Key Points
Vaccinated patients with hematologic malignancies have lower hospitalization rates for COVID-19 than unvaccinated patients.
COVID-19 vaccination did not reduce risk of death in patients with hematologic malignancies.
Abstract
Patients with hematologic malignancies have both an increased risk for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and higher morbidity/mortality. They have lower seroconversion rates after vaccination, potentially leading to inferior coronavirus disease 2019 (COVID-19) outcomes, despite vaccination. We consequently evaluated the clinical outcomes of COVID-19 infections in 243 vaccinated and 175 unvaccinated patients with hematologic malignancies. Hospitalization rates were lower in the vaccinated group when compared with the unvaccinated group (31.3% vs 52.6%). However, the rates of COVID-19–associated death were similar at 7.0% and 8.6% in vaccinated and unvaccinated patients, respectively. By univariate logistic regression, females, older patients, and individuals with higher modified Charlson Comorbidity Index scores were at a higher risk of death from COVID-19 infections. To account for the nonrandomized nature of COVID-19 vaccination status, a propensity score weighting approach was used. In the final propensity-weighted model, vaccination status was not significantly associated with the risk of death from COVID-19 infections but was associated with the risk of hospitalization. The predicted benefit of vaccination was an absolute decrease in the probability of death and hospitalization from COVID-19 infections by 2.3% and 22.9%, respectively. In conclusion, COVID-19 vaccination status in patients with hematologic malignancies was associated with a decreased risk of hospitalization but not associated with a decreased risk of death from COVID-19 infections in the pre-Omicron era. Protective strategies, in addition to immunization, are warranted in this vulnerable patient population.
Introduction
Patients with cancer, especially those with leukemias and lymphomas, are not only at a significantly increased risk for coronavirus disease 2019 (COVID-19) infections1 but also experience substantial morbidity and mortality from this infectious disease.2-4 Various vaccinations have been developed against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with a consequent decrease in the incidence and severity of COVID-19 infections in the general population.5-7 However, there have been demonstrations of decreased immune responses and lower seroconversion rates after vaccination in individuals with hematologic malignancies,8-10 leading to concern of suboptimal responses to COVID-19 immunizations.11 The efficacy of SARS-CoV-2 vaccination in preventing serious, life-threatening infection remains unknown in patients with hematologic malignancies. We aimed to determine the clinical outcomes of COVID-19 infections in both vaccinated and unvaccinated individuals in this patient population.
Methods
To study this, we performed a retrospective review of patients with hematologic malignancies, at The University of Texas MD Anderson Cancer Center, who contracted COVID-19 between December 2020 (after the availability of vaccinations) and 31 October 2021, before the emergence of the Omicron variant but encompassing the time frame over which the Delta variant became predominant in the United States. Patient demographics/clinical data, COVID-19 vaccination and infection dates, and outcome data were collected as part of the institutional Data-Driven Determinants for COVID-19 Oncology Discovery Effort, institutional review board–approved protocol 2020-0348. Data were obtained from structured and unstructured electronic medical record elements, clinical note text, and laboratory reports. Each source was identified and data were integrated and analyzed using the Palantir Foundry platform (Syntropy), which is part of the Context Engine data management system at The University of Texas MD Anderson Cancer Center. Specifics regarding the hematologic malignancies, treatment received, and causes of death were collected manually. Patients were considered to be vaccinated if they received any number of COVID-19 immunizations before their first known SARS-CoV-2 infection.
Statistical analyses were performed using R version 4.1.3.12 Categorical demographic and clinical variables were summarized by number and percentage and compared across groups using a χ2 test. Continuous demographic and clinical variables were summarized by median and range and compared across groups using a t test. Overall survival was calculated from the start of the first COVID-19 infection until death from any cause. Survival estimates were obtained using the Kaplan-Meier method and compared between groups using the log-rank test. The cumulative incidence of deaths owing to COVID-19 was computed considering deaths owing to other causes as a competing risk.12,13 Univariate logistic regression was used to determine the basic association of covariates to risk of death from COVID-19 infection, which was defined as a patient with a positive COVID-19 molecular or antigen test who developed COVID-19–related illness, usually pneumonia, and died without fully recovering from COVID-19 infections and had no alternative causes of death identified. Multivariate analysis was not performed because of low patient numbers. To account for the nonrandomized nature of COVID-19 vaccination status, because patients self-selected whether or not to be vaccinated, we relied on a propensity score weighting approach.14 Propensity score weighting was chosen over matching because matching would necessitate excluding patients and thereby discarding data. Propensity scores were obtained using a logistic regression of COVID-19 vaccination status on age, gender, and modified Charlson Comorbidity Index (CCI), with the removal of cancer scoring, disease group, race/ethnicity, and transplant status.15 Using the inverse probability of treatment weighting approach, stabilized weights were estimated based on the propensity scores. Covariate balance in the weighted sample was assessed using standardized differences. These weights were then used in a weighted logistic regression model to evaluate the association between vaccination status and risk of both death and hospitalization from COVID-19.
Results
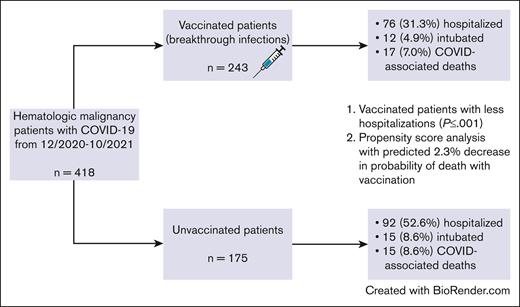
Patient characteristics
A total of 418 patients with hematologic malignancies contracted COVID-19 infections between December 2020 and October 2021, including 243 vaccinated patients that developed breakthrough COVID-19 infections and 175 unvaccinated individuals. In the vaccinated individuals, the type of immunization was available for 224 patients (92.2%); 147 patients (60.5%) received SARS-CoV-2 vaccination with Pfizer-BioNTech (Comirnaty), 65 (26.7%) with Moderna (Spikevax), and 12 (4.9%) with Johnson & Johnson/Janssen. The number of vaccine doses received before COVID-19 infections was known in 241 patients (99.2%) with a median of 2 doses (range, 1-3 doses) and a median time of 5.1 months (range, 0.1-10.2 months) from the time of last vaccination to COVID-19 infection. The median follow-up time for the entire patient cohort was 3.9 months, and 19 patients did not have follow-up information after the date of COVID-19 infection.
Table 1 summarizes baseline patient characteristics, laboratory values, and hematologic malignancy status. Vaccinated patients tended to be older with more comorbidities by the modified CCI, whereas more unvaccinated patients underwent stem cell transplantation or cellular therapy, with lower hemoglobin levels. A higher proportion of vaccinated individuals had B-cell lymphomas, whereas a greater proportion of those who were unvaccinated had plasma cell dyscrasias.
Patient characteristics
| . | N (%)/median (range) . | P value . | |
|---|---|---|---|
| Vaccinated (n = 243) . | Unvaccinated (n = 175) . | ||
| Age, y | 67 (24-89) | 56 (19-88) | < .001 |
| Gender | |||
| Female | 94 (39) | 71 (41) | .70 |
| Male | 149 (61) | 104 (59) | |
| Modified CCI∗ | 3 (0-8) | 2 (0-14) | < .001 |
| Disease type | |||
| BCL | 131 (54) | 63 (36) | <.001 |
| Chronic lymphocytic leukemia | 54 (23) | 30 (17) | |
| Low-grade BCL | 44 (18) | 16 (9) | |
| High-grade BCL | 33 (14) | 17 (9) | |
| Plasma cell dyscrasia | 37 (15) | 18 (10) | |
| Other | 75 (31) | 94 (54) | |
| Myelodysplastic syndrome/acute myeloid leukemia | 31 (13) | 37 (21) | |
| Myeloproliferative neoplasm/chronic myeloid leukemia | 18 (7) | 22 (13) | |
| Acute lymphoblastic leukemia | 8 (3) | 15 (9) | |
| Hodgkin lymphoma | 8 (3) | 10 (6) | |
| T-cell leukemia/lymphoma | 7 (3) | 5 (5) | |
| Disease/treatment status | |||
| Untreated | 31 (13) | 30 (17) | .34 |
| In remission | 150 (62) | 97 (55) | |
| With active disease | 62 (26) | 48 (27) | |
| On active therapy | 121 (50) | 86 (49) | .90 |
| Received stem cell transplantation/cellular therapy | 46 (19) | 48 (27) | .04 |
| Baseline laboratory values† | |||
| White blood cell, 103/μL | 5.3 (0.2-102.3) | 5.4 (0.1-200.7) | .88 |
| Absolute neutrophil count, 103/μL | 3.1 (0.0-41.1) | 3.0 (0.0-32.6) | .77 |
| Hemoglobin, g/dL | 12.6 (5.5-17.5) | 11.8 (6.0-16.9) | .051 |
| Platelets, 103/μL | 177 (1-600) | 162 (1-747) | .21 |
| Creatinine, mg/dL | 1.0 (0.4-5.1) | 0.9 (0.3-9.1) | .88 |
| Immunoglobulin G, mg/dL‡ | 730 (71-5230) | 772 (134-2797) | .82 |
| No. of vaccine doses received§ | |||
| 1 | 66 (27) | — | — |
| 2 | 163 (68) | — | — |
| 3 | 12 (5) | — | — |
| . | N (%)/median (range) . | P value . | |
|---|---|---|---|
| Vaccinated (n = 243) . | Unvaccinated (n = 175) . | ||
| Age, y | 67 (24-89) | 56 (19-88) | < .001 |
| Gender | |||
| Female | 94 (39) | 71 (41) | .70 |
| Male | 149 (61) | 104 (59) | |
| Modified CCI∗ | 3 (0-8) | 2 (0-14) | < .001 |
| Disease type | |||
| BCL | 131 (54) | 63 (36) | <.001 |
| Chronic lymphocytic leukemia | 54 (23) | 30 (17) | |
| Low-grade BCL | 44 (18) | 16 (9) | |
| High-grade BCL | 33 (14) | 17 (9) | |
| Plasma cell dyscrasia | 37 (15) | 18 (10) | |
| Other | 75 (31) | 94 (54) | |
| Myelodysplastic syndrome/acute myeloid leukemia | 31 (13) | 37 (21) | |
| Myeloproliferative neoplasm/chronic myeloid leukemia | 18 (7) | 22 (13) | |
| Acute lymphoblastic leukemia | 8 (3) | 15 (9) | |
| Hodgkin lymphoma | 8 (3) | 10 (6) | |
| T-cell leukemia/lymphoma | 7 (3) | 5 (5) | |
| Disease/treatment status | |||
| Untreated | 31 (13) | 30 (17) | .34 |
| In remission | 150 (62) | 97 (55) | |
| With active disease | 62 (26) | 48 (27) | |
| On active therapy | 121 (50) | 86 (49) | .90 |
| Received stem cell transplantation/cellular therapy | 46 (19) | 48 (27) | .04 |
| Baseline laboratory values† | |||
| White blood cell, 103/μL | 5.3 (0.2-102.3) | 5.4 (0.1-200.7) | .88 |
| Absolute neutrophil count, 103/μL | 3.1 (0.0-41.1) | 3.0 (0.0-32.6) | .77 |
| Hemoglobin, g/dL | 12.6 (5.5-17.5) | 11.8 (6.0-16.9) | .051 |
| Platelets, 103/μL | 177 (1-600) | 162 (1-747) | .21 |
| Creatinine, mg/dL | 1.0 (0.4-5.1) | 0.9 (0.3-9.1) | .88 |
| Immunoglobulin G, mg/dL‡ | 730 (71-5230) | 772 (134-2797) | .82 |
| No. of vaccine doses received§ | |||
| 1 | 66 (27) | — | — |
| 2 | 163 (68) | — | — |
| 3 | 12 (5) | — | — |
BCL, B-cell lymphoma.
Modified CCI incorporates all comorbidity variables of the original CCI15 except for solid tumor, leukemia, and lymphoma statuses.
Available in 219 vaccinated and 153 unvaccinated patients.
Available in 180 vaccinated and 115 unvaccinated patients.
Available in 241 vaccinated patients.
In total, 151 vaccinated (62.1%) and 116 unvaccinated patients (66.3%) received COVID-19–directed therapies (P = .44), which are summarized in Table 2. Unvaccinated patients more frequently received all therapies, with a statistically significant difference in remdesivir (P = .003) and methylprednisolone (P = .02) use, but more vaccinated patients received monoclonal antibodies (P = .04). This is consistent with the observed severity differences in infection: unvaccinated patients tended to be sicker and require admission to hospitals, in which remdesivir and methylprednisolone were used; vaccinated patients were more likely to have milder illness and thus received monoclonal antibodies with subsequent discharge from the emergency room without hospitalization.
COVID-19–directed therapies
| . | N (%) . | P value . | |
|---|---|---|---|
| Vaccinated (n = 243) . | Unvaccinated (n = 175) . | ||
| Remdesevir | 60 (25) | 67 (38) | .003 |
| Steroids | |||
| Dexamethasone | 45 (19) | 38 (22) | .42 |
| Methylprednisolone | 25 (10) | 32 (18) | .02 |
| Tocilizumab | 15 (6) | 15 (9) | .35 |
| Anakinra | 0 | 2 (1) | .17 |
| Monoclonal antibodies | 90 (37) | 47 (27) | .04 |
| . | N (%) . | P value . | |
|---|---|---|---|
| Vaccinated (n = 243) . | Unvaccinated (n = 175) . | ||
| Remdesevir | 60 (25) | 67 (38) | .003 |
| Steroids | |||
| Dexamethasone | 45 (19) | 38 (22) | .42 |
| Methylprednisolone | 25 (10) | 32 (18) | .02 |
| Tocilizumab | 15 (6) | 15 (9) | .35 |
| Anakinra | 0 | 2 (1) | .17 |
| Monoclonal antibodies | 90 (37) | 47 (27) | .04 |
Outcomes of COVID-19 infections
In the vaccinated group, 76 patients (31.3%) required hospitalization for COVID-19 infections, whereas 92 unvaccinated patients (52.6%) warranted hospitalization (P ≤ .001). Twelve vaccinated (4.9%) and 15 unvaccinated individuals (8.6%) required mechanical ventilation during their COVID-19–associated hospitalizations (P = .20). The median length of stay was 5 days for both the vaccinated and unvaccinated groups.
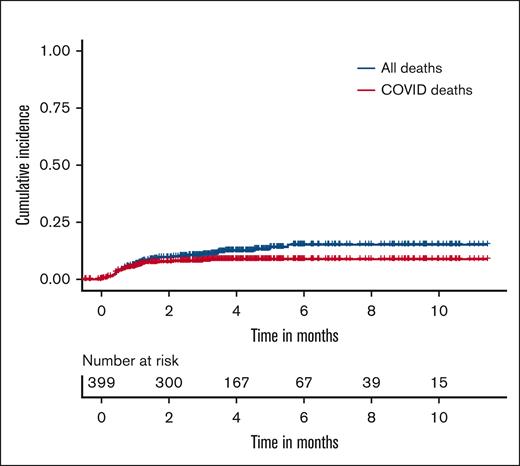
There were 47 deaths owing to any cause, with 24 deaths among vaccinated patients and 23 among unvaccinated patients, resulting in rates of death of 9.9% (95% confidence interval [CI], 6.6-14.5) and 13.1% (95% CI, 8.7-19.3), respectively. Among these, 32 deaths were due to COVID-19 infections. Figure 1 shows the cumulative incidence plot depicting deaths due to COVID-19 infections and deaths because of any cause.
Stacked cumulative incidence plot of COVID-19–associated deaths vs all deaths.
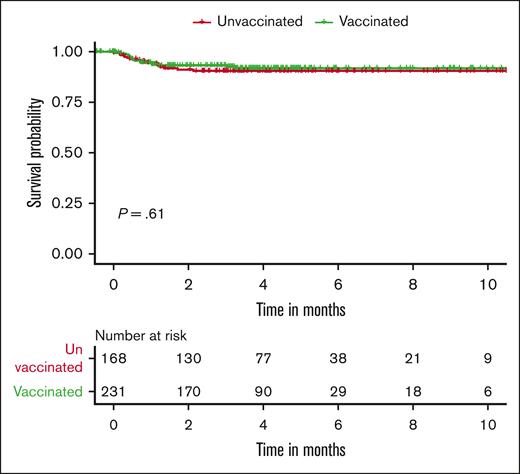
Regarding COVID-19–associated deaths, 17 occurred in the vaccinated cohort (rate of death, 7.0%; 95% CI, 4.3-11.1) and 15 in the unvaccinated group (rate of death, 8.6%; 95% CI, 5.0-14.0). Figure 2 represents the Kaplan-Meier curve for deaths due to COVID-19 infections stratified by vaccination status. There were no significant differences in COVID-19–associated deaths between the vaccinated and unvaccinated cohorts (odds ratio [OR], 0.80; 95% CI, 0.39-1.65; P = .61). The 30-day COVID-19 infection–related mortality for vaccinated and unvaccinated patients was 6.7% and 7.2%, respectively.
Exploratory analysis
Because only 27% of vaccinated patients received only 1 immunization dose before contracting SARS-CoV-2, the 175 fully vaccinated patients who received ≥2 doses were analyzed separately in comparison with the 175 unvaccinated patients. In the fully vaccinated patient group, there were 57 (32.6%) patients who required hospitalization (vs 92 unvaccinated [52.6%], P < .001), 8 (4.6%) patients who were intubated for mechanical ventilation (vs 15 unvaccinated [8.6%], P = .20), and 12 (6.9%) COVID-19–related deaths (vs 15 unvaccinated [8.5%], P = .69).
The association of vaccination status, age, gender, modified CCI, and disease group (B-cell lymphoma, plasma cell dyscrasia, and other) with the risk of death from COVID-19 infections was explored by univariate logistic regression. Females (OR, 2.77; P = .01), older patients (OR, 1.03; P = .02), and individuals with more comorbidities according to the modified CCI (OR, 1.32; P = .003) were at a higher risk of death from COVID-19 infections. There was no statistically significant association of vaccination status or disease type with the risk of death from COVID-19.
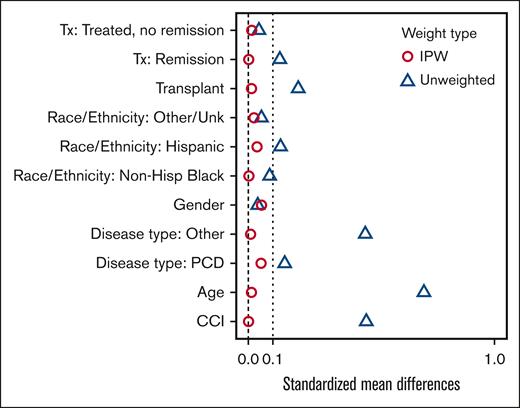
For the propensity score analysis, the standardized mean difference was controlled to be <0.1 across all covariates by inverse probability of treatment weighting, indicating that the covariates are well balanced in the weighted sample (Figure 3). In the final propensity-weighted model, vaccination status was not significantly associated with risk of death from COVID-19 infections (OR, 0.70; 95% CI, 0.33-1.50; P = .36). The predicted benefit of vaccination was an absolute decrease in the probability of death from COVID-19 infections by 2.3%. However, when applying the propensity-weighted model to hospitalization, vaccination status was significantly associated with risk of hospitalization (OR, 0.38; 95% CI, 0.25-0.57; P < .001). The predicted benefit of vaccination was an absolute decrease in the probability of hospitalization from COVID-19 infections by 22.9%.
Standardized mean differences by IPW vs unweighted covariates. Hisp, Hispanic; IPW, inverse probability of treatment weighting; PCD, plasma cell dyscrasias; Unk, unknown.
Standardized mean differences by IPW vs unweighted covariates. Hisp, Hispanic; IPW, inverse probability of treatment weighting; PCD, plasma cell dyscrasias; Unk, unknown.
Discussion
This retrospective study revealed that in 243 vaccinated and 175 unvaccinated individuals, rates of death due to COVID-19 infections were 7.0% and 8.6%, respectively, with no statistically significant differences in the risk of death due to COVID-19 between the 2 cohorts. However, a higher proportion of unvaccinated individuals required hospitalization, and showed a trend toward a higher likelihood of requiring intubation with mechanical ventilation. These results were consistent when comparing the 175 patients who received ≥2 vaccine doses with unvaccinated patients. Of note, there were more COVID-19–related deaths than patients who underwent mechanical ventilation, because some chose palliative/supportive care over intubation in the setting of their poorly controlled malignancies. By propensity score analysis, vaccination status was associated with a predicted benefit of vaccination of 2.3% that did not reach statistical significance, but a marked reduction in the absolute probability of hospitalization (22.9%).
Patients with hematologic malignancies have poor clinical outcomes from COVID-19 infections.16-22 A recent meta-analysis of 81 studies on oncologic patients and SARS-CoV-2 infections, revealed a pooled case fatality rate of 32% in patients with hematologic malignancies.23 The rates of death observed in our study are lower than those reported in the earlier literature, likely because of a combination of factors, including improved treatment options available for those with severe illness and a lesser degree of selection bias than was prevalent in early case series (in which testing was often restricted to those patients sick enough to require hospitalization). The markedly lower rates of hospitalization in vaccinated patients suggests that vaccination remains beneficial in patients with hematologic malignancies, despite the modest absolute reduction in mortality. The lack of statistically significant mortality benefit in our study may partly relate to insufficient numbers and to a relatively low number of events in both vaccinated and unvaccinated individuals. Although the death rates between vaccinated and unvaccinated patients were 7.0% and 8.6%, respectively, vaccinated individuals had significantly and markedly lower rates of hospitalization when compared with unvaccinated individuals in our study, indicating that vaccination still produces clinical benefit. There are conflicting data on the mortality benefits of COVID-19 vaccination in patients with hematologic malignancies. This lack of observed mortality benefit in our study corroborates another recent report.24 A larger study showed that 2 doses of immunization against COVID-19 may be effective against breakthrough infections, coronavirus hospitalization, and death within 28 days of a positive COVID-19 test. However, this study did not differentiate between death due to COVID-19 or other causes.25 Further investigation into the effects of SARS-CoV-2 vaccination in patients with cancer, especially those with hematologic malignancies, is warranted, with increasing importance as the SARS-CoV-2 virus evolves.
There are several limitations to the study. First, it is a nonrandomized study in which patients chose whether to be vaccinated against COVID-19. We relied on propensity score methods to reduce potential bias in associating vaccination status to the risk of death. However, propensity scores have important limitations, in particular, they can only be used to achieve balance on measured variables, and not unmeasured confounders. Socioeconomic factors, such as race/ethnicity, education levels, and health insurance; residing in urban or rural areas; previously receipt of vaccinations; and political partisanship have been shown to affect not only vaccine hesitancy, but also vaccine availability.26-28 It has also been shown that communities with lower education levels and greater percentages of black residents have higher rates of COVID-19 cases and fatalities.29 These elements are not captured or accounted for in our propensity score analysis. Secondly, because of the time frame of our study, most patients only underwent the primary SARS-CoV-2 immunization series, with 12 patients (4.9%) receiving 3 doses. Patients with hematologic malignancies have been found to have blunted immune responses to primary COVID-19 vaccination and subsequently lower vaccine effectiveness,30,31 which potentially may be because of waning humoral responses, especially in the immunosuppressed.32 Additional protection from boosters may improve vaccination results,33 and more studies regarding the potential impact of booster doses on patients with cancer are needed. Third, hematologic malignancies encompass a large variety of disease subtypes, with heterogeneity among groups; because of low patient numbers, our study was unable to assess whether particular subgroups of patients, such as those with lymphoid malignancies or those receiving B-cell–depleting therapies, may derive lesser benefit from vaccination than others. Our study was underpowered to definitively identify small absolute differences in mortality between vaccinated and unvaccinated patients. Moreover, the receipt of COVID-19–directed therapies was not balanced between the cohorts, which may have contributed to differential outcomes. Furthermore, our analysis does not capture the COVID-19 infection rates in all vaccinated vs unvaccinated patients with hematologic malignancies. Instead, we have evaluated outcomes of breakthrough infection. Part of the benefit of vaccination in the general population relates to reduction in the risk of infection, rather than just reduction in the severity of breakthrough infections. Therefore, the overall benefit of vaccination in patients with cancer may be greater than what we outline. Finally, these data may not be applicable in the era of the Omicron variant and subvariants, which have both a higher propensity to evade both vaccine-induced and previously acquired natural immunity, and a reduced risk of serious illness and death in the general population.34-37 We intend to evaluate our data from patients in the Omicron era in a subsequent publication.
In conclusion, this pre-Omicron era retrospective study demonstrated that SARS-CoV-2 vaccination in patients with hematologic malignancies had a modest impact on COVID-19–related mortality, with a predicted benefit of a 2.3% absolute decrease in the probability of COVID-19–associated death (not reaching statistical significance), but a large absolute reduction in risk of hospitalization. Although the rates of death are lower than previously reported, mortality remained high for patients with hematologic malignancies who contracted COVID-19. Protective strategies, in addition to SARS-CoV-2 immunization, are warranted in this vulnerable patient population, including preexposure prophylaxis with tixagevimab-cilgavimab38 and early use of protease inhibitors such as nirmatrelvir-ritonavir39 in patients who test positive for SARS-CoV-2. Additional data encompassing the Omicron era are required regarding the overall benefits of immunization, booster dosing, prophylactic monoclonal antibodies, and COVID-19 therapeutics, as well as the risks of COVID-19–related death for patients with hematologic malignancies.
Acknowledgments
The authors thank The University of Texas MD Anderson Data-Driven Determinants for COVID-19 Oncology Discovery Effort (D3CODE) team for providing institutional patient data.
This study was supported, in part, by a grant from The University of Texas MD Anderson (core grant P30CA016672-43).
Authorship
Contribution: K.S.C. and P.A.T. designed the study, manually collected and analyzed data, and wrote the manuscript; C.B.P. performed biostatistical analysis and helped write the manuscript; E.Y. cleaned data obtained via Data-Driven Determinants for COVID-19 Oncology Discovery Effort (institutional review board–approved protocol 2020-0348); and D.C., E.E.M., and J.L.R. assisted with study design and manuscript preparation.
Conflict-of-interest disclosure: P.A.T. has received research funding from and consulted for AstraZeneca, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Kelly S. Chien, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: kchien@mdanderson.org.
References
Author notes
Presented in abstract form at the annual meeting of the 64th American Society of Hematology, 12 December 2022, New Orleans, LA.
Data are available on request from the corresponding author, Kelly S. Chien (kchien@mdanderson.org).