TO THE EDITOR:
We were intrigued by the recent publication from Kristiansen et al1 in Blood Advances, which presents findings regarding the prevalence of the JAK2V617F mutation in patients with ischemic stroke (IS). Using a case-control design with 538 IS cases and 1613 age- and sex-matched controls free of ischemic cerebrovascular disease, the study unveiled a statistically significant disparity in the occurrence of this somatic mutation. Notably, 11% of patients with stroke harbored the JAK2V617F mutation, compared with 4.4% in the control group, yielding an odds ratio of 4.78 (P value = .001), corresponding to 2.4 times greater likelihood to harbor the JAK2V617F mutation when adjusted for other cerebrovascular risk factors.
The authors claimed that this mutation leads to a thrombophilic state characterized by excessive hematopoietic proliferation, changes in blood counts, and a concurrent state of chronic inflammation.
Notably, in previous studies the frequency of the clonal hematopoiesis of undetermined significance (CHIP) JAK2V617 mutation defined as the presence of a variant allele fraction (VAF) ≥2% was much lower than that now reported, being 0.17% among 1152 patients with IS.2 In the study of Kristiansen et al,1 75% of the carriers of JAK2V617F had a VAF <1%, suggesting that the mutation is associated with a severe vascular event even with very low VAF values.
The JAK2V617F mutation was found in patients with stroke who did not have the typical features of chronic myeloproliferative disease, a condition known as CHIP, which is itself considered a risk factor for vascular complications. These findings corroborate previous observational studies3-5 and provide a quantitative estimate of the prevalence of the JAK2V617F mutation in IS compared with controls, opening the door to prospective clinical trials of pharmacological intervention. The authors also reasoned that recurrent IS associated with the JAK2 mutation may be amenable to treatment with drugs, such as interferon-alfa (IFNα), which can modulate hematopoietic proliferation, whereas anti-inflammatory drugs, such as statins, may help alleviate the associated inflammatory state. However, no information is provided on whether patients with JAK2V617F are at higher risk to develop recurrences after stroke. Clearly there is a need for clinical trials, whose feasibility appears uncertain given the relative rarity of the JAK2 mutation in patients with CHIP without the clinical phenotype of a myeloproliferative neoplasia. Nevertheless, the rationale for future intervention studies in patient groups with clonal hematopoiesis and an increased risk of IS recurrence can be built upon recent findings in diseases with the same mutation, as in patients with overt myeloproliferative neoplasms (MPN).
Over the years, knowledge has grown in understanding the antithrombotic properties of cytoreductive drugs, such as hydroxyurea (HU), IFN, and ruxolitinib (RUXO), in the context of chronic myeloproliferative diseases,6 particularly in polycythemia vera (PV), essential thrombocythemia (ET) and myelofibrosis, in which the JAK2 mutation is prevalent in 95% and 50% to 60% of cases, respectively and is recognized as an independent risk factor for arterial vascular events in ET,7 whereas in PV, it has been associated with venous complications.8 Furthermore, recent data provided accurate quantitative estimates of vascular recurrences in patients with MPN treated with HU.9
Inspired by the study of Kristiansen et al, we reviewed the database of our recent observational study involving many European centers affiliated with the European LeukemiaNet to describe vascular recurrences in patients with index transient ischemic attack (TIA) and IS.10 The preventing ischemic stroke in myeloproliferative neoplasms (PRISM) study collected retrospective data on 597 patients with MPN, of whom 270 had TIA and 327 had IS. Secondary prophylaxis included aspirin or oral anticoagulants, with the majority of patients receiving HU (∼80%) or other cytoreductive drugs (ie, anagrelide, IFN, and RUXO in 7% of patients). Baseline blood counts and other clinical characteristics were largely similar between the TIA and IS groups, except for a higher prevalence of arterial hypertension in patients with IS and a greater incidence of microvascular disturbances in the TIA group. Criteria for recurrences in the PRISM study were those conventionally defined as a focal neurologic deficit of any duration with evidence at neuroimaging of brain ischemia located in an area not corresponding to the lesion(s) of the index stroke.
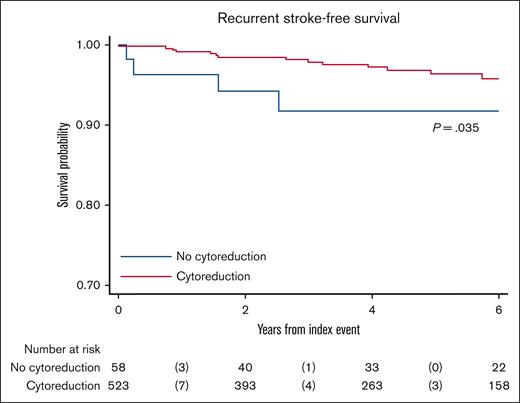
Comparison of the 1-year and 5-year cumulative incidence of recurrent strokes in PRISM study with cohorts of patients with non-MPN shows marked lower values in patients with MPN (Figure 1). A limitation of this observation may be the relatively low number of patients at risk in comparison to non-MPN cases, as reported in 1 meta-analysis and 2 registries.10-13 The figure also shows a clear reduction in stroke recurrence from 2011 to 2020 in the general population, although it never reaches the estimates obtained in patients with MPN. In the PRISM study, after adjustment for several factors, including sex, age, blood cell counts, atrial fibrillation, cardiovascular risk factors, and antithrombotic treatment, cytoreductive therapy emerged as a potent protective factor, reducing the likelihood of new IS by 76% (hazard ratio = 0.24).10 The beneficial effect by cytoreduction (mainly HU) was evident in the first months of treatment, whereas in cases without cytoreduction, recurrences occurred with equal frequency in the first 3 years after the index event (Figure 2).
Recurrent strokes in MPN and general population. Cumulative estimates of stroke recurrence at 1 and 5 years after the acute phase are reported in the PRISM study10 and compared with the results of a meta-analysis of studies from hospital–based or community–based stroke registers11 and 2 population-based registries (the South London stroke register12 and monitoring trends and determinants of cardiovascular disease stroke incidence registry).13
Recurrent strokes in MPN and general population. Cumulative estimates of stroke recurrence at 1 and 5 years after the acute phase are reported in the PRISM study10 and compared with the results of a meta-analysis of studies from hospital–based or community–based stroke registers11 and 2 population-based registries (the South London stroke register12 and monitoring trends and determinants of cardiovascular disease stroke incidence registry).13
Kaplan-Meier estimates of stroke recurrent-free survival by cytoreductive drug. Patients on cytoreductive drugs (HU in 87%; n = 523) were compared with patients without cytoreductive agents (n = 28) during follow-up since the index event (median time on follow-up, 4.2 years; range, 0–11.9). P value from the log-rank test is reported.
Kaplan-Meier estimates of stroke recurrent-free survival by cytoreductive drug. Patients on cytoreductive drugs (HU in 87%; n = 523) were compared with patients without cytoreductive agents (n = 28) during follow-up since the index event (median time on follow-up, 4.2 years; range, 0–11.9). P value from the log-rank test is reported.
In light of these findings, it appears that targeting myeloid cells with HU can effectively reduce the risk of recurrent IS. This raises the possibility that this treatment could also be beneficial for the broader non-MPN population, particularly in cases with CHIP JAK2V617F mutation and possibly other mutations. In this regard, in a recent large prospective cohort of 581 patients with IS, clonal hematopoiesis with VAF >1% was found in 41% of cases, specifically represented by mutations in the DNMT3A, TET2, and ASXL1 genes.14 Importantly, cases harboring these somatic mutations had a higher risk of recurrent vascular events and death, confirming similar findings in other vascular diseases, such as myocardial infarction, heart failure, or aortic stenosis.3-5 In this study, the clone size remained an independent risk factor for recurrence after multivariate analysis,14 so that a benefit obtained by reducing the allele burden with cytoreductive agents might be reasonably suggested.
Thus, the accumulated evidence in patients with myeloproliferative neoplasms, suggests that clonal hematopoiesis may play a role in increasing the risk of recurrent stroke and other vascular complications, and cytoreductive therapy has been shown to be effective in reducing such recurrences. One is tempted to speculate that a similar scenario occurs in non-MPN individuals with JAK2V617F CHIP, in whom cytoreductive agents could be instrumental in controlling the resulting inflammation stemming from leukocyte and platelet activation and minimizing the release of inflammatory cytokines and chemokines.15 In these cases, the use of IFN might be more appealing than HU, given its documented efficacy in reducing the JAK2V617F allele burden while normalizing blood counts and possibly preventing clonal expansion to overt MPN disease development.15
Clearly, appropriate clinical trials are needed to explore the potential benefits arising from the use of cytoreductive agents in patients with CHIP, although the feasibility of such trials remains a major matter of debate. Furthermore, before embarking on such projects, it would be prudent to identify which patient with CHIP groups are at markedly increased risk for potentially fatal or highly debilitating relapses using clinical risk criteria, hematologic variables, and appropriate biomarkers of inflammation. Moreover, considering that JAK2V617F mutation has been reported to have a thrombotic potential even at low VAF values, the role of the clone size as risk factor for recurrence should be better outlined.
Acknowledgments: The study was sponsored by Fondazione per la Ricerca Ospedale di Bergamo (FROM) - Ente del Terzo Settore and was supported by a grant from BCC Milano for the RICO project.
Contribution: T.B. and A.C. contributed equally to manuscript writing; A.C. performed statistical analysis of PRISM study; A.M.V. and V.D.S. critically revised the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tiziano Barbui, FROM Research Foundation, Papa Giovanni XXIII Hospital Piazza OMS, 1 Bergamo 24127, Italy; email: tbarbui@fondazionefrom.it.
References
Author notes
Data are available upon request from the corresponding author, Tiziano Barbui (tbarbui@fondazionefrom.it).