Key Points
This first multicenter trial of MAC haplo-BMT with PTCy in pediatric patients with acute leukemias ahowed 0% TRM and 0% grade 3 or 4 aGVHD.
Our study shows low CuIs of grade 2 aGVHD and moderate-to-severe cGVHD and similar relapse rates as those of matched-donor BMT.
Abstract
Promising results have been reported for adult patients with high-risk hematologic malignancies undergoing haploidentical bone marrow transplant (haploBMT) with posttransplant cyclophosphamide (PTCy). To our knowledge, we report results from the first multicenter trial for pediatric and young adult patients with high-risk acute leukemias and myelodysplastic syndrome (MDS) in the Pediatric Transplantation and Cellular Therapy Consortium. Nine centers performed transplants in 32 patients having acute leukemias or MDS, with myeloablative conditioning (MAC), haploBMT with PTCy, mycophenolate mofetil, and tacrolimus. The median patient age was 12 years. Diagnoses included AML (15), ALL (11), mixed-lineage leukemia (1), and MDS (5). Transplant-related mortality (TRM) at 180 days was 0%. The cumulative incidence (CuI) of grade 2 acute graft-versus-host disease (aGVHD) on day 100 was 13%. No patients developed grades 3-4 aGVHD. The CuI of moderate-to-severe chronic GVHD (cGVHD) at 1 year was 4%. Donor engraftment occurred in 27 patients (84%). Primary graft failures included 3 patients who received suboptimal bone marrow grafts; all successfully engrafted after second transplants. The CuI of relapse at 1 year was 32%, with more relapse among patients MRD positive pre-BMT vs MRD negative. Overall survival rates at 1 and 2 years were 77% and 73%, and event-free survival rate at 1 and 2 years were 68% and 64%. There was no TRM or severe aGVHD, low cGVHD, and favorable relapse and survival rates. This successful pilot trial has led to a phase 3 trial comparing MAC haploBMT vs HLA-matched unrelated donor BMT in the Children’s Oncology Group. This trial was registered at www.clinicaltrials.gov as #NCT02120157.
Introduction
HLA-haploidentical bone marrow transplant (haplo-BMT) has increased significantly in the past decade with the development of novel methods to control powerful allogeneic reactions generated in the HLA-mismatched setting. With the success of posttransplant cyclophosphamide1-11 (PTCy) and other novel graft-versus-host disease (GVHD) prophylaxis strategies12-16 in allowing for safe and effective mismatched donor transplants, almost everyone in need now has a donor option. Worldwide, studies primarily in adult patients with hematologic malignancy have shown that related haplo-BMT with PTCy provides results similar to those seen with both matched related17-20 and matched unrelated donors21-25 (MUDs). Haplo-BMT with PTCy is an affordable option readily adaptable to clinical settings around the globe and has helped fill the donor availability gap for racial and ethnic minorities. In addition, the incidence of donor-derived malignancies is not higher with PTCy than without it,26 and there is no increased risk of Epstein-Barr virus (EBV) posttransplant lymphoproliferative disease.26,27 Although haplo-BMT with PTCy has gained traction for pediatric patients with leukemia, particularly in single-center studies,28-37 there is a paucity of prospective, multicenter trial data. As such, enthusiasm for using haploBMT with PTCy in younger patients lags behind our adult colleagues.
Much of the available literature on using haplo-BMT with PTCy for adult hematologic malignancies is in the context of reduced-intensity conditioning or nonmyeloablative (NMA) conditioning regimens. In contrast, those performing pediatric BMTs generally use myeloablative conditioning (MAC), with full-dose total-body irradiation (TBI)-based conditioning for acute lymphoblastic leukemia (ALL) and chemotherapy-based conditioning for acute myeloid leukemia (AML). Potential differences in disease biology and graft source (peripheral blood vs bone marrow) add additional concern that data of adult haplo-BMT with PTCy are not directly translatable to pediatric hematologic malignancies.
Herein, to our knowledge, we report the results of the first prospective international multicenter consortium pilot trial of haplo-BMT with PTCy, using MAC in children, and adolescents and young adults (AYAs) with high-risk hematologic malignancies.
Methods
Study design
This was a Pediatric Transplantation and Cellular Therapy Consortium international, phase 2, single-arm, prospective clinical trial (#NCT02120157) using MAC T-cell–replete haplo-BMT with PTCy in children and AYA with high-risk leukemias. The study protocol was approved by the institutional review board at each participating center and by the Johns Hopkins Medicine Institutional Review Board. Written informed consent for all patients was obtained in accordance with the Declaration of Helsinki before the initiation of conditioning therapy. The primary objective was transplant-related mortality (TRM) at 180 days (TRM180), with the hypothesis that TRM180 would be <17%. Secondary end points included donor engraftment, time to platelet and neutrophil recovery, acute GVHD (aGVHD), chronic GVHD (cGVHD), event-free survival (EFS), overall survival (OS), and GVHD relapse–free survival (GRFS).
A total of 32 patients were enrolled in this pilot trial between July 2015 and November 2017. Patients aged between 6 months and 25 years with high-risk leukemia, as previously defined within the Children’s Oncology Group trials, in complete remission (defined as morphology with <5% blasts and no morphological characteristics of acute leukemia in a bone marrow with >20% cellularity), or with myelodysplastic syndrome (MDS) and without an HLA-matched sibling donor were eligible. Additional inclusion criteria included left ventricular ejection fraction >50%; for patients receiving a nonTBI regimen, a forced expiratory volume in 1 second and forced vital capacity ≥50% of predicted and for patients receiving a TBI-based preparative regimen, ≥60%; total bilirubin level <2 mg/dL, alanine aminotransferase or aspartate aminotransferase levels <3 × the laboratory upper limits of normal, and creatinine clearance >60 mL/min; and no evidence of anti–donor HLA antibodies. Grade 3 to 5 adverse events (AEs) possibly, probably, or definitely related to PTCy were collected, as were grade 3 to 5 infections, serious AEs, and unanticipated grade 3 to 5 events.
Transplant platforms
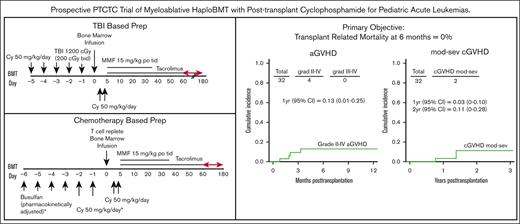
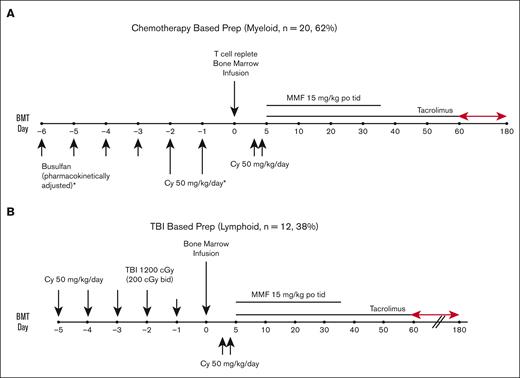
All patients received a MAC regimen with busulfan/cyclophosphamide (Bu/Cy), except those with ALL who received a Cy/TBI preparative regimen. For the patients receiving Bu/Cy (Figure 1A), Bu was given every 6 hours for 4 consecutive days, followed by Cy at 50 mg/kg per day for 2 successive days. Bu dosing was started at either 0.8 mg/kg per dose, 32 mg/m2 per dose, or 1.1 mg/kg per dose IV, in accordance with the age and weight guidelines. Dosing was adjusted for the fifth and subsequent doses based on measured pharmacokinetic variables to achieve a targeted area under the concentration curve from 800 to 1400 mmol × min/L (∼53-92 mg × h/L). Seizure prophylaxis with levetiracetam was administered for all patients receiving Bu aged >10 years. For the patients receiving Cy/TBI (Figure 1B), Cy 50 mg/kg per day was given for 2 consecutive days followed by TBI, with a total dose of 1200 cGy (either 200 cGy twice a day for 3 days beginning on day −3 or 150 cGy twice a day for 4 days starting on day −4). On day 0, patients received unmanipulated bone marrow at least 24 hours after the last dose of Cy. Allografts comprised unmanipulated bone marrow from an HLA-haploidentical (matched for at least 1 allele each of HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1) first-degree relative (full or half sibling, parent, or child), with a targeted dose of 4 × 108 marrow total nucleated cells per kilogram (TNC/kg) of recipient ideal body weight (IBW) and a recommended minimum yield of 2.5 × 108 marrow TNC/kg of recipient IBW. All patients received Cy 50 mg/kg per day on posttransplant days 3 and 4 (on day 3, Cy was given 60-72 hours after the start of bone marrow infusion). Mesna was administered with high-dose Cy at >80% of Cy dosing, per institutional standards. Additional GVHD prophylaxis consisted of mycophenolate mofetil 15 mg/kg orally, 3 times per day (maximum total daily dose, 3 g) from days 5 to 35, and tacrolimus 0.015 mg/kg per dose 2 times daily, adjusted to maintain a serum trough level of 5 to 15 ng/mL (or institutional equivalent), initially given from days 5 to 180 but subsequently ending as early as day 60 after the data with early cessation of tacrolimus were published.38 Tacrolimus was given IV until the patient tolerated oral medications. With this protocol, filgrastim was not routinely given before engraftment. Centers were instructed to notify the principal investigator if filgrastim was initiated and the reason for its start. An example of a reason to start filgrastim provided in the protocol was neutropenia with severe infection.
Supportive care was provided per standard institutional practice. Post-BMT central nervous system prophylaxis was per institutional practice.
Definitions and end points
Neutrophil engraftment was defined as the number of days from BMT to the first of 3 consecutive days with an absolute neutrophil count >0.5 × 109/L. Platelet recovery time was defined as the number of days from BMT until the platelet count was >20 × 109/L without platelet transfusion in the preceding 7 days. Primary graft failure was defined as <5% donor chimerism in the blood or bone marrow by ∼day 60 and upon all subsequent measurements. Secondary graft failure was determined based on an initial achievement of >5% donor chimerism, followed by its sustained loss with <5% donor chimerism in the blood or bone marrow. The definition and grading of aGVHD and cGVHD were in accordance with the Blood and Marrow Transplant Clinical Transplant Network’s Manual of Procedures.39 Pretransplant disease evaluation included, at minimum, bone marrow aspirate and flow cytometry. Minimal residual disease (MRD) was defined as any evidence of malignant cells via flow cytometry, fluorescence in-situ hybridization, polymerase chain reaction, or other techniques without morphological or cytogenetic evidence of disease in the blood or bone marrow.
Per the protocol, posttransplant disease evaluation was first performed on day 60. Relapse was defined as the reappearance of leukemic blast cells in the peripheral blood; or >5% blasts in the bone marrow, not attributable to another cause (eg, bone marrow regeneration); the appearance of previous new dysplastic changes (MDS-specific) within the bone marrow; the development of extramedullary leukemia or the presence of leukemic cells in the cerebral spinal fluid, or the reappearance of cytogenetic or molecular abnormalities present before transplant. Standard relapse-prevention therapies after BMT, such as tyrosine kinase inhibitors for Ph+ leukemias, and sorafenib for FLT3–internal tandem duplication leukemias, were allowed.
EFS was defined as the time from transplant to the first occurrence of any disease progression or relapse, an unplanned therapeutic maneuver for disease persistence, or death from any cause. Data of patients without any event were censored at the last clinic visit at which they were known to be disease free. OS was defined as the time from transplant to death from any cause. TRM was defined as death without evidence of disease progression or relapse after transplant. TRM was considered a competing risk for relapse and vice versa. Competing risks for GVHD included graft failure, relapse, or death. GRFS was defined as the time from BMT to the onset of grade 3 or 4 aGVHD, cGVHD requiring systemic immunosuppression, disease relapse, or death.
Statistical analysis
The proportion of patients with TRM180 was reported with an exact 95% confidence interval (CI). The cumulative incidence (CuI) of relapse and GVHD were estimated via subdistribution functions with 95% CIs via the Aalen method.40,41 Specifically, relapse was further compared using disease (myeloid vs lymphoid), disease risk index (DRI; low, intermediate, high), and pre-BMT MRD (positive vs negative). EFS and OS were estimated with the Kaplan and Meier method, and patient subgroups were compared using the log-rank statistic or Cox proportional hazards regression models. Outcomes with competing risks were assessed with the proportional subdistribution hazard regression model for competing risks.42 Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC) and R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient, donor, and allograft characteristics
Patient characteristics are summarized in Table 1. In total, 32 patients were enrolled in the study. The median recipient age was 12 years (range, 1-23 years), and the median donor age was 35 years (range, 4-53 years). Of the patients, 18 (59%) were male. The median HLA mismatch was 5 of 10 alleles. A total of 24 patients (75%) received their graft from parents, and 8 (25%) from siblings. The median (interquartile range [IQR]) TNC/kg of recipient IBW was 5.2 × 108 (2.8 × 108-6.3 × 108), and the median (IQR) of CD34+ cells per kilogram of recipient IBW was 4 × 106 (2.8 × 106-6.3 × 106). All patients received T-cell–replete, haploidentical bone marrow. The conditioning regimen for all patients consisted of MAC therapy. Twenty patients (62%) received a Bu-based preparative regimen, whereas 12 received a TBI-based regimen. The Center for International Blood and Marrow Transplant Research Pediatric DRI assignments43 included 1 patient (3%) at the low risk, 16 patients (50%) at intermediate risk, and 15 patients (47%) at high risk. Four patients with AML and 1 with ALL had evidence of MRD before BMT. Five patients with MDS had evidence of disease, including 3 with multilineage dysplasia and 2 with excess blasts.
Patient, donor, and graft characteristics
| Variable . | Entire cohort (n = 32) . |
|---|---|
| Sex, n (%) | |
| Male | 18 (59) |
| Female | 14 (41) |
| Median age, Y (range) | |
| Recipient | 12 (1-23) |
| Donor | 35 (4-53) |
| MRD results, n (%) | |
| Negative | 22 (69) |
| Positive | 10 (31) |
| DRI, n (%) | |
| Low/intermediate | 17 (53) |
| High | 15 (47) |
| Diagnosis, n (%) | |
| AML | 15 (47) |
| MDS | 5 (16) |
| ALL | 11 (34) |
| Mixed lineage | 1 (3) |
| Donor relation, n (%) | |
| Sibling | 8 (25) |
| Parent | 24 (75) |
| HLA alleles, n (range) | |
| Median HLA match | 5/10 (5-8/10) |
| No. of HLA alleles match | n (%) |
| 8/10 | 2 (6) |
| 7/10 | 2 (6) |
| 6/10 | 6 (19) |
| 5/10 | 22 (69) |
| Cell dose infused, n (range) | |
| TNC/kg BM infused | 4.8 × 108 (1.2 × 108-6.8 × 108) |
| CD34+/kg BM infused | 4.3 × 106 (0.9 × 106-9.3 × 106) |
| Conditioning regimen, n (%) | |
| Bu/Cy | 20 (62) |
| Cy/TBI | 12 (38) |
| Variable . | Entire cohort (n = 32) . |
|---|---|
| Sex, n (%) | |
| Male | 18 (59) |
| Female | 14 (41) |
| Median age, Y (range) | |
| Recipient | 12 (1-23) |
| Donor | 35 (4-53) |
| MRD results, n (%) | |
| Negative | 22 (69) |
| Positive | 10 (31) |
| DRI, n (%) | |
| Low/intermediate | 17 (53) |
| High | 15 (47) |
| Diagnosis, n (%) | |
| AML | 15 (47) |
| MDS | 5 (16) |
| ALL | 11 (34) |
| Mixed lineage | 1 (3) |
| Donor relation, n (%) | |
| Sibling | 8 (25) |
| Parent | 24 (75) |
| HLA alleles, n (range) | |
| Median HLA match | 5/10 (5-8/10) |
| No. of HLA alleles match | n (%) |
| 8/10 | 2 (6) |
| 7/10 | 2 (6) |
| 6/10 | 6 (19) |
| 5/10 | 22 (69) |
| Cell dose infused, n (range) | |
| TNC/kg BM infused | 4.8 × 108 (1.2 × 108-6.8 × 108) |
| CD34+/kg BM infused | 4.3 × 106 (0.9 × 106-9.3 × 106) |
| Conditioning regimen, n (%) | |
| Bu/Cy | 20 (62) |
| Cy/TBI | 12 (38) |
NRM
The primary objective of our study was TRM180. The incidence of TRM at 6 and 12 months was 0% (exact 95% CI, 0-10.9).
Engraftment/chimerism
The median day of neutrophil engraftment was 22 days (range, 14-58 days) and 21 days (range, 12-44 days) for platelets. Engraftment of donor cells with donor chimerism of >95% at 60 days was achieved in 27 patients (84%). Of these patients, 2 received filgrastim (both for slow count recovery at the discretion of the treating physicians).
Patients who did not achieve >95% donor chimerism included 3 patients with MDS, who did not undergo pretransplant disease reduction, and 2 patients with AML. The median (IQR) TNC/kg of recipient IBW for those patients who did not achieve engraftment was statistically significantly lower at 2.6 × 108 (2.0 × 108-2.9 × 108) than those who achieved engraftment, at 5.2 × 108 (3.9 × 108-6.7 × 108; P = .0147). All patients who failed to achieve engraftment received a salvage haplo-BMT, which engrafted, and at the time of analysis, all 5 patients were alive, 4 of them with no evidence of disease.
GVHD, relapse, and transplant-related complications
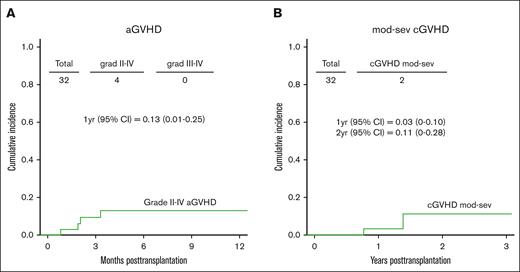
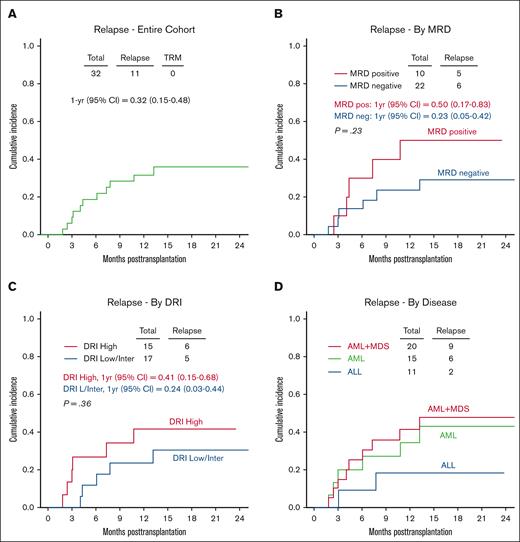
The CuI of grade 2 aGVHD at 1 year was 13% (95% CI, 1-25; Figure 2A). No patients experienced grade 3 to 4 aGVHD. Two patients developed moderate-to-severe cGVHD with a CuI at 1 and 2 years of 3% (95% CI, 0-10) and 11% (95% CI, 0-28), respectively (Figure 2B). The CuI of relapse at 1 and 2 years was 32% (95% CI, 15-48) and 36% (95% CI, 18-54), respectively (Figure 3A). Although not statistically significant, patients with pre-BMT positive MRD results and high DRI had higher relapse incidences than patients with MRD-negative results and those with a low/intermediate DRI (Figure 3B-C). The CuI of relapse at 1 year in patients with pre-BMT MRD positive results was 50% (95% CI, 17-83) compared with 23% (95% CI, 5-42) in patients with MRD-negative results. Patients with a high DRI had a CuI of relapse at 1 year of 41% (95% CI, 15-68) vs 24% (95% CI, 3-44) in patients with a low/Intermediate DRI. The estimated CuI of relapse at 1 year in ALL was 18% (95% CI, 6-42), in AML it was 35% (95% CI, 9-60), and in AML+ MDS it was 41% (95% CI, 18-64) (Figure 3D). Infections were the most common transplant-related complication and included catheter-associated grade 3–related infections (n = 5), enterocolitis (n = 2), Clostridium difficile (n = 2), bacteremia (n = 3), sepsis (n = 1), cytomegalovirus reactivation (n = 2), EBV reactivation (n = 1), parainfluenza (n = 1), and varicella-zoster virus infection (n = 1). Sinusoidal obstruction syndrome (SOS) occurred in 4 patients (common terminology criteria for AEs, grade 3 = 3 and grade 4 = 1), which fully resolved. Two patients developed grade 3 hemorrhagic cystitis, both cases BK virus-positive that presented on days 27 and 40 after transplantation, respectively. Grade 3 mucositis was reported in 13 patients. Most importantly, none of these complications resulted in mortality. No patients with transplant-associated thrombotic microangiopathy (TA-TMA) were reported.
Relapse. (A) CuI of relapse; (B) CuI of relapse based on the MRD results; (C) CuI of relapse based on the DRI; and (D) CuI of based on the by disease type.
Relapse. (A) CuI of relapse; (B) CuI of relapse based on the MRD results; (C) CuI of relapse based on the DRI; and (D) CuI of based on the by disease type.
OS, EFS, and GRFS
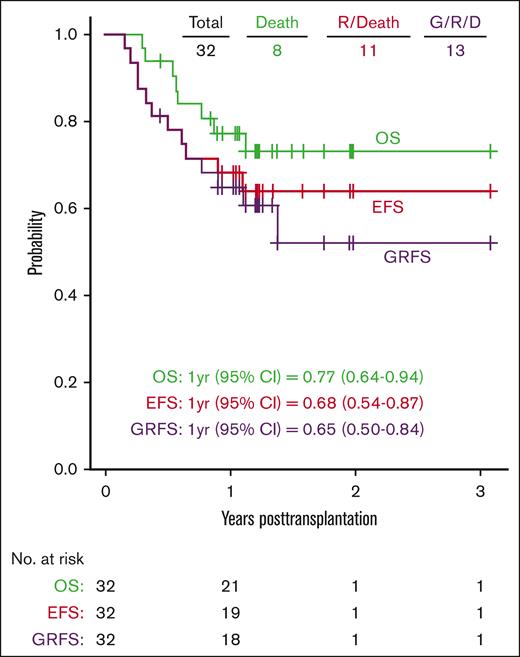
The median follow-up was 15 months, ranging from 5 months to 3.1 years. The OS probabilities at 1 and 2 years were 77% (95% CI, 64-94) and 73% (95% CI, 59-91), respectively. The estimated 1-year and 2-year EFSs were 68% (95% CI, 54-87) and 64% (95% CI, 49-84), respectively, and GRFSs were 65% (95% CI, 50-84) and 52% (95% CI, 34-79), respectively (Figure 4).
Discussion
We report the results from the first international, multicenter phase 2 Pediatric Transplantation and Cellular Therapy Consortium trial using MAC, T-cell–replete haplo-BMT, and PTCy in children and AYA patients with high-risk hematologic malignancies. Impressively, for our primary end point, we had no TRM, supporting the safety and tolerability of this regimen. Albeit with small numbers, having a CuI of 0% TRM compares very favorably with other published regimens.15,44,45 Moreover, the CuIs of severe aGVHD and moderate-to-severe cGVHD requiring systemic immunosuppression are lower than those of other successful BMT platforms and donor sources.15,45-48 This provides an opportunity to continue to shorten the duration of posttransplant tacrolimus for all patients in future trials using MAC regimens, as has already been safely accomplished in the NMA setting.38 This is especially important given that relapse remains challenging after BMT regardless of donor type, graft source, or conditioning regimen intensity. Incorporating novel agents early after the transplant to prevent or treat relapse is optimized when patients discontinue immunosuppression and are without GVHD.49 We did not collect data on the use of posttransplant relapse–prevention therapy in this trial.
Several aspects of our data warrant further discussion. Firstly, graft failure was higher than expected in this trial: 3 patients with untreated MDS and 2 with AML, all receiving chemotherapy-based conditioning with Bu/Cy, failed to engraft. These patients underscore 2 key factors when using haploidentical donors: (1) the importance of graft cell dose and (2) the challenge of engraftment for patients who are naive to any cytoreductive therapy, even after MAC. All 5 patients received a TNC/kg cell dose lower than the recommended target yield; in fact, the 2 patients with AML received a TNC/kg lower than the minimum recommended target yield (2.5 × 108 TNC/kg of recipient IBW). The necessity of adequate cell dose to facilitate engraftment is evident in this small data set, as in other trials.50 Continuing to provide harvest training and education regarding the importance of graft cell dose will be important for future trials. Three patients with MDS also had not received any pretransplant disease reduction therapy before BMT. Strategies to improve engraftment for these patients could also include using peripheral blood stem cells (PBSCs) or pretransplant therapy with agents such as azacytidine, as has been used in the NMA setting, or both.38,51 Ultimately, all 5 patients achieved engraftment after salvage haplo-BMT. Four of them received PBSCs, 2 from the same donor, 2 from a second donor, and one of the patients received bone marrow stem cells from the same donor. Salvage conditioning regimens included alemtuzumab (0.2 mg/kg per dose), fludarabine (flu; 30 mg/m2 per dose), Cy (2 grams/m2 per dose), and 200 cGy TBI (n = 2), all on day −1; antithymocyte globulin (3.5 mg/kg divided over 2 doses) and flu (200 mg/m2 divided over 5 doses; n = 1); flu (150 mg/m2 divided over 5 doses), Cy (28 mg/kg divided over 2 doses), and 200 cGy TBI (n = 1); and alemtuzumab, flu, and Cy (n = 1, doses not provided).
Secondly, the incidences of transplant-related complications in this study are similar to those in other published allogeneic BMT studies in the HLA-matched and haploBMT with PTCy settings.35,52-54 Infections were the most common transplant-related complication. They included bacterial infections; BK virus–associated hemorrhagic cystitis and viral reactivations of EBV, cytomegalovirus, and varicella-zoster virus with no end-organ disease. SOS was reported in 4 patients. In this pilot trial, we did not collect data of which SOS diagnostic criteria were used. Most importantly, none of these complications resulted in mortality. There were no reports of TA-TMA; it is possible that the collection of our AEs/serious AEs, as stated in “Methods,” was not conducive to capturing these patients, and we did not specifically ask centers to report TA-TMA in this trial. Thus, we cannot conclusively report its incidence. A prior publication using haplo-BMT with PTCy reported an extremely low TA-TMA incidence (1.4%).55 Therefore, it is also possible that there were no patients with TA-TMA.
The pediatric BMT community has expressed general concern regarding the CuI of relapse using PTCy. The CuI of relapse reported in published studies using haploidentical donors, MUDs, cord, and matched sibling donors has ranged from 12% to 70%15,44-46,51,56-60 with pre-BMT MRD positivity and a high DRI associated with a higher incidence of relapse. The CuI of relapse of 32% and 36% at 1 and 2 years, respectively, in our small, multicenter study, with lower but not statistically significant CuI of relapse in MRD negative and low-intermediate DRI patients, is directly comparable with that reported in the pediatric BMT published literature and does not support that haplo-BMT with PTCy is inferior. It is important to remember that more than one-third of our patients MRD positive before transplant, almost one-half had a high DRI, and patients with MDS with >10% blasts were eligible and enrolled. Other single-center haplo-BMT trials suggest similar supporting evidence.28,29,32-37 The current standard of discontinuing tacrolimus at day 60 after haplo-BMT was implemented toward the end of this trial, and consideration of PBSC grafts for patients with high-risk disease may provide additional relapse prevention.
Limitations of this study include patient heterogeneity and small numbers. It is difficult to directly compare our results with that of other haplo-BMT platforms, such as the megadose CD34+ selection61 and α-β T-cell–depleted haplo-BMT13,15 without randomized controlled trials. Nevertheless, the absolute number of longitudinal experiences with pediatric patients who have undergone haplo-BMT after MAC is low compared with the number of longitudinal experiences among those who have received MAC allogeneic BMT from HLA-matched donors and cord blood. This underscores the importance of continuing to study MAC haplo-BMT with PTCy, laying the groundwork for prospective randomized controlled BMT trials using MAC, different donor sources, and other haploidentical graft methods, especially for pediatric and AYA populations.
In conclusion, this first (to our knowledge) multicenter, international consortium trial demonstrates that myeloablative HLA–haplo-BMT with PTCy for pediatric and AYA high-risk acute leukemias offers a widely available platform that is safe and feasible. Our findings are an essential contribution to the literature, serving as background for the recently opened Children’s Oncology Group randomized controlled trial of haplo (using both PTCy and α-β T-cell–depleted platforms) vs MUD hematopoietic cell transplants in pediatric patients with high-risk hematologic malignancies.
Acknowledgments
Funding for this trial was provided by National Heart, Lung, and Blood Institute/National Cancer Institute grant UG1HL069254 and a Johnny Crisstopher Children’s Charitable Foundation St. Baldrick’s Consortium grant. M.A.P. was supported by National Cancer Institute grant P30CA040214.
Authorship
Contribution: J.C.F.-P. interpreted data and wrote the manuscript; H.-L.T. and A.B. designed the trial, analyzed and interpreted data, performed the statistical analysis, and reviewed the manuscript; J. Dalal, M.E., M.A.P., S.S.K., D.L., L.L., and L.M. designed the trial, enrolled patients, and reviewed the manuscript; A.C., E.C., J. Davis, J.H., A.K., J.K., and B.O. enrolled patients and reviewed the manuscript; T.F. and M.A.P. designed the trial, interpreted data, and reviewed the manuscript; M.H. designed the trial, enrolled patients, and reviewed the manuscript; and H.J.S. designed the trial, enrolled patients, collected data, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: M.H. reports advisory board activities for Novartis, Mesoblast, Jazz Pharmaceuticals, and Kadmon Pharmaceuticals. D.L. reports research funding, consulting, and licensing fees from Kiadis Pharma. B.O. is a full-time employee of ImmunoGen Inc. M.A.P. reports advisory board activities for Novartis, Gentibio, bluebird bio, Vertex, Medexus, and Equillium; educational honoraria from Novartis; and study support from Miltenyi and Adaptive. H.J.S. reports speaker bureau activity for Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Heather Symons, Pediatric Blood and Marrow Transplantation, Johns Hopkins University, 1650 Orleans St, CRB I 2M-52, Baltimore, MD 21287; e-mail: hsymons2@jhmi.edu.
References
Author notes
Deidentified individual participant data that underlie the reported results will be made available 3 months after publication for a period of 5 years after the publication date on the Pediatric Transplantation and Cellular Therapy Consortium website (theptctc.org).
The study protocol is available at https://www.theptctc.org/pastprotocols.