Key Points
NRD is not inferior to traditional PD during neutropenia after HSCT.
Multiple myeloma diagnosis, antibiotic prophylaxis, and absence of mucositis reduce the risk of grade ≥2 infections.
Abstract
Infections are a major cause of morbidity and mortality during neutropenia after hematopoietic stem cell transplantation (HSCT). The use of a low-microbial protective diet (PD) in the peritransplantation period is a standard of care, although its efficacy has never been tested prospectively. We conducted a multicenter, randomized, noninferiority trial, enrolling all consecutive adult patients undergoing high-dose induction chemotherapy or HSCT with the objective to compare nonrestrictive diet (NRD) vs PD. Overall, 222 patients were enrolled, randomly assigned, and analyzed. One hundred seventy-five subjects (79%) received autologous HSCT (auto-HSCT), 41 (18%) received allogeneic HSCT (allo-HSCT), and 6 (3%) patients received high-dose induction chemotherapy. There was no significant difference in terms of incidence of grade 2 infections and death during neutropenia in the 2 arms. In multivariable analysis, only multiple myeloma diagnosis, fluoroquinolone prophylaxis, and the absence of mucositis were associated with a lower incidence of grade 2 infections. We did not report any significant variation in terms of hospitalization length, incidence of mucositis and gastrointestinal infections, body weight, and serum albumin variations in the 2 arms. In allo-HSCT recipients, the incidence of acute graft-versus-host disease grade 3 was similar. NRD was associated with higher patient-reported satisfaction. In conclusion, NRD is not inferior to a traditional PD during neutropenia after HSCT, and our results demonstrated that implementing a restrictive diet unnecessary burdens patients' quality of life. The clinical trial was registered prospectively in the clinical trial registry of the Istituto Nazionale dei Tumori of Milan as INT54/16.
Introduction
High-dose chemotherapy followed by autologous or allogeneic hematopoietic stem cell transplantation (auto- or allo-HSCT) is a potentially curative treatment for several hematological malignancies.1 The damage to the gastrointestinal mucosa, prolonged neutropenia consequent to chemotherapy, and immunosuppression represent major risk factors for the development of severe infections.2-5 Despite the advances in supportive care, infections remain a major cause of morbidity and mortality in the setting of HSCT.6
Various measures have been adopted to minimize the incidence of infections, including the use of a low-microbial protective diet (PD). PD traditionally consists of a cooked food diet, which restricts raw foods, fresh fruits, and vegetables.7 The rationale behind the use of PD is to prevent the introduction of dangerous bacteria through food into a gastrointestinal tract damaged by high-dose chemotherapy,7 thus limiting bacteria translocation into the bloodstream.
Recent surveys demonstrated that more than 80% of bone marrow transplantation centers continue to adopt PD in the neutropenic phase after HSCT, although a standardization of diet prescriptions is lacking.8,9 Moreover, a low-microbial PD is extensively used even though its efficacy was never tested prospectively and evidence-based results are lacking.7,10 Furthermore, a retrospective study reported a significant negative correlation between oral feeding and severe graft-versus-host disease (GVHD) incidence.11 It can be postulated that the use of a less palatable, restrictive diet could reduce the oral food intake of patients undergoing allo-HSCT, exposing them to a higher risk of GVHD.10
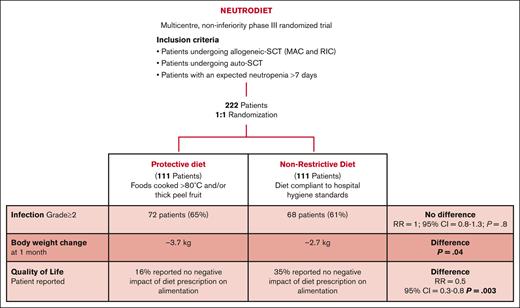
Given the potentially harmful effect of this commonly adopted procedure and the lack of prospective evidence demonstrating its efficacy in infection prevention, we designed a prospective, multicenter, noninferiority, randomized study (NEUTRODIET) that aimed to assess the impact of diet on infection incidence and transplantation outcomes in patients undergoing auto or allo transplantation and patients in whom a period of neutropenia >7 days was expected.
Methods
Study design and patients
Since July 2016, we have conducted a prospective, multicenter, randomized (1:1), parallel-group, noninferiority phase 3 trial, enrolling all consecutive hospitalized adult patients undergoing auto- (standard myeloablative conditioning)12 or allo-HSCT (reduced intensity conditioning [RIC] and myeloablative conditioning [MAC])13 and adult patients in whom a period of neutropenia >7 days was expected (ie, patients receiving induction chemotherapies for acute leukemias).
The exclusion criteria were (1) the presence of an active infection before neutropenia, (2) inability to feed orally, (3) transplantation for nonmalignant diseases, (4) haploidentical HSCT, and (5) HSCT from cord blood.
The study was conducted in Italy (3 centers located in the cities of Milan, Verona, and Cuneo) and registered prospectively in the clinical trial registry of Istituto Nazionale dei Tumori of Milan (registry number INT 54/16). The trial was approved by the local ethics committee and was conducted in accordance with principles of the Declaration of Helsinki. All the patients provided written informed consent. The study protocol synopsis is available as supplemental Table 1.
Randomization and diet
Eligible patients were randomly assigned in a 1:1 ratio to receive either PD or a nonrestrictive diet (NRD) from the start of chemotherapy and during the period of severe neutropenia (absolute neutrophil count <500/μl). Block randomization and stratification for transplantation type (auto-HSCT vs allo-HSCT) were planned.
The PD allowed only foods cooked at >80°C and thick peel fruits washed and peeled. The foods characterizing NRD were fresh fruits and vegetables washed and manipulated according to safe food handling practices. Extended information about diet composition is provided in Table 1. Adherence to the study was defined as daily consumption of at least 1 food among those considered to characterize PD and NRD, except for clinical limitations (mucositis, nausea, etc). Data regarding feeding information were collected using a diary filled out by the patient and checked by the nursing staff.
Diet details
| . | PD . | NRD . |
|---|---|---|
| Fish and meat | Only well cooked | Only well-cooked |
| Vegetables | Only cooked above 80°C | Fresh vegetables allowed∗ |
| Fruit | Cooked or thick peel fruit washed and peeled | Fresh fruit allowed∗ |
| Milk | Only pasteurized | Only pasteurized |
| Cheese | Only pasteurized | Pasteurized and seasoned cheese without mold |
| Yogurt | No | Only pasteurized |
| Eggs | Only freeze-dried | Only cooked |
| Bread | Allowed | Allowed |
| Dessert and ice cream | Only industrial preparation | Only industrial preparation |
| Honey | No | Only pasteurized |
| Cold cuts and sausages | No | Yes single portioned |
| . | PD . | NRD . |
|---|---|---|
| Fish and meat | Only well cooked | Only well-cooked |
| Vegetables | Only cooked above 80°C | Fresh vegetables allowed∗ |
| Fruit | Cooked or thick peel fruit washed and peeled | Fresh fruit allowed∗ |
| Milk | Only pasteurized | Only pasteurized |
| Cheese | Only pasteurized | Pasteurized and seasoned cheese without mold |
| Yogurt | No | Only pasteurized |
| Eggs | Only freeze-dried | Only cooked |
| Bread | Allowed | Allowed |
| Dessert and ice cream | Only industrial preparation | Only industrial preparation |
| Honey | No | Only pasteurized |
| Cold cuts and sausages | No | Yes single portioned |
Manipulated according to safe food handling procedures.
End points and assessments
The primary end point was the investigator-assessed incidence of infection of grade ≥2 (G ≥2) or death during the period of neutropenia.14 The secondary preplanned end points were summarized as follows: (1) incidence of infections (both incidence of gastrointestinal tract infections and incidence of fever of unknown origin from the beginning of neutropenia until discharge), (2) nutritional status (analyzed as the variation in body weight and biochemical indicators from admission to discharge and use of parenteral/enteral nutrition), (3) duration of hospital stay (days from admission to discharge), (4) estimated overall survival at 30 days from the onset of neutropenia, and (5) incidence of acute GVHD (aGVHD) according to standard criteria14 (only applicable to patients receiving allo-HCT). Infectious and adverse events were coded and graded according to the Common Terminology Criteria for Adverse Events version 4.0.The planned follow-up was 100 days after transplantation for allo recipients and 30 days after the resolution of neutropenia for the other patients.
Microbiological surveillance
All microbial cultures that were tested as positive from the onset of neutropenia were included in the analysis. Routine surveillance was conducted weekly with a rectal swab to investigate the presence of Salmonella species, Shigella species, methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococci, Pseudomonas species, carbapenem-resistant Enterobacteriaceae, extended-spectrum β-lactamase–producing Enterobacteriaceae, fungi, and yeast. A nasal swab for respiratory virus was performed according to clinical symptoms. From 2020, all patients required a negative molecular severe acute respiratory syndrome coronavirus 2 test result before hospitalization. Blood cultures and specific fluid cultures were collected only when clinically indicated.
Statistical analysis
It was estimated that 40% of patients receiving PD during neutropenia would develop a clinically relevant infection or die in the first 30 days of hospitalization.15 A noninferiority trial was designed. It was planned to enroll 222 patients with a 1:1 allocation ratio in the 2 arms (111 in the control group and 111 in the experimental group). This sample size allowed to obtain a power of 80% under the following assumptions: a significance level of 10%, a proportion of 40% of developing infections in the control group, and a noninferiority margin equal to a relative risk (RR) of 1.35. Considering a dropout rate of 10%, a total of 244 patients were to be enrolled (122 in the control group and 122 in the experimental group).
Analyses were performed in the intention-to-treat population, which included all patients who underwent randomization. For group comparison of categorical data, Fisher exact test or χ2 test was used. Mann-Whitney test was performed for comparison of continuous variables. Multivariable logistic regression was used to study the association of different covariates with the incidence of infection. Covariates included in the model were sex, age (>55 vs ≤55 years), allo-HSCT, dietary regimen, ≥2 previous lines of therapy, antibiotic prophylaxis, multidrug resistant (MDR) bacteria colonization at admittance, use of parenteral nutrition, any grade of mucositis, and type of diagnosis. Interactions of the dietary regimen with allo-HSCT and antibiotic prophylaxis were also explored in the multivariable model. All the reported P values are 2-sided and were considered statistically significant when P < .05. Plots and statistical analysis were performed using GraphPad Prism version 9.00 (license number RRID:SCR_002798).
Results
Patients
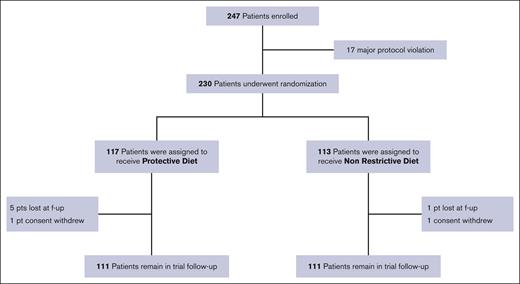
Overall, 247 patients were enrolled. Twenty-five patients were excluded from the final analysis: 2 patients withdrew consent, 17 were improperly enrolled for protocol violation, and 6 were lost to follow-up. Between July 2016 and March 2022, a total of 222 patients were randomly assigned and analyzed at the data cutoff: 111 were assigned to the PD arm and 111 to the NRD arm (Figure 1).
The clinical and demographic characteristics were similar between the 2 arms and are reported in Table 2. The median age was 57 years (range, 22-72 years), and 96 patients were female (43%). Surveillance rectal swabs at admission were tested positive for antibiotic-resistant gram-negative bacteria in 24 patients (11%), equally distributed between the 2 arms; extended-spectrum beta-lactamases-producing Enterobacteriaceae were the most frequently isolated strains (12 out of 24 patients). Gut decontamination was not proposed to any patients, whereas 125 individuals (56%) received antibiotic prophylaxis (95% with fluoroquinolones), 219 (99%) received antiviral prophylaxis (acyclovir), and 212 (95%) received antifungal prophylaxis.
Patients’ characteristics based on the diet assigned
| . | Total 222, n (%) . | Diet . | P value∗ . | |
|---|---|---|---|---|
| PD 111, n (%) . | NRD 111, n (%) . | |||
| Sex (female) | 96 (43) | 46 (41) | 50 (45) | .7 |
| Age, median (range) | 57 (22-72) | 57 (26-72) | 57 (22-71) | .1 |
| Disease | .7554 | |||
| Lymphomas | 105 (47) | 51 (46) | 54 (49) | |
| NHL aggressive | 65 (62) | 31 (61) | 34 (63) | |
| NHL indolent | 17 (16) | 10 (20) | 7 (13) | |
| HL | 23 (22) | 10 (20) | 13 (24) | |
| Multiple myeloma | 89 (40) | 48 (43) | 41 (37) | |
| AML | 9 (4) | 4 (4) | 5 (4) | |
| Other | 19 (9) | 8 (7) | 11 (10) | |
| Type of procedure | .9851 | |||
| Auto-HSCT | 175 (79) | 88 (79) | 87 (78) | |
| Allo-HSCT | 41 (18) | 20 (18) | 21 (19) | |
| High-dose CHT | 6 (3) | 3 (3) | 3 (3) | |
| Previous lines (≥2) | 106 (48) | 52 (47) | 54 (49) | .9 |
| Disease status at enrollment | .1233 | |||
| Complete response | 124 (56) | 66 (60) | 58 (52) | |
| Partial response | 74 (33) | 38 (34) | 36 (32) | |
| Stable disease | 10 (5) | 2 (2) | 8 (7) | |
| Progressive disease | 10 (5) | 3 (3) | 7 (7) | |
| Not applicable | 4 (2) | 2 (2) | 2 (2) | |
| Antimicrobial prophylaxis | ||||
| Antiviral | 219 (99) | 108 (97) | 111 (100) | .2 |
| Antibiotic† | 125 (56) | 61 (55) | 64 (57) | .7 |
| Antifungal | 212 (95) | 106 (95) | 106 (95) | > .99 |
| MDR colonization | 24 (11) | 12 (11) | 12 (11) | > .99 |
| Duration of neutropenia (days), median (range) | 6 (3-22) | 6 (3-20) | 6 (3-22) | .4 |
| . | Total 222, n (%) . | Diet . | P value∗ . | |
|---|---|---|---|---|
| PD 111, n (%) . | NRD 111, n (%) . | |||
| Sex (female) | 96 (43) | 46 (41) | 50 (45) | .7 |
| Age, median (range) | 57 (22-72) | 57 (26-72) | 57 (22-71) | .1 |
| Disease | .7554 | |||
| Lymphomas | 105 (47) | 51 (46) | 54 (49) | |
| NHL aggressive | 65 (62) | 31 (61) | 34 (63) | |
| NHL indolent | 17 (16) | 10 (20) | 7 (13) | |
| HL | 23 (22) | 10 (20) | 13 (24) | |
| Multiple myeloma | 89 (40) | 48 (43) | 41 (37) | |
| AML | 9 (4) | 4 (4) | 5 (4) | |
| Other | 19 (9) | 8 (7) | 11 (10) | |
| Type of procedure | .9851 | |||
| Auto-HSCT | 175 (79) | 88 (79) | 87 (78) | |
| Allo-HSCT | 41 (18) | 20 (18) | 21 (19) | |
| High-dose CHT | 6 (3) | 3 (3) | 3 (3) | |
| Previous lines (≥2) | 106 (48) | 52 (47) | 54 (49) | .9 |
| Disease status at enrollment | .1233 | |||
| Complete response | 124 (56) | 66 (60) | 58 (52) | |
| Partial response | 74 (33) | 38 (34) | 36 (32) | |
| Stable disease | 10 (5) | 2 (2) | 8 (7) | |
| Progressive disease | 10 (5) | 3 (3) | 7 (7) | |
| Not applicable | 4 (2) | 2 (2) | 2 (2) | |
| Antimicrobial prophylaxis | ||||
| Antiviral | 219 (99) | 108 (97) | 111 (100) | .2 |
| Antibiotic† | 125 (56) | 61 (55) | 64 (57) | .7 |
| Antifungal | 212 (95) | 106 (95) | 106 (95) | > .99 |
| MDR colonization | 24 (11) | 12 (11) | 12 (11) | > .99 |
| Duration of neutropenia (days), median (range) | 6 (3-22) | 6 (3-20) | 6 (3-22) | .4 |
AML, acute myeloid leukemia; high-dose CHT, high-dose chemotherapy with an expected period of neutropenia >7 days; NHL, non-Hodgkin lymphoma.
P value resulting from a Mann-Whitney test for continuous variables and χ2 test for categorical variables.
Among the 125 patients who received antibiotic prophylaxis, 119 (95%) received fluoroquinolones.
One hundred seventy-five subjects (79%) received auto-HSCT (88 in the PD arm and 87 in the NRD arm), among whom the main indication was multiple myeloma (87 out of 175; 49%), followed by non-Hodgkin lymphomas (65 out of 175; 37%). In contrast, 41 subjects (18%) received allo-HSCT (20 in the PD arm and 21 in the NRD arm), and the main indication was lymphoma (20 out of 41; 49%). In this subset, the 2 arms were well balanced in terms of donor type, conditioning regimen, GVHD prophylaxis, the use of total body irradiation, and antithymocyte globulin (supplemental Table 2). Twenty-six allo-HSCT recipients (63%) received RIC.15
Infections
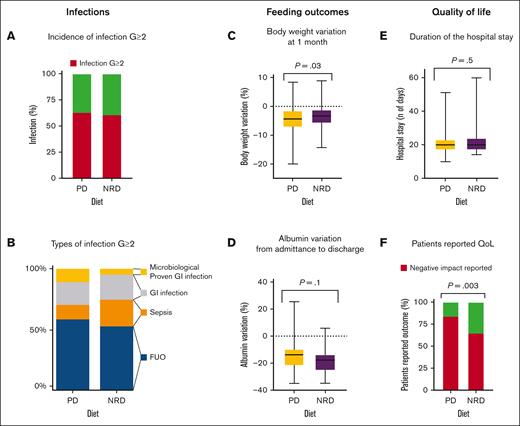
No difference was found between the 2 study arms in investigator-assessed cumulative incidence of G ≥2 infections and death during the period of neutropenia. Globally, 140 cases of G ≥2 infections were reported; 72 of them occurred in patients receiving PD and 68 in those receiving NRD (65% vs 61%; RR = 1.0; 95% confidence interval [CI], 0.9-1.3; P = .7; Figure 2A). Only 1 death was reported at 30 days, and it was secondary to multiorgan failure in the context of cytokine release syndrome after early suspension of ruxolitinib in a patient randomly assigned to the NRD arm and affected by myeloproliferative neoplasm while undergoing allo-HSCT.
Infection, feeding outcomes, and QoL. (A) Incidence of infection G ≥2 in the 2 study arms; (B) types of infection G ≥2; (C) body weight variations at 1 month: change in normalized weight from baseline, expressed as a percentage; (D) albumin variation from admittance to discharge: change in normalized serum albumin from baseline, expressed as a percentage; (E) duration of the hospital stay expressed in days; and (F) patient-reported QoL. QoL, quality of life.
Infection, feeding outcomes, and QoL. (A) Incidence of infection G ≥2 in the 2 study arms; (B) types of infection G ≥2; (C) body weight variations at 1 month: change in normalized weight from baseline, expressed as a percentage; (D) albumin variation from admittance to discharge: change in normalized serum albumin from baseline, expressed as a percentage; (E) duration of the hospital stay expressed in days; and (F) patient-reported QoL. QoL, quality of life.
Considering auto-HSCT and allo-HSCT recipients separately, we did not find any evidence of a difference in infection incidence in the 2 diet arms. In particular, in allo-HSCT recipients, G ≥2 infections occurred in 95% of the patients receiving PD vs 76% of patients receiving NRD (RR = 1.2; 95% CI, 0.9-1.7; P = .2).
Globally, the incidence of fever of unknown origin, including febrile neutropenia with unknown origin) and sepsis was comparable in the 2 arms, with 48 cases (43%) in the PD and 37 cases (33%) in the NRD arm (RR = 1.3; 95% CI, 0.9-1.8; P = .1) and 10 cases (9%) in the PD and 15 (13%) in the NRD arms (RR = 0.7; 95% CI, 0.3-1.4; P = .4), respectively (Figure 2B). The use of antibiotic prophylaxis did not prevent severe infection (G ≥3): 57 out of 118 (48%) patients who received antibiotic prophylaxis vs 47 (45%) among patients who did not receive it had a severe infection (RR = 1.1; 95% CI, 0.8-1.4; P = .7).
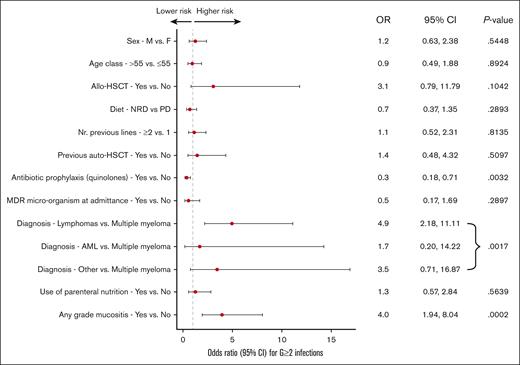
To further assess the impact of the dietary regimen on the risk of G ≥2 infection development, we carried out a multiple logistic regression analysis (Figure 3). The only covariates significantly associated with a lower incidence of G ≥2 infections were the diagnosis of multiple myeloma (P = .001) and the use of antibiotic prophylaxis with quinolones (odds ratio [OR], 0.35; 95% CI, 0.17-0.71; P = .003), whereas the presence of any grade of mucositis was strongly associated with a higher incidence of G ≥2 infections (OR, 3.95; 95% CI, 1.94-8.04; P = .0002).
Multivariate analysis. NRD, non-restrictive diet; PD, protective diet; F, female; M, male; Nr, number.
Multivariate analysis. NRD, non-restrictive diet; PD, protective diet; F, female; M, male; Nr, number.
To investigate how diet interacts with allo-HSCT and antibiotic prophylaxis, 2 multivariable models with interaction terms were fitted. In the multivariable model for the interaction between diet and allo-HSCT (supplemental Table 3), a trend of higher infections G ≥2 in patients undergoing allo-HSCT among those receiving PD was observed (OR, 12.04; 95% CI, 1.19-122.09) than among those receiving NRD, even though the interaction term did not reach statistical significance (interaction P = .0710). The multivariable model for interaction between diet and antibiotic prophylaxis (supplemental Table 4) demonstrated that the impact of antibiotic prophylaxis on infections G ≥2 was similar in the 2 study arms (interaction P = .5444).
With regard to gastrointestinal infections, no significant differences in the incidence of gut infection and any grade mucositis were found in the 2 arms, respectively, 14% in PD vs 14% in NRD (RR = 1.1; 95% CI, 0.6-2.0; P > .99) and 67% in PD vs 71% in NRD (RR = 0.9; 95% CI, 0.8-1.1; P = .6). Notably, in allo-HSCT recipients, the incidence of G ≥2 mucositis between MACand RIC was similar (53% vs 46%; RR = 1.2; 95% CI, 0.6-2.1; P = .7). Surprisingly, a trend for higher GI infections with confirmed microbiological isolation in PD arms was observed but did not reach statistical significance (9% in PD vs 2.7% in NRD; RR = 3.3; 95% CI, 1.02-11; P = .09).
allo-HSCT: GVHD
We analyzed the frequency of GVHD in patients receiving allo-HSCT. The incidence of aGVHD G ≥3 was similar between the 2 arms (20% in PD vs 9.5% in NRD [P = .4]; RR = 2.1; 95% CI, 0.5-9.1; P = .4). No differences were found between the 2 arms in terms of any grade aGVHD (35% in PD vs 29% in NRD; RR = 1.2; 95% CI, 0.5-3; P = .7) or intestinal aGVHD (15% in PD vs 10% in NRD; RR = 1.5; 95% CI, 0.3-7.3; P = .7). Dietary regimen did not affect neutrophil and platelet engraftment after allo-HSCT (data not shown).
Feeding outcomes
The incidence of nausea and diarrhea was similar between the 2 arms, respectively, 17% in PD vs 15% in NRD (RR = 1.1; 95% CI, 0.6-2.0; P = .9) and 31% in PD vs 34% in NRD (RR = 0.9; 95% CI, 0.6-1.3; P = .7). Patients randomly assigned to the NRD arm lost significantly less body weight at 1 month compared with patients in the PD arm (mean, −3.7 kg in the PD arm vs −2.7 kg in the NRD arm; P = .04; equal to a mean percentage of weight loss from baseline of −4.6% in the PD arm vs −3.4% in the NRD arm; P = .03; Figure 2C). No differences were found between arms in terms of body weight variations from admittance to discharge (mean, −3.6kg in the PD arm vs −3.2kg in the NRD arm; P = .3), body mass index variations from admittance to discharge (mean, −0.9 in the PD arm vs −0.8 in the NRD arm; P = .7), serum albumin variation from admittance to discharge (mean, −14.5% in the PD arm vs −18% in the NRD arm; P = .1; Figure 2D), or hospitalization length (mean, 21 days in the PD arm vs 22 days in the NRD arm; P = .6; Figure 2E). From the analysis of daily diaries filled out by patients, NRD was associated with higher satisfaction, because 16% of patients receiving PD vs 35% receiving NRD reported that “diet prescription did not negatively affect my alimentation” (RR = 0.5; 95% CI, 0.3-0.8; P = .003; Figure 2F).
In patients requiring nutritional support, the use of parenteral nutrition was preferred over enteral nutrition (3 patients received enteral nutrition vs 55 patients received parenteral nutrition). The use of parenteral nutrition, 26 (23%) in the PD arm and 29 (26%) in the NRD arm (RR = 0.9; 95% CI, 0.6-1.4; P = .8) as well its duration (6.9 days in the PD arm and 6.7 days in the NRD arm; P = .8) was similar between the study arms.
Microbiological isolations
Details regarding microbiological isolation are listed in Table 3. The most frequently isolated bacteria from blood cultures belonged to Enterobacteriaceae family, and the incidence of MDR bacteria was similar in the 2 arms (1% in both arms). The most frequently isolated microorganism via the stool test was Clostridium difficile in both arms (3% in the PD arm vs 2% in the NRD arm).
Microbiological isolation
| . | Total . | PD . | NRD . |
|---|---|---|---|
| Stool culture and stool test | 12 | 9 | 3 |
| Clostridium difficile | 5 | 3 | 2 |
| Campylobacter jejuni | 1 | 1 | 0 |
| Pseudomonas aeruginosa | 2 | 1 | 1 |
| Adenovirus | 3 | 3 | 0 |
| Rotavirus | 1 | 1 | 0 |
| Blood culture | 38 | 16 | 22 |
| Enterobacteriaceae | 13 | 6 | 7 |
| Escherichia coli | 8 (2 ESBL) | 3 (2 ESBL) | 5 |
| Klebsiella pneumoniae | 3 | 2 | 1 |
| Enterobacter cloacae | 2 | 1 | 1 |
| Staphilococcus species | 11 (0 MRSA) | 3 | 8 |
| Pseudomonas aeruginosa | 2 | 1 | 1 |
| Other | 12 | 6 | 6 |
| . | Total . | PD . | NRD . |
|---|---|---|---|
| Stool culture and stool test | 12 | 9 | 3 |
| Clostridium difficile | 5 | 3 | 2 |
| Campylobacter jejuni | 1 | 1 | 0 |
| Pseudomonas aeruginosa | 2 | 1 | 1 |
| Adenovirus | 3 | 3 | 0 |
| Rotavirus | 1 | 1 | 0 |
| Blood culture | 38 | 16 | 22 |
| Enterobacteriaceae | 13 | 6 | 7 |
| Escherichia coli | 8 (2 ESBL) | 3 (2 ESBL) | 5 |
| Klebsiella pneumoniae | 3 | 2 | 1 |
| Enterobacter cloacae | 2 | 1 | 1 |
| Staphilococcus species | 11 (0 MRSA) | 3 | 8 |
| Pseudomonas aeruginosa | 2 | 1 | 1 |
| Other | 12 | 6 | 6 |
ESBL, extended-spectrum beta-lactamases; MRSA, methicillin-resistant Staphylococcus aureus.
Regardless of the diet, the use of antibiotic prophylaxis with fluoroquinolones correlates with a lower incidence of bloodstream infection (9.3% in patients receiving antibiotic prophylaxis vs 23% in patients who did not receive it; RR = 0.4; 95% CI, 0.2-0.8; P = .002) but, as shown earlier, it did not correlate with a lower incidence of severe infection (G ≥3). The use of antibiotic prophylaxis did not affect the incidence of mucositis or stool culture positivization rate (data not shown).
Discussion
Despite the lack of a true standardization of dietary prescription during HSCT, many centers continue to adopt a low-microbial PD.8 To the best of our knowledge, this is the first prospective randomized trial investigating an NRD approach in the setting of auto- and allo-HSCT.
Results of our study demonstrated that the frequency of infections, deaths, nutritional outcomes, and aGVHD were not different between patients receiving a PD vs NRD during the period of neutropenia after HSCT or high-dose chemotherapy. Specifically, the primary end point of the study was met: the incidence of G ≥2 infections and deaths within 30 days after HSCT was not different between the 2 arms (RR = 1; 95% CI, 0.8-1.4; P = .9). The noninferiority of NRD in terms of infection frequency was confirmed in all subgroups considered, with particular interest in allo-HSCT recipients (76% vs 95%), although the trial was not planned to explore this objective. Moreover, considering different conditioning regimens, we did not report any detrimental effect of NRD compared with PD, in patients receiving either MAC or RIC. The G ≥2 mucositis incidence in allo-HSCT recipients treated with a thiotepa-based RIC was 46%, similar to the 1 reported in literature.16
The multivariable analysis identified mucositis of any grade, the absence of quinolone prophylaxis, and any diagnosis other than multiple myeloma as independent factors for the development of G ≥2 infections. The negative role of mucositis has been widely described by several groups and is determined by the gut damage facilitating the entrance of some microorganisms into the bloodstream with consequent bacteremia.17
In the early 2000s, a prospective randomized trial reported the efficacy of quinolones as prophylaxis in patients with neutropenia in terms of the prevention of neutropenic fever and microbiologically defined infections.18 More recently, the European Conference on Infections in Leukemia suggested caution on quinolone prophylaxis because of a lack of advantage on survival and the risk of selecting MDR strains.19 Therefore, after 2018, only a minority of transplant recipients received quinolones as bacterial prophylaxis. Regardless of the dietary regimen, we reported a reduced bacteriemia frequency in patients treated with quinolones but no differences in terms of G ≥3 infections.
The protective role of multiple myeloma diagnosis in our cohort can be explained by the absence of previous cytotoxic chemotherapies in the treatment history of these patients who usually receive auto-HSCT in the first line after an induction phase with targeted therapies (ie, bortezomib, thalidomide, or lenalidomide). A previous report suggested a correlation between induction chemotherapy and dysbiosis, which may lead to an increased risk of gastrointestinal infection or sepsis.20
As previously reported,21 in our population, colonization with MDR gram-negative bacteria influenced infection incidence or overall survival neither in the PD nor in the NRD arm. A trend toward higher gastrointestinal infections with confirmed microbiological isolation in PD arms was observed, suggesting a detrimental effect of this diet on the constitution of a functional intestinal microbiota, consistent with previously reported data.22,23 Furthermore, the presence of a functional microbiota has been correlated with the outcomes of allogeneic transplantations and the incidence of GVHD.24 It is particularly interesting to note that in our study, the risk ratio for GVHD was twofold higher in patients who received the PD than that in those who received NRD, even if this difference did not reach statistical significance.
After HSCT, weight loss and malnutrition can arise rapidly and have long-term effects.25 Nutrition is an important determinant of the patient's quality of life, and the consumption of some foods, including fresh fruit, traditionally excluded from restrictive diets, is associated with an improvement in gastrointestinal symptoms, including nausea.26 Our study showed that nutritional outcomes with the use of NRD were not inferior to those of PD and that patients receiving NRD reported higher satisfaction with the diet.
These results, together with data published in settings other than after transplantation,27-33 demonstrate that the use of a restrictive diet is an unnecessary burden for patients’ quality of life. Undoubtedly, our data are more mature in the setting of auto-HSCT, in which the use of a restrictive diet should no longer be recommended. Subgroup analysis on allo-HSCT recipients is not powered to draw practice-changing conclusions; however, it is important to note that no safety concerns have emerged regarding the use of an NRD. Consequently, further trials are needed to validate the use of NRD in the context of allo-HSCT, including haploidentical HSCT.
Acknowledgments
The authors thank the patients, their families, nurses, and data managers.
This study was supported, in part, by AIL Milano and Provincia OdV.
Authorship
Contribution: F.S., V.M., and P.C. designed the research; F.S., V.M., M.P., G.V.L., A.G., C.V., C.T., N.M., G.G., L.S., and S.L. performed the research; R.M. and S.L. provided statistical analysis; and F.S., V.M., A.D.F., and P.C. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paolo Corradini, Hematology Division, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico, Istituto Nazionale Tumori and University of Milano, Via Venezian 1, 20133 Milan, Italy; e-mail: paolo.corradini@unimi.it.
References
Author notes
∗F.S. and V.M. contributed equally as joint first authors.
Deidentified patient data are available on request from the corresponding author, Paolo Corradini (paolo.corradini@unimi.it).
The full-text version of this article contains a data supplement.