, hematocrit, and platelet counts compared with those without the mutation. Furthermore, the JAK2Stroke cohort presented with increased hematocrit, platelets, and white blood cells compared with the matched control cohort. This indicates that blood cell hyperproliferation plays a role in the risk of thrombosis; however, using the standard reference range to distinguish individuals at risk of having the JAK2V617F mutation is insufficient. Notably, 7 out of 8 patients who were subsequently diagnosed with MPN had elevated levels of at least 1 blood cell line, but only 2 had mild leukocytosis. To detect patients with undiagnosed MPN, screening patients with stroke with elevated blood cell levels could be effective. However, more than half of the patients with JAK2V617F-CHIP would then be left out of identification. Based on our results, these individuals are at an increased risk of thrombosis and need special attention and, possibly, a specific treatment strategy. Particularly smoking cessation is important in this population of patients, as revealed by our results.
Previous studies
Several other studies have assessed the prevalence of the JAK2V617F mutation in patients with ischemic stroke50-57 (Table 5), yet they differ from our study in 3 important ways. First, most previous studies only included patients with ischemic stroke who were referred for thrombophilia testing. This represents a selected group of typically young patients with stroke and limited competing cerebrovascular risk factors. Studies presumably build on the assumption that the JAK2V617F mutation is more prevalent in young and otherwise healthy patients with stroke than in those with known cerebrovascular risk factors. The contrary is likely to be the case because the risk of thrombosis in patients with MPN is associated with the same cardiovascular risk factors as those in the general population, and patients with MPN have a significant burden of these comorbidities.58-60 In agreement, our results underline that patients with stroke and the JAK2V617F mutation were as likely to have other cerebrovascular risk factors and were of the same age as the average patients with stroke. Only 1 further study evaluated consecutively enrolled patients with stroke (N = 153) and found a prevalence of 8.5%, a high prevalence in line with our results.55 Secondly, all previous studies were based on relatively small study populations (N < 200); thus, the estimated frequencies were based on a small number of individuals who had the mutation. The last significant difference was in the methodology and detection limits of molecular JAK2V617F analysis. Previous studies used allele-specific PCR or quantitative PCR, with detection limits varying from 0.01% to 5%. Using ddPCR, we quantified mutated alleles with a median detection limit of 0.0036%.
Previous studies of JAK2V617F mutation prevalence in patients with stroke
| Author, y . | Population . | N . | No. of patients with JAK2V617F mutation (%) . | Age (median) . | Exclusion . | Assay detection limit . |
|---|---|---|---|---|---|---|
| Pardanani et al, 200750 | Patients with stroke referred for thrombophilia testing | 136 | 1 (0.7%) | 56 | Overt MPN | ≤1% |
| Xavier et al, 200851 | Patients with stroke referred for thrombophilia testing | 178 | 2 (1%) | 37 | Overt MPN | 3% |
| Smalberg et al, 200852 | Patients with first ever ischemic stroke, below age 75, with nonatherosclerotic cause | 51 | 0 | NR | Overt MPN | NR |
| Abel et al, 200853 | Patients with stroke referred for thrombophilia testing, without factor V leiden and protrombin gene mutation | 10 | 0 | NR | Overt MPN, malignancy, and HIT | >5% |
| Zerjavic et al, 201054 | Patients with stroke referred for thrombophilia testing | 60 | 2 (3.3%) | 49 | Hematological malignancy, and SVT | NR |
| Chen et al, 201755 | Consecutive patients with ischemic stroke | 153 | 13 (8.5%) | 66.3 (mean) | NR | 0.1% |
| Levraut et al, 202056 | Patients with stroke referred for thrombophilia testing | 30 | 3 (10%) | NR | Laboratory results suggestive of MPN | 0.05% |
| Janjetovic et al, 202157 | Patients with stroke referred for thrombophilia testing | 129 | 8 (6.2%) | NR | Overt MPN | 0.01% |
| Author, y . | Population . | N . | No. of patients with JAK2V617F mutation (%) . | Age (median) . | Exclusion . | Assay detection limit . |
|---|---|---|---|---|---|---|
| Pardanani et al, 200750 | Patients with stroke referred for thrombophilia testing | 136 | 1 (0.7%) | 56 | Overt MPN | ≤1% |
| Xavier et al, 200851 | Patients with stroke referred for thrombophilia testing | 178 | 2 (1%) | 37 | Overt MPN | 3% |
| Smalberg et al, 200852 | Patients with first ever ischemic stroke, below age 75, with nonatherosclerotic cause | 51 | 0 | NR | Overt MPN | NR |
| Abel et al, 200853 | Patients with stroke referred for thrombophilia testing, without factor V leiden and protrombin gene mutation | 10 | 0 | NR | Overt MPN, malignancy, and HIT | >5% |
| Zerjavic et al, 201054 | Patients with stroke referred for thrombophilia testing | 60 | 2 (3.3%) | 49 | Hematological malignancy, and SVT | NR |
| Chen et al, 201755 | Consecutive patients with ischemic stroke | 153 | 13 (8.5%) | 66.3 (mean) | NR | 0.1% |
| Levraut et al, 202056 | Patients with stroke referred for thrombophilia testing | 30 | 3 (10%) | NR | Laboratory results suggestive of MPN | 0.05% |
| Janjetovic et al, 202157 | Patients with stroke referred for thrombophilia testing | 129 | 8 (6.2%) | NR | Overt MPN | 0.01% |
HIT, heparin-induced thrombocytopenia; NR, not reported; SVT, splanchnic venous thrombosis.
Low allele burdens
In most patients in our study (75%), the JAK2V617F allele burden was <1%. The clinical significance of a mutation allele burden <1% is uncertain. However, it is essential to emphasize that a subset of citizens in the GESUS cohort with a mutational load of ∼1% without elevated blood cell numbers displayed abnormal bone marrow changes, albeit not being diagnostic for MPN.61 Previous follow-up population studies have shown that individuals with a JAK2V617F mutation allele burden >2% have an increased risk of developing MPN.62 However, we are yet to determine the long-term consequences of the very low JAK2V617F allele burden. It is reasonable to believe that the mutated clone is expanding over time, inducing a hyperproliferative state and eventually progressing to overt MPN. This evolution is likely influenced by several competing factors, including germ line predisposition, lifestyle factors (eg, overweight and smoking), and an increased frequency of infections.63,64 This also implies that a large proportion of citizens may harbor the JAK2V617F mutation without developing MPN. Even if these individuals never develop MPN, they may be susceptible to other inflammation-mediated diseases, such as age-related macular degeneration,65,66 cardiovascular diseases (eg, stroke, coronary disease, aortic and valve calcifications, aneurysms, and peripheral arterial insufficiency),15-17 inflammatory bowel diseases,67 or chronic kidney disease.68
Smoking
Smoking is a risk factor for the development of MPN and likely also for the acquisition or expansion of the JAK2V617F mutation.64,69 Similarly, smoking is a major risk factor for the development of ischemic stroke and other cardiovascular diseases.70 To examine the impact of this confounder, we performed a new matching according to smoking status and a subsequent stratified conditional logistic regression analysis. This revealed that the association between the JAK2V617F mutation and ischemic stroke is driven mainly in current smokers, emphasizing the importance of smoking cessation, particularly in this group of patients.
Strengths and limitations
The major strength of our study was that a large number of patients with ischemic stroke were included. In addition, consecutive enrollment and limited exclusion criteria ensured a representative stroke population. Furthermore, we used a highly sensitive ddPCR method. Patients who could not provide informed consent were excluded. This led us to exclude patients with aphasia, dementia, or severe stroke that resulted in reduced consciousness. Because the JAK2V617F mutation might promote an increased inflammatory response to cerebrovascular insult, we cannot rule out the possibility that some severely affected patients may have this mutation. However, this would only strengthen our finding of a strong association between JAK2V617F mutation and stroke. The control group was created based on registration data, whereas the variables for the cases were registered based on the manual assessment of patient journals; hence, there is a risk of inconsistency in how covariates are reported. We recognize that participants in the population cohorts are typically healthier than the average population; thus, this design entails a risk of participation bias. We matched the cases based on age and sex and further adjusted the conditional logistic regression analysis according to the cerebrovascular risk factors. Based on these means, we sought to minimize the risk that the association between the JAK2V617F mutation and stroke was based on the heavier burden of cerebrovascular comorbidities in these cases. Notably, there was a 10-year time difference between the sampling of cases and controls; however, neither the molecular nor biochemical analyses nor the treatment or registration of the diseases changed markedly during this period. As mentioned, the JAK2V617F mutation possibly occurs and/or expands due to chronic or severe infections. From this perspective, the recent COVID-19 pandemic might have increased the overall prevalence of the mutation compared with the background prevalence 10 years ago. To address this, we assessed the number of patients who had been hospitalized for COVID-19 before inclusion. We found that only 10 patients in the entire cohort had a history of admission for COVID-19, none of whom had the JAK2V617F mutation. Although we were not able to account for mild and self-limitating infections, based on these low numbers, it does not seem as if the increased prevalence in cases is explained by the COVID-19 pandemic.
Conclusions and perspectives
Interestingly, we find the JAK2V617F mutation was associated with ischemic stroke when adjusted for other cerebrovascular risk factors. This suggests that the JAK2V617F mutation itself induces a prothrombotic state that goes beyond the hyperproliferation of blood cells alone, possibly through the activation of thromboinflammatory systems, as has been observed in patients with MPN.7,22,71
Our results emphasize the need for a systematic JAK2V617F screening of patients with ischemic stroke and other thrombotic events and a subsequent routine follow-up of individuals with JAK2V617F mutation, preferably in specialized outpatient clinics ("CHIP clinics"). This would benefit many individuals with undiagnosed or prestage MPN who suffer from repeated life-invalidating or life-threatening thrombotic episodes several years before MPN diagnosis.27 Our results imply that individuals with CHIP-JAK2V617F have an increased risk of stroke. In patients with MPN, early treatment with interferon–alfa 2 may reduce this risk by lowering elevated blood cell counts and JAK2V617F allelic burden.22,72 In the general population, statins protect against MPN development.73 Thus, it is intriguing to consider whether treating individuals with CHIP receiving interferon–alfa 2 and/or a statin might have a reduction or even elimination of the JAK2V617F mutation, prohibiting CHIP-JAK2V617F development toward overt MPN.74 Considering the huge health care costs associated with treatment and rehabilitation after stroke, such a perspective will benefit both patients and the society. We believe that our results are of utmost significance, hopefully paving the path for promoting the JAK2V617F mutation as a novel risk factor for cerebrovascular disease and a compulsory part of stroke evaluation.
Thus, further research on the pathophysiological mechanisms of the JAK2V617F mutation leading to thrombosis is required. In addition, there are potential future targeted treatments for individuals with the JAK2V617F mutation, such as the possibility of treatment with pegylated interferon.
Acknowledgments
The authors thank the Department of Clinical Biochemistry for performing the molecular analysis and the study nurse Sarah Berg Brink Jensen for her assistance in collecting data. The authors thank Editage for the English language editing.
Grants were provided by the Greater Copenhagen Health Science Partners, Region Zealand Research Foundation, Toyota Foundation, Harboe Foundation, A.P. Moeller Foundation, Carpenter Sophus Jacobsen and Spouse Astrid Jacobsen Scholarship, Manufacturer Einar Willumsens Scholarship, Merchant A.V. Lykfeldt Scholarship, and the Department of Neurology, Zealand University Hospital, Denmark. GESUS was funded by the Region Zealand Research Foundation, Naestved Hospital Foundation, Naestved Municipality, Johan and Lise Boserup Foundation, TrygFonden, Johannes Fog’s Foundation, Region Zealand, Naestved Hospital, The National Board of Health, and The Local Government Denmark Foundation; Denmark. C.E. is partly funded by the Laboratory Medicine Endowment Fund of Boston Children's Hospital.
M.H.K. is a PhD Candidate at the University of Copenhagen. This work is submitted in partial fulfillment of the requirement for the PhD.
This work was supported by public and nonprofit organizations that support science in general. They had no role in gathering, analyzing, or interpreting the data.
Authorship
Contribution: M.H.K., L.K., V.S., M.K.L., C.E., H.C.H., and T.W. designed the study; M.H.K. and M.K.L. analyzed and interpreted the data; and M.H.K. collected the data and wrote the manuscript, with all coauthors contributing to revisions and improvements.
Conflict-of-interest disclosure: H.C.H. has received a research grant from Novartis. The remaining authors declare no competing financial interest.
Correspondence: Marie Hvelplund Kristiansen, Department of Neurology, Zealand University Hospital, Vestermarksvej 11, 4000 Roskilde, Denmark; e-mail: mariehvelplundkristiansen@gmail.com.
References
Author notes
Data are available on request from the corresponding author, Marie Hvelplund Kristiansen (mariehvelplundkristiansen@gmail.com).
The full-text version of this article contains a data supplement.