Key Points
Etranacogene dezaparvovec induced stable, sustained mild/normal FIX levels and eliminated bleeding and need for prophylaxis over 3 years.
No late-emergent safety events were observed over 3 years.
Abstract
Etranacogene dezaparvovec (AMT-061) is a recombinant adeno-associated virus serotype 5 (AAV5) vector containing a codon-optimized Padua variant human factor IX (FIX) transgene with a liver-specific promoter. Here, we report 3-year outcomes from a phase 2b, open-label, single-dose, single-arm, multicenter trial conducted among adults with severe or moderately severe hemophilia B (FIX ≤2%). All participants (n = 3) received a single intravenous dose (2 × 1013 gene copies per kg) and will be followed up for 5 years. The primary end point of FIX activity ≥5% at 6 weeks was met. Secondary end points included bleed frequency, FIX concentrate use, joint health, and adverse events (AEs). All participants required routine FIX prophylaxis and had neutralizing antibodies to AAV5 before etranacogene dezaparvovec treatment. After administration, FIX activity rose to a mean of 40.8% in year 1 and was sustained in year 3 at 36.9%. All participants discontinued FIX prophylaxis. Bleeding was completely eliminated in 2 out of 3 participants. One participant required on-demand FIX replacement therapy per protocol because of elective surgical procedures, for 2 reported bleeding episodes, and twice for a single self-administered infusion because of an unreported reason. One participant experienced 2 mild, self-limiting AEs shortly after dosing. During the 3-year study period, there were no clinically significant elevations in liver enzymes, no requirement for steroids, no FIX inhibitor development, and no late-emergent safety events in any participant. Etranacogene dezaparvovec was safe and effective in adults with hemophilia B over 3 years after administration. This trial was registered at www.clinicaltrials.gov as #NCT03489291.
Introduction
Hemophilia B is an inherited disorder resulting from mutations in the gene for the essential blood clotting factor IX (FIX), leading to insufficient expression for hemostasis. The current standard of care for the treatment of people with hemophilia B is lifelong prophylactic factor replacement requiring frequent, burdensome intravenous injections.1 Substantial progress has been made in the treatment of hemophilia B, such as the development of extended half-life FIX products. Although these advances have reduced the frequency of treatment, regular infusions are still required.2,3 Despite treatment, people with moderate (FIX activity 1%-5%) and severe (FIX activity level <1%) FIX deficiency are still at risk of bleeding events, notably into joints, leading to degenerative joint disease.4 FIX activity levels are generally correlated with bleeding and, therefore, largely determine the severity of the bleeding phenotype.1,5
Gene therapy affords the prospect of a one-time infusion potentially modifying the hemophilia phenotype, thereby offering the potential for long-term treatment for people with hemophilia B, through the endogenous production of functional FIX clotting factor by the patient’s liver.6 Adeno-associated virus (AAV) vector–based gene therapy for hemophilia B has resulted in sustained FIX expression for at least 1 year in several studies.7-11 However, the durability of AAV-based, liver-directed gene therapy for hemophilia B has not yet been established.12-14
AMT-060, a recombinant adeno-associated viral vector serotype 5 (AAV5) containing the wild-type human FIX gene, has been evaluated in an ongoing phase 1/2 trial in hemophilia B.15 A single administration of AMT-060 resulted in the stable and durable expression of wild-type FIX up to 5 years. Overall, FIX activity, on an average, was 5.2% in cohort 1 (5 × 1012 gene copies [gc] per kg) and 7.4% in cohort 2 (2 × 1013 gc per kg).15 No safety concerns have been reported. Etranacogene dezaparvovec (AAV5-Padua human FIX) was developed by introducing a 2-nucleotide change in the transgene coding sequence of AMT-060 (causing an R338L substitution during protein translation), resulting in the expression of the highly active Padua FIX variant.16,17 All other aspects of the FIX expression cassette of AMT-060, including the promoter and other transcriptional regulatory elements and the codon optimization, are identical in etranacogene dezaparvovec.
Previously, we reported interim efficacy and safety data for etranacogene dezaparvovec in this phase 2b clinical trial up to 26 weeks.17 The primary end point to confirm that a single dose of 2 × 1013 gc per kg etranacogene dezaparvovec (AMT-061) will result in FIX activity levels of ≥5%, measured by the 1-stage assay, at 6 weeks after dosing was met, with an average FIX activity at 47% by 26 weeks. This FIX expression was achieved despite all trial participants having anti-AAV5 neutralizing antibodies (NAbs; mean titer at screening = 39) before treatment.17 No bleeds were observed, and all participants receiving FIX before treatment stopped prophylaxis.
Here, we report the planned interim analysis at 3 years of this phase 2b study.
Methods
Etranacogene dezaparvovec: design and production
Etranacogene dezaparvovec (AMT-061) is a recombinant AAV5 gene therapy vector encoding a codon-optimized human FIX transgene with a naturally occurring mutation (FIX-Padua), which results in increased specific activity of the protein produced. Its design, characterization, and manufacturing have been described elsewhere.17
Study design and participants
This phase 2b, open-label, single-arm, multicenter trial evaluates the efficacy and safety of a single intravenous dose (2 × 1013 gc per kg) of etranacogene dezaparvovec. The study was designed with screening, treatment, and posttreatment follow-up phases, including long-term follow-up for a total of 5 years, as previously described.17 Three adults with moderately severe to severe hemophilia B (FIX activity ≤2% of normal) receiving prophylactic FIX were recruited.17 The recruitment of 3 participants in this study was supported by the US Food and Drug Administration and the European Medicines Agency to address the change of transgene product and to inform the dose choice for etranacogene dezaparvovec in phase 3.
Seropositivity for pre-existing anti-AAV5 NAbs was not an exclusion criterion for this clinical trial. Details of the assays used for measuring anti-AAV5 NAbs are described in supplemental Methods. Full inclusion/exclusion criteria are listed in supplemental Table 1. The study was approved by the institutional review board/institutional ethics committee at each center. All participants provided written informed consent. The trial was performed according to the Declaration of Helsinki and the principles of good clinical practice. The biopharmaceutical companies, uniQure Biopharma B.V. and CSL Behring, collaborated with the authors on the study design, data collection, data analysis, and/or data interpretation. All authors have access to the primary clinical trial data. This clinical trial is listed in the following registry: ClinicalTrials.gov identifier: NCT03489291.
Long-term follow-up of etranacogene dezaparvovec treatment
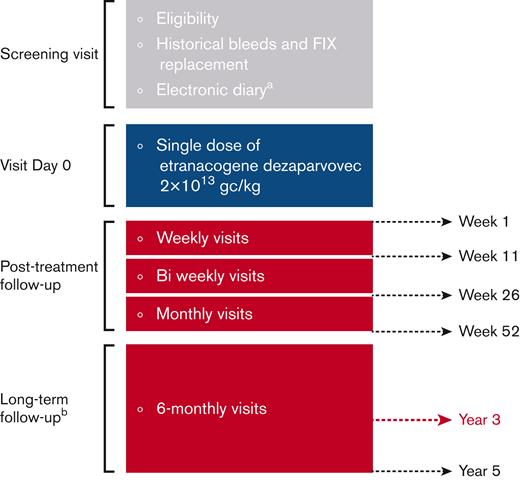
Efficacy and safety outcomes up to week 26 have been previously reported.17 Monthly visits were scheduled until week 52. Long-term follow-up visits occurred or were planned every 6 months thereafter up to 5 years (Figure 1).
Study design. (A) Recording of bleeds and FIX replacement before and after etranacogene dezaparvovec treatment. Assessment of bleeds and FIX replacement before screening were based on medical records. (B) No e-diary recording during long-term follow-up. Figure adapted from Von Drygalski A et al.17
Study design. (A) Recording of bleeds and FIX replacement before and after etranacogene dezaparvovec treatment. Assessment of bleeds and FIX replacement before screening were based on medical records. (B) No e-diary recording during long-term follow-up. Figure adapted from Von Drygalski A et al.17
Long-term outcome measures
Efficacy
The primary efficacy aim was the achievement of ≥5% FIX activity by 6 weeks after treatment.17 Secondary efficacy outcome measures included confirmation of ≥5% FIX activity maintenance at week 52 after treatment. FIX activity was assessed using a 1-stage activated partial thromboplastin time–based assay and a chromogenic assay. Details of assays and biochemical assessments are given in the supplemental Methods. Bleeding event data and FIX concentrate use were prospectively recorded from screening up to week 52 by participants using an electronic diary and onward using a paper diary. These entries were reviewed by investigators. FIX replacement data for the year before etranacogene dezaparvovec treatment were obtained by reviewing medical records. Joint health was assessed at baseline and annually thereafter as part of long-term follow-up, using the Hemophilia Joint Health Score (HJHS) version 2.1.18
Safety outcome measures
Safety assessments included the occurrence of adverse events (AEs). Definitions of AEs, their severity, and relationship to treatment (treatment-related AEs [TRAEs]) are contained in supplemental Table 2. Abdominal ultrasounds were evaluated by qualified personnel at each site at every visit, starting at the month 30 visit, to monitor participants for liver fibrosis and potential occurrences of liver malignancies. Long-term assessments include anti-AAV5 antibody titers (total and NAbs, supplemental Methods), T-cell response, presence of antibodies to FIX (including FIX inhibitors), liver enzymes (aspartate transaminase [AST] and alanine aminotransferase [ALT]), α-fetoprotein (AFP), and routine blood and coagulation parameters. Vector DNA was longitudinally assessed in semen and blood using quantitative real-time polymerase chain reaction. Vector clearance was confirmed either via the measurement of zero vector DNA or a reading below the limit of assay detection for 3 consecutive assessments, with the date of clearance being the first of the 3 (refer to Figure 1 for visit timings). Methods for the above analyses are provided in the supplemental Methods.
Patient-related outcome measures
Health-related quality of life was assessed as an exploratory end point using the Hemophilia Quality of Life Questionnaire for Adults (Hem-A-QoL) at baseline, week 26, week 52, and then yearly.
Data analysis
The data presented herein were collected from study initiation to 14 December 2021. No statistical hypothesis testing was conducted because of the study sample size (n = 3). All results are reported using descriptive statistics.
Results
Study population
Baseline demographics and disease characteristics are shown in Table 1. Participant 1 had a moderate-to-severe FIX deficiency, and participants 2 and 3 had severe hemophilia B. All participants required regular FIX prophylaxis before etranacogene dezaparvovec treatment and had preexisting anti-AAV5 NAbs (Table 1).
Baseline demographics and disease characteristics
| Characteristic . | Participant 1 . | Participant 2 . | Participant 3 . |
|---|---|---|---|
| Age at enrollment (y) | 43 | 50 | 47 |
| Weight (kg) | 89 | 81 | 82 |
| HIV status | Nonreactive | Reactive (controlled) | Reactive (controlled) |
| HBV status | Negative | Negative | Negative |
| HCV status | Resolved | Resolved | Resolved |
| Baseline FIX activity levels (%) | 1 | <1 | <1 |
| Prescreening FIX treatment | Prophylaxis (EHL) | Prophylaxis (EHL) | Prophylaxis (EHL) |
| ABR 1 y before screening∗ | 3 | 1 | 5 |
| Anti-AAV5 NAb status at screening∗ (titer)†,‡ | Positive (48) | Positive (44) | Positive (25) |
| Anti-AAV5 NAb status at day of dosing∗ (titer)†,‡ | Positive (22) | Positive (33) | Positive (20) |
| Characteristic . | Participant 1 . | Participant 2 . | Participant 3 . |
|---|---|---|---|
| Age at enrollment (y) | 43 | 50 | 47 |
| Weight (kg) | 89 | 81 | 82 |
| HIV status | Nonreactive | Reactive (controlled) | Reactive (controlled) |
| HBV status | Negative | Negative | Negative |
| HCV status | Resolved | Resolved | Resolved |
| Baseline FIX activity levels (%) | 1 | <1 | <1 |
| Prescreening FIX treatment | Prophylaxis (EHL) | Prophylaxis (EHL) | Prophylaxis (EHL) |
| ABR 1 y before screening∗ | 3 | 1 | 5 |
| Anti-AAV5 NAb status at screening∗ (titer)†,‡ | Positive (48) | Positive (44) | Positive (25) |
| Anti-AAV5 NAb status at day of dosing∗ (titer)†,‡ | Positive (22) | Positive (33) | Positive (20) |
Participants 2 and 3 were previously excluded from another AAV-based gene therapy trial for hemophilia B based on anti-AAV NAb titer.
ABR, annualized bleeding rate; EHL, extended half-life; HBV, hepatitis B virus; HCV, hepatitis C virus.
Total bleeds (treated + untreated).
AAV5 NAb data considered positive if titer was ≥2.
Luciferase cell-based assay.
Efficacy
Stable and durable endogenous FIX activity
After etranacogene dezaparvovec administration, mean endogenous FIX activity was 36.9% (min-max, 32.3%-41.5%) at 3 years (Figure 2). At the last time point for which uncontaminated FIX activity was available, participants 1 (at 3 years) and 3 (at 2.5 years) maintained FIX activity in the nonhemophilic range (≥40%). Participant 2 (at 3 years) maintained FIX activity at 32.3% (mild range: >5 to <40%).
Sustained increases in FIX activity after etranacogene dezaparvovec administration. Uncontaminated FIX activity measured by using a 1-stage aPTT assay. ∗Samples at baseline and Week 1 may have included activity from exogenous FIX replacement. aPTT, activated partial thromboplastin time.
Sustained increases in FIX activity after etranacogene dezaparvovec administration. Uncontaminated FIX activity measured by using a 1-stage aPTT assay. ∗Samples at baseline and Week 1 may have included activity from exogenous FIX replacement. aPTT, activated partial thromboplastin time.
The measured mean (min-max) FIX antigen level at 3 years was 3.7% (3.1%-4.2%) in participants (all cross-reacting material [CRM]–negative). The FIX activity-to-antigen ratio was 10.2.
Sustained reduction in bleeds and FIX use
All participants remained prophylaxis-free for 3 years after treatment. By year 3, bleeding events were substantially reduced (for all participants), with an annualized bleeding rate of 0.22. There were no joint bleeds among the 3 participants. No bleeds (100% reduction relative to pre-etranacogene dezaparvovec treatment) were reported for 2 of the 3 trial participants (Table 2).
Comparative outcomes at baseline and at 3 years after etranacogene dezaparvovec treatment in the 3 participants
| Outcome . | Participant 1 . | Participant 2 . | Participant 3 . | |||
|---|---|---|---|---|---|---|
| At baseline . | At 3 y . | At baseline . | At 3 y . | At baseline . | At 3 y . | |
| ABR (all bleeds) | 3∗,† | 0 | 1∗ | 0 | 6∗ | 0.64 |
| Mean on-demand FIX use (IU/y) | 24 000∗ | 0 | 7 681∗ | 0 | 63 000∗ | 2 143.9‡ |
| Total HJHS score | 35§ | 31 | 36§ | 32 | 1§ | NA |
| Hem-A-QoL total score | 26.7§ | 8.3 | 13.0§ | 5.0 | 13.5§ | 15.7 |
| Outcome . | Participant 1 . | Participant 2 . | Participant 3 . | |||
|---|---|---|---|---|---|---|
| At baseline . | At 3 y . | At baseline . | At 3 y . | At baseline . | At 3 y . | |
| ABR (all bleeds) | 3∗,† | 0 | 1∗ | 0 | 6∗ | 0.64 |
| Mean on-demand FIX use (IU/y) | 24 000∗ | 0 | 7 681∗ | 0 | 63 000∗ | 2 143.9‡ |
| Total HJHS score | 35§ | 31 | 36§ | 32 | 1§ | NA |
| Hem-A-QoL total score | 26.7§ | 8.3 | 13.0§ | 5.0 | 13.5§ | 15.7 |
ABR, annualized bleeding rate; NA, not available.
Data collected retrospectively 1 year before screening from medical records.
One bleed occurred after enrollment but before dosing.
Except for FIX use for the prevention of bleeds during operations.
At day of dosing
Over 3 years, 1 participant had 2 reported bleeds in the left lower leg muscle (1 suspected and 1 confirmed); 1 was a mild spontaneous bleed and 1 was a traumatic bleed, and each was successfully treated with a single FIX infusion, representing a 92% reduction in bleeds when compared with pre-etranacogene dezaparvovec treatment (Table 2). The same participant received additional FIX infusions for the management of hip surgeries, as described in “Joint health.”
Overall, the annualized mean on-demand FIX use was 714.6 IU/y over 3 years of follow-up post–etranacogene dezaparvovec administration, compared with 306 205 IU/y in the 1 year before screening (ie, before vector infusion). The annualized on-demand FIX usage for participants 1, 2, and 3 was 0, 0, and 2144 IU/y over 3 years, respectively.
Joint health
Total joint health scores (assessed using HJHS 2.1) improved for participants 1 and 2 from 35 and 36 at baseline to 31 and 32 at 3 years after vector infusion, respectively (Table 2). Participant 3, who had a low baseline score of 1, had an increased score of 6 at 2 years after treatment. This participant reported left knee and left ankle muscle atrophy and loss of strength (each rated 1), as well as alteration of global gait (rated 2), 1 month after he received a left hip surgery. No HJHS scores were available for Participant 3 beyond 2 years after treatment. Neither participant presented with target joints at baseline or at 3 years.
Safety
AEs
Two previously reported TRAEs occurred in the first year of follow-up (Participant 1: headache and a transient elevation of C-reactive protein that resolved without intervention).17 No additional TRAEs or serious TRAEs have been reported. One serious AE deemed unrelated to treatment occurred in year 1 after treatment and was previously reported17: Participant 3 experienced worsening avascular necrosis, which necessitated 2 left hip operations. FIX replacement therapy was used for perioperative management as required per protocol, with dose and duration adjusted to account for the participant’s current endogenous FIX levels.
No FIX inhibitor development was observed, and no clinically significant ALT or AST elevations or AAV5-specific T-cell responses were reported for the first year of follow-up in all 3 participants.10,17 One participant (Participant 2) had an isolated AST elevation at the 18-month visit (62 U/L; upper limit of normal 34 at the central laboratory), which was not accompanied by ALT elevations. The AST elevation was mild, asymptomatic, and transient (at month 24, AST was 25 U/L), did not affect FIX activity, did not require treatment, was not reported as AEs nor considered clinically significant, and was assessed as unrelated to treatment by the investigator. No viral workup or liver ultrasound was performed. To evaluate long-term safety (with respect to hepatotoxicity), AFP levels were measured at each visit from year 1 onward, and abdominal ultrasounds were performed from month 30 onward. All recorded AFP measurements were in the normal range. No clinically significant liver abnormalities were seen in the abdominal ultrasounds up until month 36 for all participants.
Immune and inflammatory biomarkers
Using an enzyme-linked immunosorbent assay method for the detection of antibodies directed to AAV5, none of the participants had total anti-AAV5 immunoglobulin G (IgG) antibodies at screening or on the day of dosing. Participant 1 had anti-AAV5 IgM antibodies at screening and on the day of dosing. All participants demonstrated rapid elevation of total anti-AAV5 IgG and IgM titers within 1 week of gene transfer. At 3 years, all participants demonstrated sustained and elevated anti-AAV5 IgG antibody titers.
Using a cell-based transduction inhibition assay for the measurement of anti-AAV5 NAbs,17 all 3 participants had preexisting anti-AAV5 NAbs with a mean (min-max) NAb titer of 39 (25-48) at screening and of 25 (20-33) at the day of dosing, respectively. The mean (min-max) duration period between screening and dosing was 25 days (15-31) (Table 1). Titers ≥2 were considered to be positive for this assay.17
Anti-AAV5 NAbs rose to titers of 36 450 (the maximum limit of detection of the assay) by week 2 after administration and remained >36 450 for the remainder of the posttreatment period.
Participant 2 had an isolated, positive AAV5-capsid–specific T-cell assay result (17 spot-forming units per 300 000 peripheral blood mononuclear cells; supplemental Methods) at week 48, not associated with liver transaminase elevation or a decrease in circulating plasma FIX activity. No participant exhibited a clinically significant cytotoxic T-cell–mediated immune response to the capsid. No clinically relevant changes in inflammatory biomarkers were observed.17
Vector biodistribution
Vector DNA was cleared from semen in Participants 1 and 2 by week 26 after treatment. No additional data are available for Participant 3 after the positive test result in semen at 12 weeks after treatment.
Vector DNA was cleared from blood samples in Participant 2 by week 31, and in Participant 1 by month 18 after treatment. Participant 3 had positive test results at all visits post–etranacogene dezaparvovec administration up to month 30 (although below the lower limit of quantification of the assay from week 36) and a negative test result at month 36. Given the requirement for 3 consecutive negative results, confirmation of vector clearance in Participant 3 will take an additional 12 months. The presence of AAV vector DNA in bodily fluids was not associated with any adverse safety or efficacy findings.
Patient-related outcomes
The mean (min-max) total Hem-A-QoL scores decreased from 17.6 (13.0-26.7) at the day of dosing to 9.6 (5.0-15.5) at 3 years after treatment, representing an improvement in Hem-A-QoL scores (Table 2). Participants 1 and 2 had a decrease of −14.5 and −8, respectively, and Participant 3 had an increase of +2.2 of the total Hem-A-QoL score at 3 years after treatment as compared with the day of dosing. Improvement in the domain “treatment” primarily contributed to the difference in total score for all participants, with a mean (min-max) “treatment” score ranging from 23.9 (18.8-28.1) at the day of dosing to 0 (0-0) at 3 years after treatment.
Discussion
In this phase 2b study of liver-directed gene therapy for hemophilia B, 3 participants were treated with a single infusion of etranacogene dezaparvovec, resulting in stable, durable, and clinically relevant increases in FIX activity. After treatment, FIX activity rapidly increased to a mean of 31% at week 6, reaching ∼47% by 26 weeks and ∼37% by 3 years.17 Etranacogene dezaparvovec was well tolerated and enabled a durable change in disease phenotype from a severe or moderately severe classification to the upper end of the mild/nonhemophilic range. All participants successfully discontinued FIX prophylaxis after the administration of etranacogene dezaparvovec. Etranacogene dezaparvovec resulted in an overall 100% reduction in bleeds in 2 out of 3 participants (Participants 1 and 2) and a 92% reduction in bleeds in Participant 3 compared with the year before administration. Subsequently, both Participants 1 and 2 showed a 9% reduction in the total HJHS score, highlighting a joint health improvement over 3 years after the etranacogene dezaparvovec infusion. However, Participant 3 had an increased total HJHS score of 5 over the first 2 years of follow-up due to his previous history of avascular necrosis, necessitating 2 hip surgeries.
A previous study of joint health in 62 participants with moderate or severe hemophilia assessed using HJHS scoring showed that 37% of participants experienced joint degeneration over the study period (5-10 years).19 An increase in the total HJHS score of >4 was defined as joint deterioration.19 In this study, Participant 3 had worsening avascular left hip necrosis, which required 2 left hip surgeries over the first 2 years of follow-up. In this participant, the increase in the total HJHS score at year 2 due to muscle atrophy and loss of strength in the left lower limb reported 1 month after the second hip surgery is unlikely to be a result of bleed-related joint deterioration. This would be in line with the absence of any joint bleeds over 3 years after the administration of etranacogene dezaparvovec in all participants.
Similarly, the mean total Hem-A-QoL score decreased (lower scores indicate better quality of life) after etranacogene dezaparvovec treatment. Improvements in the total Hem-A-QoL score for 2 out of 3 participants of ≥7 meet the previously established responder definition indicating health-related QoL improvement, whereas Participant 3, who experienced deterioration of a preexisting comorbid condition (avascular necrosis), did not demonstrate health-related QoL improvement.20 The domain “treatment” improved for all participants, consistent with what has been observed in other gene therapy clinical trials for hemophilia B21 and hemophilia A.22
A potential impediment to the successful administration of AAV-based therapies is the presence of preexisting vector-specific NAbs.14,23-26 Consequently, seropositivity for AAV is often an exclusion criterion in AAV gene therapy studies.8,27-29 However, studies using AAV5 serotype vectors (AMT-060) and etranacogene dezaparvovec (AMT-061) have demonstrated FIX expression in participants with preexisting anti-AAV5 NAbs, as determined by our specific NAb assays, at up to 5 and 3 years, respectively.9,17,30,31 The colorimetric anti-AAV5 IgG/M assay (enzyme-linked immunosorbent assay)17,31 used to evaluate anti-AAV5 antibodies in this study did not detect low levels of these antibodies in the study participants at baseline, except for IgM in Participant 1. However, the luciferase-based anti-AAV5 NAb (cell-based transduction inhibition) assay31 measured anti-AAV5 NAbs in all participants before treatment. By week 2 after treatment, anti-AAV5 NAb titers had risen to 36 450 (the maximum limit of detection of the assay) and were maintained for the duration of follow-up. The increased anti-AAV5 antibodies did not have direct clinical consequences or affect long-term transgene expression. Other AAV gene therapy studies reported the development of anti-capsid antibodies after treatment, which also persist for many years,7,32 potentially precluding redosing with AAV vectors in the event of FIX expression decreasing back to baseline.
The first hemophilia B study to use the hyperactive Padua FIX variant was BAX 335 (AAV8-FIX Padua).12 FIX-Padua expression was initially measurable in 7 of 8 participants. Participants in the highest dose cohort exhibited peak FIX activity (32.0%-58.5%). However, FIX activity was not sustained beyond 11 weeks in 7 of the 8 participants. The loss of BAX 335 transgene expression was attributed to the number of CpG motifs introduced during transgene codon optimization.12 CpG motifs can initiate an innate immune response via toll-like receptor 9 signaling, potentially resulting in transduced cell elimination.33 Etranacogene dezaparvovec has no CpG motifs, whereas BAX 335 contains 99 CpGs in the open reading frame of FIX.34
Other clinical trials using AAV-based vectors demonstrated durable FIX-Padua expression, comparable to that demonstrated by etranacogene dezaparvovec. Fidanacogene elaparvovec, an AAV-Spk-100 vector, was reported to sustain FIX activity of 33.7% over 28 to 78 weeks follow-up in 8 out of 10 participants.8 Verbrinacogene setparvovec (FLT 180), a liver-targeted (AAVS3) vector encoding FIX Padua, demonstrated FIX activity ranging from 24% to 168% for all doses by week 3 after treatment.11,29,35 Two participants treated with the lowest vector dose demonstrated FIX activity levels of 37.5% at 2 years.29
The disparity in transgene expression noted between clinical trials using AAV vectors expressing FIX Padua remains unexplained. However, the constructs used differ in many respects, including serotype (AAV8, AAV-Spk-100, AAV5, and AAVS3), CpG content, and dose administered.8,17,29,35 All these factors could potentiate an immune response that limits transgene expression, as reviewed elsewhere.36
Simioni et al 37 determined that FIX Padua has a specific activity (activity-to-antigen ratio) of >8. The FIX activity-to-antigen ratio in CRM-negative participants administered fidanacogene elaparvovec was ∼12.8 Both ratios are comparable to our estimation of a FIX activity-to-antigen ratio of 10.2 in all participants (all CRM-negative). The 2 × 1013 gc per kg cohort participants (n = 5) in a study of AMT-060 (AAV5–wild-type human FIX) had a mean FIX activity of 7.4% at 5 years,15 which is fivefold lower than that achieved by etranacogene dezaparvovec (36.9%) at 3 years; this estimate is also comparable to the findings of Simioni et al. 37
Given that etranacogene dezaparvovec (AMT-061) is a near-identical construct to AMT-060 (except for a 2 nucleotide change), its durability is likely to reflect that of AMT-060, which has demonstrated sustained transgene expression over 5 years accompanied by clinical benefits (reduction in bleeds and FIX use, no requirement for prophylaxis) that were also maintained over multiple years.9,30,38
No study participants developed inhibitors against FIX Padua in the 3 years after treatment. However, the study recruited participants at low risk of inhibitor development (ie, individuals who have had >150 exposure days to replacement therapy and have never developed inhibitors). To date, the presence and/or history of inhibitors are often an exclusion criterion in gene therapy studies.39,40 Therefore, it remains unknown whether inhibitor formation could occur after gene transfer in treatment-naïve individuals.
Although this study included a limited number of participants, there was no indication of clinically relevant liver toxicity. No participant required corticosteroids for transaminase elevations after treatment, and no liver ultrasound abnormalities were found. The liver safety profile of etranacogene dezaparvovec is being explored more fully in the ongoing phase 3 study: 17% of participants have required a short course of corticosteroids because of liver enzyme elevations.41 No elimination of FIX activity was seen, in contrast to some cases in other studies in hemophilia B.8,11,28 Immune responses to gene transfer, indicated by liver transaminase elevations, were observed in 17% to 40% of participants in other hemophilia B studies8,11,15,28,41 and up to 86% in a hemophilia A study.42,43
Recombinant AAV secretion in study participants is monitored because of the potential risk of either vertical transmission to offspring (from vector shedding into semen) or horizontal transmission to other contacts via other body fluids, such as blood. Vector DNA was most persistent in whole blood. One participant demonstrated clearance by 31 weeks, 1 by 18 months, and the third had a negative test result at 36 months after treatment. Vector DNA was cleared from semen in 2 participants by 18 months after treatment (no long-term data are available from the third participant). In a dose-escalation hemophilia A study using an AAV5-hFVIII-SQ construct, all participants in cohorts treated with the highest vector doses (4 × 1013 [cohort 4] and 6 × 1013 vg/kg [cohort 3]) still exhibited measurable vector in blood at 3 years. In semen, the minimum/maximum time to clearance was 15/83 weeks in cohort 3 and 14/60 weeks in cohort 4.44,45 Participants in the high-dose cohort (2 × 1013 gc per kg) of an ongoing study evaluating AMT-060 demonstrated vector clearance from blood and semen of all (high-dose cohort) participants by 44 months and 15 months, respectively.9,46 The persistence of etranacogene dezaparvovec in the blood and semen of participants seems comparable to other studies using AAV5 vectors.9,44-46 Of note, vector clearance may reflect a number of variables, including dose, route of administration, target organ, as well as various individual factors. Furthermore, the risk posed by a treated individual is considered negligible given that the AAV vector is nonpathogenic and replication-incompetent.
In conclusion, a single infusion of etranacogene dezaparvovec (AMT-061) safely resulted in stable and durable expression of endogenous FIX at a near-normal/normal range in all participants over 3 years, although they were all seropositive to anti-AAV5 NAbs before treatment. Sustained FIX endogenous expression at therapeutic levels without requirements for immunosuppression, clinical benefits, and FIX prophylaxis discontinuation were achieved in all participants, with no late-emergent safety events over 3 years.
Acknowledgments
The authors thank the following individuals for their assistance with this study, including the individuals who participated in the study and the study site staff: Yanyan Li and Ling Chen, both full-time employees at CSL Behring, who aided with biostatistics and figure design, and Jacqueline Tarrant, full-time employee at CSL Behring, who aided with methods description and interpretation of data.
This work was supported by CSL Behring, which provided funding for the writing support provided by Lesley Taylor and Adam Taylor on behalf of Bioscript Science (Macclesfield, United Kingdom).
Authorship
Contribution: A.v.D., A.G., G.C., N.S.K., F.W.G.L., S.U.L., W.A.M., M.R., E.G., and S.W.P. were study investigators; A.v.D., A.G., E.G., and S.W.P. enrolled and treated participants and conducted clinical follow-up; R.G., R.D., P.E.M., and S.L.Q. were involved in the design of the research, interpretation of the data, and the development of the paper; the study sponsor, uniQure biopharma BV and CSL Behring, collaborated with the authors on the study design, data collection, data analysis, and data interpretation; and all authors provided critical feedback on the manuscript and approved the final version of the paper before submission.
Conflict-of-interest disclosure: A.v.D. is a consultant for BioMarin, Sanofi Genzyme, Novo Nordisk, Pfizer, uniQure, and Hematherix. A.G. is a consultant for Bioverativ, Genentech/Roche, BioMarin, and uniQure and serves as a speaker bureau of Bioverativ and Genentech/Roche. G.C. received grants/research support from CSL Behring, Pfizer, and Sobi and serves as a speaker bureau of Bayer, BioMarin, Roche, Sobi, Grifols, LFB, Novo Nordisk, Werfen, Kedrion, and uniQure. N.S.K. received grants/research support from Grifols and Takeda and is a consultant for uniQure, BioMarin, and Novo Nordisk. S.U.L is a consultant for uniQure. F.W.G.L. received grants/research support from CSL Behring, Shire/Takeda, Roche, and Sobi and is a consultant for uniQure, Takeda, Novo Nordisk, and BioMarin. W.A.M. received grants/research support from Bayer, Biotest, Takeda, LFB, Octapharma, Novo Nordisk, Pfizer, and uniQure and is a consultant for Bayer, BioMarin, Freeline, LFB, Octapharma, Novo Nordisk, Pfizer, and uniQure. M.R. received research funding from Bayer, BioMarin, CSL Behring, Genentech, Grifols, Hema Biologics, LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark Therapeutics, Takeda, and uniQure; is a consultant for Catalyst Biosciences, CSL Behring, Genentech, Hema Biologics, Kedrion, Novo Nordisk, Pfizer, Sanofi, Takeda, and uniQure; and is on the board of directors for Foundation for Women and Girls with Blood Disorders, Partners in Bleeding Disorders Education, and Thrombosis and Hemostasis Societies of North America. E.G. serves as a speaker bureau of Global Blood Therapeutics. R.G. is an employee of uniQure. R.D. is an employee of uniQure. S.L.Q. is an employee of CSL Behring. P.E.M. is an employee of CSL Behring. S.W.P. received a grant/research support from Bayer, BioMarin, Freeline, Novo Nordisk, and Roche/Genentech and is a consultant for ApcinteX, ASC Therapeutics, Bayer, BioMarin, CSL Behring, GeneVentiv, HEMA Biologics, Freeline Therapeutics, Novo Nordisk, Pfizer, Regeneron/Intellia, Roche/Genentech, Sanofi, Spark Therapeutics, Takeda, and uniQure.
Correspondence: Annette von Drygalski, Division of Hematology/Oncology, Department of Medicine, University of California San Diego, 9333 Genesse Ave, Suite 310, San Diego, CA 92121; e-mail: avondrygalski@health.ucsd.edu.
References
Author notes
Individual participant data will not be shared. CSL Behring can provide scientific researchers with access to deidentified participant data collected in clinical trials to improve participant care to support the advancement of medical science.
Data are available on request from the corresponding author, Annette von Drygalski (avondrygalski@health.ucsd.edu).
The full-text version of this article contains a data supplement.