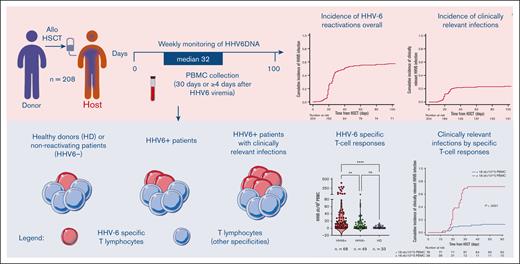
Key Points
HHV-6 reactivation can be life-threatening for patients who are immunocompromised.
Posttransplant HHV-6–specific T cells, detectable via ELISpot assay, are significantly associated with clinically relevant infection.
Abstract
Human herpesvirus 6 (HHV-6) can reactivate after allogeneic hematopoietic stem cell transplant (allo-HSCT) and may lead to severe symptoms. HHV-6–specific immune responses after HSCT are largely unexplored. We conducted a prospective observational study on 208 consecutive adult patients who received allo-HSCT to investigate HHV-6 reactivations and specific immune responses. Interferon gamma–producing HHV-6–specific T cells were quantified using enzyme-linked immunospot assay (ELISpot). HHV-6 reactivation occurred in 63% of patients, at a median of 25 days from allo-HSCT. Only 40% of these presented a clinically relevant infection, defined by the presence of classical HHV-6 end-organ diseases (EODs), based on European Conference on Infections in Leukaemia (ECIL) guidelines, and other possible HHV6-related EODs. Using multivariate analysis, we identified risk factors for HHV-6 reactivation: previous allo-HSCT, posttransplant cyclophosphamide (PT-Cy), and time-dependent steroids introduction. The use of PT-Cy and steroids were associated with clinically relevant infections, whereas higher CD3+ cell counts seemed to be protective. Interestingly, circulating HHV-6–specific T cells were significantly higher in patients with reactivated virus. Moreover, HHV-6–specific T-cell responses, quantified at >4 days after the first viremia detection, predicted clinically relevant infections (P < .0001), with higher specificity (93%) and sensitivity (79%) than polyclonal CD3+ cells per μL. Overall survival and transplant-related mortality were not affected by time-dependent HHV-6 reactivation, whereas a significant association was observed between clinically relevant infections and acute graft-versus-host disease. These results shed light on the role of HHV-6 in allo-HSCT and may affect HHV-6 monitoring and treatment.
Introduction
Over the past decade, the use of hematopoietic stem cell transplant (HSCT) has been constantly growing, along with the introduction of different conditioning regimens and graft-versus-host disease (GVHD) prophylaxis platforms.1 Despite improvements in HSCT outcomes, double-stranded DNA viruses’ reactivation still represents an unsolved issue, contributing substantially to early posttransplant morbidity,2,3 particularly in the context of posttransplant cyclophosphamide (PT-Cy).4,5 Although the introduction of letermovir might be a turning point in cytomegalovirus (CMV) prevention, other double-stranded DNA viruses are still lacking specific management.
Post-HSCT reactivation of human herpesvirus 6 (HHV-6) has been associated with impaired immune reconstitution and end-organ diseases (EODs).6,7 HHV-6 is a member of the β-herpesvirus subfamily associated with roseola infantum. After primary infection, the virus remains latent and can be reactivated during lymphopenia and immune depression. HHV-6 subtype B is responsible for majority of post-HSCT reactivation cases.8 Genome-integrated HHV-6 has been reported, but its pathogenic role is less defined.9 Known risk factors for HHV-6 reactivation in transplant recipients include the use of umbilical cord and HLA-mismatched donors as graft source, T-cell depletion, steroid administration, and acute GVHD.10 Among different clinical symptoms, limbic encephalitis is the most life-threatening HHV-6–related complication.11 Although no antivirals have been approved, foscarnet, ganciclovir, and cidofovir are often used,3 and initial evidence of brincidofovir activity has been reported.12
Knowledge on posttransplant HHV-6–specific T-cell immunoreconstitution is still limited.13,14 Despite HHV-6 high seroprevalence, specific interferon gamma (IFN-γ)-producing T cells are rarely detected in healthy donors (HDs) (<0.1% of the total CD413) and low frequencies of specific spot forming cells (SFCs) were reported upon stimulation with immunodominant proteins (mean, 3 SFCs per 105 peripheral blood mononuclear cells [PBMCs]).15,16 After allo-HSCT, the level of response to HHV-6 immunodominant targets was between 21 and 47 SFCs per 105 PBMCs in an adult patient directly evaluated ex vivo at the time of acute HHV-6 infection (day 29 after HSCT),17 and between 0 and 36 SFCs per 105 PBMCs in children who experienced a viral reactivation at a median of 14 days after HSCT and were evaluated ex vivo at 2 months.18
Early T-cell reconstitution protects against HHV-6 reactivation in transplant recipients,19 with high CD3+ counts at the time of viral reactivation being associated with less severe infections and improved outcomes.20 De Pagter et al reported high HHV-6–specific CD8+ T-cell proliferation and IFN-γ production in patients with early HHV-6 reactivation after HSCT.18 However, they did not investigate any association between the magnitude of HHV-6–specific T-cell response and the severity of the viral reactivation. Furthermore, Hanson et al documented a higher HHV-6 viremia in patients with HSCT who received 10-fold fewer donor-derived HHV-6–specific CD4+ T cells than in patients experiencing milder (<200 copies per mL) or no viral reactivations, corroborating the hypothesis of a potential protective role of HHV-6–specific T cells.21
The aim of this study was to prospectively analyze the clinical aspects and the risk factors for HHV-6 reactivation, its influence on main transplant outcomes and, finally, to investigate the role of HHV-6–specific T-cell immune responses upon viral reactivation.
Materials and methods
Study design and definitions
In this prospective, monocentric, noninterventional study, we analyzed all adult consecutive allogenic HSCT recipients who received the transplant between 2013 and 2015 in our center. A total of 208 consecutive HSCT recipients affected by hematological malignancies (Table 1; supplemental Material) were included in the study. The median follow-up was 4 years after HSCT (range, 0.4-5.7).
Patient and transplant characteristics (n = 208)
| Patient age, y, median (range) | 53 (19-77) |
| Sex, male (%) | 129 (62) |
| Diagnosis, n (%) | |
| Myeloid diseases | |
| AML | 122 (59) |
| MDS or MPN | 32 (15) |
| Lymphoid diseases/myeloma | 54 (26) |
| Disease status at HSCT, n (%) | 57 (27) |
| CR1 | |
| CR > 1 | 28 (14) |
| Active disease | 123 (59) |
| R-DRI at HSCT, n (%) | |
| Not applicable | 10 (5) |
| Low or intermediate | 82 (39) |
| High | 93 (45) |
| Very high | 23 (11) |
| HCT-CI score, median (range) | 2 (0-8) |
| Type of donor, n (%) | |
| MRD | 39 (19) |
| UD | 63 (30) |
| MMRD | 99 (48) |
| CBU | 7 (3) |
| Stem cell source, n (%) | |
| PB | 181 (87) |
| BM | 20 (10) |
| CBU | 7 (3) |
| Conditioning regimen, n (%) | |
| MAC | 169 (81) |
| RTC | 39 (19) |
| CMV serostatus (H/D), n (%) | |
| Pos/pos | 115 (55) |
| Pos/neg | 66 (32) |
| Neg/pos | 7 (3) |
| Neg/neg | 20 (10) |
| GVHD prophylaxis, n (%) | |
| In vivo T-cell depletion | |
| PT-Cy–based | 101 (49) |
| ATG-based | 84 (40) |
| None | 23 (11) |
| Immunesuppression | |
| Sirolimus-based | 161 (78) |
| CsA-based | 47 (22) |
| Patient age, y, median (range) | 53 (19-77) |
| Sex, male (%) | 129 (62) |
| Diagnosis, n (%) | |
| Myeloid diseases | |
| AML | 122 (59) |
| MDS or MPN | 32 (15) |
| Lymphoid diseases/myeloma | 54 (26) |
| Disease status at HSCT, n (%) | 57 (27) |
| CR1 | |
| CR > 1 | 28 (14) |
| Active disease | 123 (59) |
| R-DRI at HSCT, n (%) | |
| Not applicable | 10 (5) |
| Low or intermediate | 82 (39) |
| High | 93 (45) |
| Very high | 23 (11) |
| HCT-CI score, median (range) | 2 (0-8) |
| Type of donor, n (%) | |
| MRD | 39 (19) |
| UD | 63 (30) |
| MMRD | 99 (48) |
| CBU | 7 (3) |
| Stem cell source, n (%) | |
| PB | 181 (87) |
| BM | 20 (10) |
| CBU | 7 (3) |
| Conditioning regimen, n (%) | |
| MAC | 169 (81) |
| RTC | 39 (19) |
| CMV serostatus (H/D), n (%) | |
| Pos/pos | 115 (55) |
| Pos/neg | 66 (32) |
| Neg/pos | 7 (3) |
| Neg/neg | 20 (10) |
| GVHD prophylaxis, n (%) | |
| In vivo T-cell depletion | |
| PT-Cy–based | 101 (49) |
| ATG-based | 84 (40) |
| None | 23 (11) |
| Immunesuppression | |
| Sirolimus-based | 161 (78) |
| CsA-based | 47 (22) |
AML, acute myeloid leukemia; ATG, antithymocyte globulin; BM, bone marrow; CBU, cord blood unit; CR, complete remission; CsA, cyclosporine A; HCT-CI, hematopoietic cell transplant–comorbidity index; H/D, host/donor; HL, Hodgkin lymphoma; MAC, myeloablative conditioning22,23; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; MRD, matched-related donor; MMRD, mismatched related donor; neg, negative; PB, peripheral blood; pos, positive; R-DRI, refined-disease risk index; RTC, reduced-toxicity conditioning24,25; UD, unrelated donor.
Polyclonal immune reconstitution was evaluated via flow cytometry and analyzed with the FCS Express software (de novo software).
HHV-6 was monitored weekly via real-time quantitative polymerase chain reaction (PCR; commercial kit HSV6 Elite MGB from ELITECH group SPA, target gene U67; sensitivity limit between 100 and 2.5 ×106 copies per mL) using plasma samples until day 100 after HSCT; HHV-6 was monitored twice weekly in case of a positive result. All patients and donors were screened before HSCT26; chromosomally integrated HHV-6 with persistent high levels of HHV-6 DNAemia, registered in 6 cases (2.7% of the entire cohort), were excluded from the study. All patients received acyclovir as an antiviral prophylaxis. Our center’s policy, based on European Conference on Infections in Leukaemia (ECIL) guidelines8 and center experience,4,20,27 adopts a multidisciplinary model based on different tools for the diagnosis of HHV-6 reactivation and clinically relevant HHV-6 infection (supplemental Material).
Briefly, HHV-6 reactivation was defined as the detection of HHV6 DNA in the peripheral blood, with 2 consecutive positive PCR samples and an eventual association with fever and rash, whereas clinically relevant HHV-6 infection was arbitrarily defined by the presence of (1) classical HHV-6 related EODs recognized by ECIL-consensus guidelines (proven encephalitis, delayed engraftment, or myelosuppression [detailed definitions are given in supplemental Material]); or (2) possible HHV-6–related EODs for clinical entities with a weaker association with HHV-6 infection (probable or possible encephalitis, pneumonitis, hepatitis, and gastrointestinal disease), prospectively ruling out potential concomitant causes and always in the presence of HHV-6 DNA-positive samples (detailed definitions in supplemental Material).
HHV6 DNA encephalitis were treated with ganciclovir and/or foscarnet per international recommendations,8 whereas antiviral therapy of HHV-6 cases was based on clinician judgment per the institutional guidelines.
HHV-6–specific T cells were enumerated at a median of 32 days after HSCT in all patients experiencing viral reactivation within +60 days, in the presence of >25 circulating CD3+ cells per μL, with available samples stored in the institutional biobank. Concomitantly, we evaluated a cohort of patients who tested negative for HHV-6 as the control group. Inclusion criteria for HHV-6 negative patients were (1) availability of PBMC samples at 30 ± 10 days after allo-HSCT (median +33 days), (2) adequate numbers of circulating CD3+ cells (>25 cells per μL), and (3) survival beyond day 30 after allo-HSCT. Patients developing a disease relapse within day 30 after allo-HSCT were excluded from both groups.
Sampling and IFN-γ enzyme-linked immunospot assay (ELISpot)
Peripheral blood samples were collected either on day 30 after HSCT in patients who tested negative for HHV-6 or starting from the fourth day after the first HHV-6 DNAemia. PBMCs were isolated from patients and HD samples by density gradient centrifugation (Lymphoprep, Sentinel diagnostics) and frozen. Frequencies of IFN-γ–producing HHV-6–specific T cells were determined via ELISpot assay28 using cryopreserved PBMCs (details in supplemental Materials). Cells were stimulated overnight at 37°C and 5% CO2 with a library of overlapping peptides covering the entire sequence of the immunodominant viral protein U54 from HHV-6B strain (10 μg/mL; acquired from JPT).17,29 CMV-specific responses were also assessed using the glycine extract of a human fibroblast cell line infected with the CMV strain AD169.2 (CMV antigen), as previously reported.30 Polyclonal stimulation with phytohemagglutinin (Sigma) was used as the positive control. Specific SFCs were counted using a computer-assisted video image analysis (Ely Analyze V4.2 Reader). Negative controls (medium alone and unstimulated PBMCs) were subtracted, and the frequency of HHV-6–specific T cells was expressed as a specific SFC per 105 PBMCs.
To enrich samples from HDs for HHV-6–specific T cells, PBMC were stimulated with a library of overlapping peptides covering the U54 protein (10 μg/mL; JPT)16 in Iscove modified Dulbecco medium (Lonza) supplemented with 1% glutamine, 1% penicillin/streptomycin, and 10% human serum. After 3 days, recombinant human IL-2 (60 UI/mL; Novartis) was added, and the medium was replaced every 2 or 3 days for a 14-day culture. Cells were tested via IFN-γ ELISpot for the recognition of HHV-6–specific peptides before and after the in vitro enrichment.
Statistical analysis
The following outcomes were measured from the time of stem cell infusion: overall survival (OS), transplant-related mortality (TRM), and progression-free survival (PFS). Definitions are provided in supplemental Materials.
Cumulative incidences (CIs) were estimated for HHV-6 reactivation and clinically relevant infection, GVHD (acute and chronic), relapse, and TRM, to accommodate the competing risks. Relapse/progression was a competing risk for TRM. Relapse/progression and death from any cause were competing risks for GVHD and HHV-6 reactivation or clinically relevant infection.
The probabilities of OS and PFS were estimated using the Kaplan-Meier estimator. Log-rank test was used for univariate comparisons of survival curves, whereas the Gray test was conducted to compare CIs of competing-risk end points.
Cox regression models were used to study factors predicting HHV-6 and analyze whether HHV-6 reactivation influenced the risks of GVHD, TRM, relapse, PFS, and OS. Included variables are defined in supplemental Materials. The proportional hazard assumption was met for all variables. A P value of .05 was considered significant for determination of factors associated with the time to event.
Receiver operating characteristic curve allowed for the selection of the cut-off value of continuous variables able to segregate among different outcomes with the best compromise between sensitivity and specificity. Comparison between 2 groups were carried out using nonparametric Mann-Whitney test or paired t test, as appropriate. In case of >2 groups, Kruskal-Wallis followed by Dunn multiple comparison test or Wilcoxon test were used.
Statistical analyses were performed using R 4.0.4 (R Development Core Team, Vienna, Austria) and GraphPad PRISM version 9.
Ethics statement
All patients were treated in accordance with the institutional programs upon obtaining written informed consent for transplant procedures, use of medical records for research, and immunological studies for patients undergoing allo-HSCT within the noninterventional ALMON study approved by the San Raffaele Institutional Ethical Committee on 19 October 2007.
Results
Transplant outcomes
Engraftment was obtained in 202 patients after a median of 18 days (range, 10-83 days) since HSCT for neutrophils and 32 days since HSCT for platelets (range, 8-93 days). Six patients did not achieve engraftment because of early transplant-related death (n = 4), disease relapse (n = 1), or graft rejection (n = 1).
Overall, 107 patients (51%) reached the threshold of >100/μL CD3+ cells within 30 days since HSCT. On day 30, we observed medians of 65/μL (range, 0-2650/μL) CD4+ T cells, 92/μL (range, 0-6450/μL) CD8+ T cells, and 0/μL (range, 0-109/μL) CD19+ cells.
Transplant outcomes are shown in Table 2. The primary causes of death were disease relapse (n = 53) and TRM (n = 51); there was no death because of HHV-6–related infection.
Transplant outcomes
| . | 30 days . | 100 days . | 1 y . | 4 y . |
|---|---|---|---|---|
| HHV-6 reactivation (CI) | 46% ± 7% | 57% ± 7% | ||
| Clinically relevant HHV-6 infection (CI) | 20% ± 5% | 24% ± 6% | ||
| Grade 2, 3 or 4 aGVHD (CI) | 28% ± 6% | |||
| Grade 3 or 4 aGVHD (CI) | 13% ± 4% | |||
| cGVHD (CI) | 25% ± 6% | 33% ± 6% | ||
| TRM (CI) | 18 % ± 5% | 26% ± 6% | ||
| Relapse (CI) | 26% ± 6% | 32% ± 6% | ||
| OS | 64% ± 6% | 47% ± 7% | ||
| PFS | 56% ± 7% | 42% ± 7% |
| . | 30 days . | 100 days . | 1 y . | 4 y . |
|---|---|---|---|---|
| HHV-6 reactivation (CI) | 46% ± 7% | 57% ± 7% | ||
| Clinically relevant HHV-6 infection (CI) | 20% ± 5% | 24% ± 6% | ||
| Grade 2, 3 or 4 aGVHD (CI) | 28% ± 6% | |||
| Grade 3 or 4 aGVHD (CI) | 13% ± 4% | |||
| cGVHD (CI) | 25% ± 6% | 33% ± 6% | ||
| TRM (CI) | 18 % ± 5% | 26% ± 6% | ||
| Relapse (CI) | 26% ± 6% | 32% ± 6% | ||
| OS | 64% ± 6% | 47% ± 7% | ||
| PFS | 56% ± 7% | 42% ± 7% |
aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease.
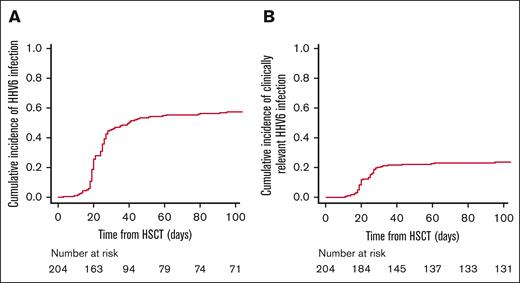
HHV-6 reactivation
HHV-6 reactivation occurred in 130 patients (63%; CI, 57% ± 7% at 100 days; Figure 1A). The median time from allo-HSCT to the first HHV-6 reactivation was 25 days (range, 3-538 days). At onset, the median value of viremia in the peripheral blood was 885 (range, 26-207 042) copies per mL, reaching a peak of 1721 (range, 26-1 537 606) copies per mL. At the time of viral reactivation, 68 patients were on steroid therapy: in 22 patients, ≤0.5 mg/kg (17%); in 28 patients, 1 mg/kg (21%); and in 18 patients, 2 mg/kg (14%).
HHV-6 reactivation and clinically relevant infection. (A) CI of total HHV-6 reactivation; (B) CI of HHV-6 clinically relevant infection.
HHV-6 reactivation and clinically relevant infection. (A) CI of total HHV-6 reactivation; (B) CI of HHV-6 clinically relevant infection.
Within the group of HHV-6–positive patients, clinically relevant HHV-6 infections were observed in 52 patients (40%; CI, 24% ± 6% at 100 days; Figure 1B). Furthermore, we observed the following classical HHV-6–related EODs: delayed engraftment or myelosuppression (n = 39) and proven encephalitis (n = 4), with a concomitant syndrome of inappropriate antidiuretic hormone secretion (SIADH) in 1 case. Moving to possible HHV-6 related EODs, we observed: probable/possible encephalitis (n = 5; probable n = 3 and possible n = 2), with concomitant SIADH in 3 cases: pneumonitis (n = 3), gastrointestinal diseases (upper n = 18 and lower n = 28), and hepatitis (n = 1). Some patients had >1 symptom compatible with EOD. At onset, the median value of viremia in the peripheral blood was 1091 (range, 181-6875) copies per mL, reaching a peak of 5259 (range, 475-22 519) copies per mL. At the time of clinically relevant HHV-6 infection, 27 patients were on steroid therapy: in 15 patients ≤0.5 mg/kg, in 6 patients, 1 mg/kg; and in 6 patients, 2 mg/kg.
The timeframe from first HHV-6 DNA positivity in the peripheral blood to the development of clinical entities was at least 15 days (median, 1 month). Overall, the 52 patients who developed clinically relevant HHV-6 infections received antiviral therapy for a median of 21 days, consisting of ganciclovir (n = 32), foscarnet (n = 9), or combination therapy (n = 11).
Via multivariate analyses (Table 3), HHV-6 reactivation was significantly associated with a history of prior allo-HSCT (hazard ratio [HR], 2.89; confidence interval [CI], 1.49-5.60; P < .01), use of PT-Cy as GVHD prophylaxis (HR, 2.59; CI, 1.50-4.47; P < .01), and the time-dependent introduction of any dose of steroids after HSCT (1 mg/kg vs no steroids: HR, 3.64; CI, 2.34-5.66; 2 mg/kg vs no steroids: HR, 4.2; CI, 2.29-7.70; P < .01). Moreover, CD3+ cell counts >100 cell per μL upon viral reactivation (HR, 0.23; CI, 0.07-0.81; P = .02) were protective against clinically relevant HHV-6 infection, whereas the use of PT-Cy (HR, 3.77; CI, 1.47-9.64; P < .01) and of steroids at any dose (1 mg/kg vs no steroids: HR, 2.74; CI, 1.44-5.24; 2 mg/kg vs no steroids: HR, 3.54; CI, 1.31-9.53; P < .01) increased the risk of developing clinically relevant HHV-6 infection significantly (Table 3).
Risk factors for HHV-6 reactivation (multivariate analysis)
| Risk factors . | Any HHV-6 reactivation . | Clinically relevant HHV-6 infection . | ||
|---|---|---|---|---|
| P value . | HR (ci 95%) . | P value . | HR (ci 95%) . | |
| CD3 counts >100 cell per μL | .09 | 0.55 (0.27-1.10) | .02 | 0.23 (0.07-0.81) |
| Age > 53 y | .06 | 1.47 (0.99-2.19) | .20 | 1.51 (0.81-2.82) |
| Disease (vs acute leukemia) | .16 | .14 | ||
| Lymphoma/MM | .75 | 1.08 (0.67-1.74) | .76 | 0.90 (0.44-1.82) |
| MDS/MPN | .08 | 0.59 (0.33-1.06) | .05 | 0.34 (0.12-0.98) |
| Disease status (vs CR1) | .62 | .22 | ||
| Active disease | .57 | 0.83 (0.43-1.60) | .19 | 0.48 (0.16-1.43) |
| CR > 1 | .33 | 0.78 (0.48-1.28) | .11 | 0.55 (0.27-1.43) |
| HCT-CI > 2 | .14 | 0.75 (0.51-1.10) | .41 | 0.78 (0.43-1.41) |
| Previous allo-HSCT | <.01 | 2.89 (1.49-5.60) | .68 | 1.26 (0.42-3.82) |
| MAC (vs RTC) | .12 | 1.59 (0.81-3.13) | .61 | 1.35 (0.42-4.32) |
| Donor (vs haploidentical) | .40 | .95 | ||
| MRD | .97 | 0.99 (0.53-1.83) | .57 | 1.29 (0.54-3.10) |
| MUD | .24 | 1.44 (0.78-2.66) | .78 | 1.16 (0.41-3.31) |
| CBU | .33 | 0.47 (0.10-2.14) | .33 | 0.47 (0.11-2.14) |
| PT-Cy (vs ATG) | <.01 | 2.59 (1.50-4.47) | <.01 | 3.77 (1.47-9.64) |
| Steroids | <.01 | <.01 | ||
| 1 mg/kg | <.01 | 3.64 (2.34-5.66) | <.01 | 2.74 (1.44-5.24) |
| 2 mg/kg | <.01 | 4.2 (2.29-7.70) | <.01 | 3.54 (1.31-9.53) |
| Risk factors . | Any HHV-6 reactivation . | Clinically relevant HHV-6 infection . | ||
|---|---|---|---|---|
| P value . | HR (ci 95%) . | P value . | HR (ci 95%) . | |
| CD3 counts >100 cell per μL | .09 | 0.55 (0.27-1.10) | .02 | 0.23 (0.07-0.81) |
| Age > 53 y | .06 | 1.47 (0.99-2.19) | .20 | 1.51 (0.81-2.82) |
| Disease (vs acute leukemia) | .16 | .14 | ||
| Lymphoma/MM | .75 | 1.08 (0.67-1.74) | .76 | 0.90 (0.44-1.82) |
| MDS/MPN | .08 | 0.59 (0.33-1.06) | .05 | 0.34 (0.12-0.98) |
| Disease status (vs CR1) | .62 | .22 | ||
| Active disease | .57 | 0.83 (0.43-1.60) | .19 | 0.48 (0.16-1.43) |
| CR > 1 | .33 | 0.78 (0.48-1.28) | .11 | 0.55 (0.27-1.43) |
| HCT-CI > 2 | .14 | 0.75 (0.51-1.10) | .41 | 0.78 (0.43-1.41) |
| Previous allo-HSCT | <.01 | 2.89 (1.49-5.60) | .68 | 1.26 (0.42-3.82) |
| MAC (vs RTC) | .12 | 1.59 (0.81-3.13) | .61 | 1.35 (0.42-4.32) |
| Donor (vs haploidentical) | .40 | .95 | ||
| MRD | .97 | 0.99 (0.53-1.83) | .57 | 1.29 (0.54-3.10) |
| MUD | .24 | 1.44 (0.78-2.66) | .78 | 1.16 (0.41-3.31) |
| CBU | .33 | 0.47 (0.10-2.14) | .33 | 0.47 (0.11-2.14) |
| PT-Cy (vs ATG) | <.01 | 2.59 (1.50-4.47) | <.01 | 3.77 (1.47-9.64) |
| Steroids | <.01 | <.01 | ||
| 1 mg/kg | <.01 | 3.64 (2.34-5.66) | <.01 | 2.74 (1.44-5.24) |
| 2 mg/kg | <.01 | 4.2 (2.29-7.70) | <.01 | 3.54 (1.31-9.53) |
Significant values are shown in bold.
ATG, antithymocyte globulin; CBU, cord blood unit; CR, complete remission; HCT-CI, hematopoietic cell transplant–comorbidity index; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MM, multiple myeloma; MPN, myeloproliferative neoplasm; MRD, matched-related donor; MUD, matched-unrelated donor; RTC, reduced-toxicity conditioning.
Correlations between HHV-6 clinically relevant infection and transplant outcomes are shown in Table 4. In our cohort, we observed a significant correlation with grade 2, 3, or 4 (HR, 3.32; CI, 1.65-6.69; P < .01) and 3 or 4 (HR, 4.13; CI, 1.58-10.79; P < .01) acute GVHD. No relation with chronic GVHD was found, in line with early post-HSCT timing of HHV-6 reactivation. Furthermore, no association with TRM and OS was documented.
Multivariate analysis of the association between patient/transplant characteristics and HSCT outcomes
| . | OS . | PFS . | TRM . | Relapse . | aGVHD 2-4 . | aGVHD 3-4 . | cGVHD . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% ci) . | P value . | HR (95% ci) . | P value . | HR (95% ci) . | P value . | HR (95% ci) . | P value . | HR (95% ci) . | P value . | HR (95% ci) . | P value . | HR (95% ci) . | P value . | |
| Clinically relevant HHV-6 infection | 1.13 (0.69-1.84) | .63 | 0.94 (0.58-1.54) | .82 | 1.08 (0.52-2.25) | .84 | 0.88 (0.45-1.71) | .70 | 3.32 (1.65-6.69) | <.01 | 4.13 (1.58-10.79) | <.01 | 0.84 (0.42-1.69) | .62 |
| Age > 53 y | 1.17 (0.77-1.78) | .47 | 1.19 (0.79-1.79) | .41 | 1.81 (0.96-3.41) | .07 | 0.82 (0.47-1.45) | .49 | 0.76 (0.44-1.30) | .31 | 0.81 (0.39-1.67) | .56 | 1.08 (0.62-1.89) | .80 |
| Disease (vs AML) | .45 | .13 | .62 | .05 | .14 | .92 | <.01 | |||||||
| Lymphoma/MM | 1.05 (0.61-1.81) | .86 | 1.20 (0.72-2.01) | .49 | 1.40 (0.64-3.09) | .40 | 0.97 (0.48-1.97) | .94 | 0.76 (0.35-1.65) | .48 | 1.00 (0.38-2.67) | .99 | 2.66 (1.24-5.71) | .01 |
| MDS/MPN | 0.68 (0.35-1.33) | .26 | 0.59 (0.30-1.14) | .11 | 1.42 (0.58-3.49) | .44 | 0.25 (0.08-0.77) | .02 | 1.92 (0.87-4.22) | .11 | 1.27 (0.39-4.15) | .70 | 3.08 (1.32-7.22) | <.01 |
| Disease status (vs CR1) | <.01 | <.01 | .25 | <.01 | .49 | .26 | .19 | |||||||
| Active disease | 1.06 (0.46-2.45) | .90 | 1.12 (0.49-2.52) | .79 | 1.50 (0.53-4.26) | .45 | 0.70 (0.17-2.83) | .62 | 1.44 (0.53-3.90) | .48 | 1.31 (0.33-5.18) | .70 | 0.79 (0.32-1.99) | .62 |
| CR>1 | 2.95 (1.66-5.25) | <.01 | 3.28 (1.84-5.82) | <.01 | 1.99 (0.88-4.46) | .10 | 4.77 (2.09-10.86) | <.01 | 1.52 (0.76-3.02) | .24 | 2.18 (0.82-5.83) | .12 | 1.56 (0.76-3.18) | .22 |
| HCT-CI>2 | 1.57 (1.03-2.40) | .04 | 1.30 (0.86-1.94) | .21 | 1.18 (0.64-2.17) | .60 | 1.29 (0.74-2.26) | .36 | 0.94 (0.54-1.62) | .82 | 0.89 (0.42-1.90) | .77 | 1.19 (0.66-2.12) | .57 |
| Previous allo-HSCT | 0.59 (0.26-1.34) | .20 | 0.54 (0.24-1.23) | .14 | 0.35 (0.08-1.55) | .17 | 0.72 (0.27-1.94) | .52 | 1.05 (0.40-2.71) | .93 | 1.24 (0.37-4.11) | .73 | 1.46 (0.58-3.69) | .43 |
| MAC (vs RTC) | 1.01 (0.47-2.13) | .99 | 0.97 (0.45-2.06) | .93 | 0.94 (0.34-2.55) | .90 | 1.20 (0.36-3.97) | .76 | 0.67 (0.29-1.55) | .35 | 0.52 (0.16-1.67) | .27 | 1.08 (0.40-2.96) | .88 |
| Donor (vs haplo) | .29 | .65 | 1.00 | .82 | .31 | .67 | .01 | |||||||
| MRD | 0.57 (0.26-1.23) | .15 | 0.73 (0.35-1.52) | .40 | 0.00 (0.00-5.81) | .97 | 1.30 (0.58-2.89) | .53 | 0.44 (0.15-1.30) | .14 | 0 (0-1.61) | .97 | 0.32 (0.09-1.18) | .09 |
| MUD | 1.12 (0.53-2.34). | .77 | 1.08 (0.53-2.19) | .84 | 1.04 (0.38-2.83) | .95 | 1.06 (0.37-3.02) | .91 | 0.73 (0.29-1.84) | .50 | 0.56 (0.16-1.98) | .37 | 0.29 (0.12-1.69) | <.01 |
| PBSC (vs BM) | 2.72 (1.07-6.91) | .04 | 2.54 (1.08-5.99) | .03 | 1.30 (0.43-3.89) | .64 | 4.53 (1.07-19.23) | .04 | 1.26 (0.47-3.35) | .65 | 2.98 (0.39-22.75) | .29 | 2.52 (0.71-8.91) | .15 |
| PT-Cy (vs ATG) | 0.74 (0.39-1.41) | .36 | 0.69 (0.37-1.30) | .25 | 1.22 (0.50-2.98) | .67 | 1.58 (0.64-3.93) | .32 | 0.95 (0.43-2.11) | .90 | 0.60 (0.21-1.74) | .35 | 0.81 (0.39-1.67) | .57 |
| CMV status neg/neg (vs others) | 0.76 (0.36-1.60) | .47 | 0.80 (0.39-1.64) | .54 | 0.63 (0.21-1.89) | .41 | 0.95 (0.36-2.53) | .92 | 0.87 (0.32-2.37) | .79 | 0.57 (0.12-2.63) | .47 | 1.36 (0.60-3.12) | .46 |
| . | OS . | PFS . | TRM . | Relapse . | aGVHD 2-4 . | aGVHD 3-4 . | cGVHD . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% ci) . | P value . | HR (95% ci) . | P value . | HR (95% ci) . | P value . | HR (95% ci) . | P value . | HR (95% ci) . | P value . | HR (95% ci) . | P value . | HR (95% ci) . | P value . | |
| Clinically relevant HHV-6 infection | 1.13 (0.69-1.84) | .63 | 0.94 (0.58-1.54) | .82 | 1.08 (0.52-2.25) | .84 | 0.88 (0.45-1.71) | .70 | 3.32 (1.65-6.69) | <.01 | 4.13 (1.58-10.79) | <.01 | 0.84 (0.42-1.69) | .62 |
| Age > 53 y | 1.17 (0.77-1.78) | .47 | 1.19 (0.79-1.79) | .41 | 1.81 (0.96-3.41) | .07 | 0.82 (0.47-1.45) | .49 | 0.76 (0.44-1.30) | .31 | 0.81 (0.39-1.67) | .56 | 1.08 (0.62-1.89) | .80 |
| Disease (vs AML) | .45 | .13 | .62 | .05 | .14 | .92 | <.01 | |||||||
| Lymphoma/MM | 1.05 (0.61-1.81) | .86 | 1.20 (0.72-2.01) | .49 | 1.40 (0.64-3.09) | .40 | 0.97 (0.48-1.97) | .94 | 0.76 (0.35-1.65) | .48 | 1.00 (0.38-2.67) | .99 | 2.66 (1.24-5.71) | .01 |
| MDS/MPN | 0.68 (0.35-1.33) | .26 | 0.59 (0.30-1.14) | .11 | 1.42 (0.58-3.49) | .44 | 0.25 (0.08-0.77) | .02 | 1.92 (0.87-4.22) | .11 | 1.27 (0.39-4.15) | .70 | 3.08 (1.32-7.22) | <.01 |
| Disease status (vs CR1) | <.01 | <.01 | .25 | <.01 | .49 | .26 | .19 | |||||||
| Active disease | 1.06 (0.46-2.45) | .90 | 1.12 (0.49-2.52) | .79 | 1.50 (0.53-4.26) | .45 | 0.70 (0.17-2.83) | .62 | 1.44 (0.53-3.90) | .48 | 1.31 (0.33-5.18) | .70 | 0.79 (0.32-1.99) | .62 |
| CR>1 | 2.95 (1.66-5.25) | <.01 | 3.28 (1.84-5.82) | <.01 | 1.99 (0.88-4.46) | .10 | 4.77 (2.09-10.86) | <.01 | 1.52 (0.76-3.02) | .24 | 2.18 (0.82-5.83) | .12 | 1.56 (0.76-3.18) | .22 |
| HCT-CI>2 | 1.57 (1.03-2.40) | .04 | 1.30 (0.86-1.94) | .21 | 1.18 (0.64-2.17) | .60 | 1.29 (0.74-2.26) | .36 | 0.94 (0.54-1.62) | .82 | 0.89 (0.42-1.90) | .77 | 1.19 (0.66-2.12) | .57 |
| Previous allo-HSCT | 0.59 (0.26-1.34) | .20 | 0.54 (0.24-1.23) | .14 | 0.35 (0.08-1.55) | .17 | 0.72 (0.27-1.94) | .52 | 1.05 (0.40-2.71) | .93 | 1.24 (0.37-4.11) | .73 | 1.46 (0.58-3.69) | .43 |
| MAC (vs RTC) | 1.01 (0.47-2.13) | .99 | 0.97 (0.45-2.06) | .93 | 0.94 (0.34-2.55) | .90 | 1.20 (0.36-3.97) | .76 | 0.67 (0.29-1.55) | .35 | 0.52 (0.16-1.67) | .27 | 1.08 (0.40-2.96) | .88 |
| Donor (vs haplo) | .29 | .65 | 1.00 | .82 | .31 | .67 | .01 | |||||||
| MRD | 0.57 (0.26-1.23) | .15 | 0.73 (0.35-1.52) | .40 | 0.00 (0.00-5.81) | .97 | 1.30 (0.58-2.89) | .53 | 0.44 (0.15-1.30) | .14 | 0 (0-1.61) | .97 | 0.32 (0.09-1.18) | .09 |
| MUD | 1.12 (0.53-2.34). | .77 | 1.08 (0.53-2.19) | .84 | 1.04 (0.38-2.83) | .95 | 1.06 (0.37-3.02) | .91 | 0.73 (0.29-1.84) | .50 | 0.56 (0.16-1.98) | .37 | 0.29 (0.12-1.69) | <.01 |
| PBSC (vs BM) | 2.72 (1.07-6.91) | .04 | 2.54 (1.08-5.99) | .03 | 1.30 (0.43-3.89) | .64 | 4.53 (1.07-19.23) | .04 | 1.26 (0.47-3.35) | .65 | 2.98 (0.39-22.75) | .29 | 2.52 (0.71-8.91) | .15 |
| PT-Cy (vs ATG) | 0.74 (0.39-1.41) | .36 | 0.69 (0.37-1.30) | .25 | 1.22 (0.50-2.98) | .67 | 1.58 (0.64-3.93) | .32 | 0.95 (0.43-2.11) | .90 | 0.60 (0.21-1.74) | .35 | 0.81 (0.39-1.67) | .57 |
| CMV status neg/neg (vs others) | 0.76 (0.36-1.60) | .47 | 0.80 (0.39-1.64) | .54 | 0.63 (0.21-1.89) | .41 | 0.95 (0.36-2.53) | .92 | 0.87 (0.32-2.37) | .79 | 0.57 (0.12-2.63) | .47 | 1.36 (0.60-3.12) | .46 |
Risk factors with a significant influence on outcomes and their respective HRs are shown in bold.
aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; ATG, antithymocyte globulin; AML, acute myeloid leukemia; BM, bone marrow; CR, complete remission; haplo, haploidentical; HCT-CI, hematopoietic cell transplant–comorbidity index; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MM, multiple myeloma; MPN, myeloproliferative neoplasms; MRD, matched-related donor; MUD, matched-unrelated donor; neg, negative; PBSC, peripheral blood stem cells; RTC, reduced-toxicity conditioning.
HHV-6–specific immune reconstitution
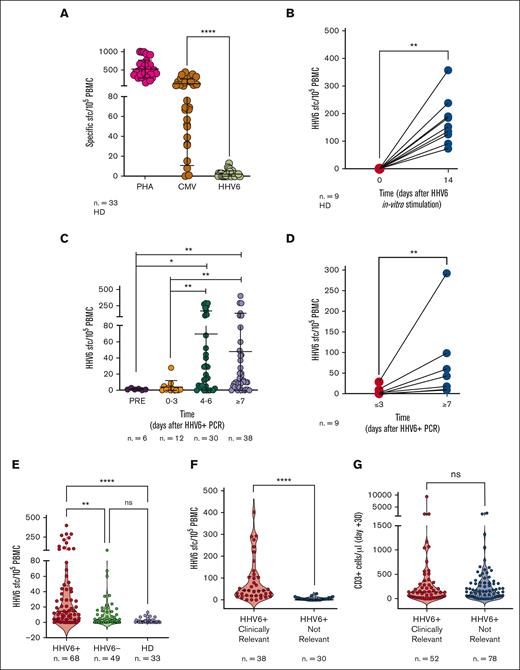
Given the association between low CD3+ cell counts (<100/μL) and high risk of developing clinically relevant HHV-6 infections, we further investigated HHV6-specific T-cell responses. We collected data from 150 individuals screened for HHV-6–specific T-cell immunity, including 33 HDs and 117 HSCT recipients fulfilling the inclusion criteria described in “Methods” (49 controls and 68 patients with virus reactivation). To date, limited data on the frequency of HHV-6–specific T cells in both HDs and allo-HSCT recipients are available. Therefore, we initially stimulated PBMCs harvested from HDs with a library of overlapping peptides covering the entire sequence of the immunodominant viral protein U54, a tegument protein expressed during the lytic cycle of viral replication and described as a relevant antigen in the context of allo-HSCT.17 Although the response to both polyclonal stimulation (phytohemagglutinin) and CMV viral antigens was sustained as expected, the immunological response to HHV-6 in HDs was undetectable ex vivo (n = 33; Figure 2A). HHV-6–specific T cells could be uncovered only after in vitro enrichment, obtained after a 14-day culture in presence of HHV-6 viral peptides (n = 9; P < .01; Figure 2B). In allo-HSCT recipients, HHV-6–specific T cells were undetectable before reactivation (n = 6; median, 1 SFC per 105 PBMCs) or between 0 and 3 days after the first viremia detection (n = 12; median, 1 SFC per 105 PBMCs), and increased within the first 4, 5, or 6 days (n = 30; median, 24 SFCs per 105 PBMCs; P < .05) or ≥7 days after the initial viremia detection (n = 38; median, 11 SFCs per 105 PBMCs; P < .01; Figure 2C). In selected patients (n = 9), in whom HHV-6–specific T-cell responses could be evaluated at 2 different time points, we observed that the frequency of HHV-6–specific T cells increased 18-fold at later time points compared with at an earlier time point (day 0-3 after viremia; P < .01; Figure 2D).
IFN-γ ELISpot for HHV-6–specific T cells. (A) Ex vivo T-cell responses in HDs upon stimulation with the phytohemagglutinin control (PHA), CMV antigens, and HHV-6. Results are expressed as SFC/105 PBMC. Lines indicate medians with interquartile range. (B) HHV-6–specific T-cell responses in HD at steady state (day 0) and 14 days after in vitro stimulation. (C) Frequencies of HHV-6–specific T cells at the indicated time points from the first day of viremia in HSCT recipients. (D) HHV-6–specific SFC/105 PBMC measured at ≥3 or at ≥7 days from the first day of viremia in samples collected from the same patient. (E) Frequencies of HHV-6–specific T cells in different groups: reactivating patients (HHV6+, red), controls (nonreactivating patients; HHV6-, green) and HD (violet). (F) Frequencies of HHV-6–specific T cells in patients developing HHV-6 clinically relevant infection (red) and in patients experiencing HHV-6 subclinical reactivation (blue). (G) Absolute counts of polyclonal CD3+ cells in patients developing HHV-6 clinically relevant infection (red) and in patients experiencing HHV-6 subclinical reactivation (blue). Statistical analysis in panels B and D were performed by Wilcoxon t test. Statistical analyses in panel E were performed using Kruskal-Wallis test, and in panels A, C, F, and G using Mann-Whitney test. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. ns, not significant.
IFN-γ ELISpot for HHV-6–specific T cells. (A) Ex vivo T-cell responses in HDs upon stimulation with the phytohemagglutinin control (PHA), CMV antigens, and HHV-6. Results are expressed as SFC/105 PBMC. Lines indicate medians with interquartile range. (B) HHV-6–specific T-cell responses in HD at steady state (day 0) and 14 days after in vitro stimulation. (C) Frequencies of HHV-6–specific T cells at the indicated time points from the first day of viremia in HSCT recipients. (D) HHV-6–specific SFC/105 PBMC measured at ≥3 or at ≥7 days from the first day of viremia in samples collected from the same patient. (E) Frequencies of HHV-6–specific T cells in different groups: reactivating patients (HHV6+, red), controls (nonreactivating patients; HHV6-, green) and HD (violet). (F) Frequencies of HHV-6–specific T cells in patients developing HHV-6 clinically relevant infection (red) and in patients experiencing HHV-6 subclinical reactivation (blue). (G) Absolute counts of polyclonal CD3+ cells in patients developing HHV-6 clinically relevant infection (red) and in patients experiencing HHV-6 subclinical reactivation (blue). Statistical analysis in panels B and D were performed by Wilcoxon t test. Statistical analyses in panel E were performed using Kruskal-Wallis test, and in panels A, C, F, and G using Mann-Whitney test. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. ns, not significant.
Based on these results, the test was then performed starting from the fourth day after the first HHV-6 DNAemia detection. Because HHV-6 reactivations and symptoms usually occur early after HSCT,4,8,31 and considering that the high variability of T-cell counts across different time points after the transplant might affect the magnitude of T-cell responses, we focused on patients developing the viral reactivation within 60 days after HSCT. Accordingly, among the 130 patients experiencing HHV-6 reactivation, 115 cases occurred in the first 60 days after HSCT. Of these, 38 patients could not be tested because of the absence of biobanked samples (28 patients) or low CD3+ cell counts (10 patients). The test was performed in the remaining 77 patients evaluable for the quantification of HHV-6–specific T cells. In 9 cases, the test was not informative because of internal controls failure. Overall, 68 patients were analyzed (median, 32 days after allo-HSCT) via IFN-γ ELISpot, accounting for ∼90% of the 77 evaluable patients in whom HHV-6 reactivated within 60 days after HSCT. Results were compared with those of a cohort of 49 patients who tested negative for HHV-6 and were evaluated at a median of 33 days after HSCT.
In HHV-6–positive patients, the frequency of viral-specific T cells (median, 14.5 SFCs per 105 PBMCs) was higher than that in patients in whom HHV-6 did not reactivate (median, 4.0 SFCs per 105 PBMCs; P = .0013) and in HDs (median, 1.0 SFC per 105 PBMCs; P < .0001; Figure 2E). Given the high degree of homology between HHV-6 and CMV, patients’ samples were tested in parallel for both HHV-6 and CMV-specific responses. Linear regression analysis performed on all tested samples (n = 109) showed that there was no association between the 2 specificities (P = .88; supplemental Figure 1A). Interestingly, although in the cohort of patients experiencing a HHV-6 reactivation, the frequency of HHV-6–specific T cells was measurable and comparable with that of CMV-specific T cells (supplemental Figure 1B); patients who did not develop HHV-6 reactivations displayed a lower amount of HHV-6–specific T cells with respect to CMV-specific ones (P < .0001; supplemental Figure 1C), similarly to what was observed in HDs (Figure 2A). The lack of correlations between the 2 specificities highlights that the emergence of HHV-6–specific T-cell responses during reactivation episodes was independent of CMV-specific T-cell responses.
Notably, we observed a remarkably higher HHV-6–specific response in patients who would later develop clinically relevant infection (median, 44 SFCs per 105 PBMCs) than that in patients who had subclinical reactivation (median, 4 SFCs per 105 PBMCs; P < .0001; Figure 2F). In contrast, the comparison of CD3 cell counts on day 30 in patients developing clinically relevant or subclinical reactivations showed no significant differences (Figure 2G).
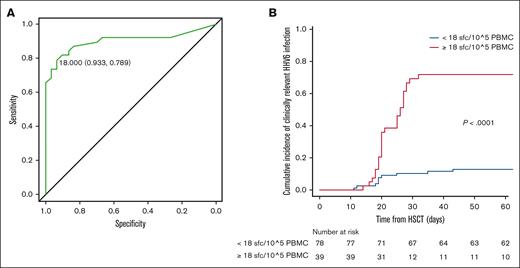
Interestingly, the frequency of HHV-6–specific T cells was compared in patients displaying classical HHV-6–related EODs (ie, proven encephalitis and delayed engraftment or myelosuppression) and other possible EODs, without significant differences (supplemental Figure 2). The receiver operating characteristic analysis identified the specific threshold of 18 SFCs per 105 PBMCs, found to be highly significant in distinguishing the 2 outcomes (area under the curve, 0.902; 95% ci, 0.822-0.981; Figure 3A) with higher specificity (93%) and sensitivity (79%) than polyclonal CD3+ cell counts (<100/μL; specificity = 59%; sensitivity = 36%; supplemental Table 1). Accordingly, we report a significant correlation between high levels of HHV-6–specific T cells at ≥4 days after HHV-6 reactivation (≥18 SFCs per 105 PBMCs) and the risk of developing clinically relevant HHV-6 infection by day 60 (P < .0001; Figure 3B; supplemental Table 2).
Correlation of HHV-6 reactivation and specific immune reconstitution. (A) Receiver operating characteristic (ROC) analysis for HHV-6–specific immune reconstitution by ELISpot (threshold of 18 SFCs per 105 PBMCs; specificity 0.933; sensitivity 0.789); (B) CI of clinically relevant HHV-6 reactivations by the identified threshold (in blue, patients below threshold and in red, patients above the threshold of 18 HHV-6–specific SFC/105 PBMC).
Correlation of HHV-6 reactivation and specific immune reconstitution. (A) Receiver operating characteristic (ROC) analysis for HHV-6–specific immune reconstitution by ELISpot (threshold of 18 SFCs per 105 PBMCs; specificity 0.933; sensitivity 0.789); (B) CI of clinically relevant HHV-6 reactivations by the identified threshold (in blue, patients below threshold and in red, patients above the threshold of 18 HHV-6–specific SFC/105 PBMC).
When comparing HHV-6 viremia at onset between patients developing clinically relevant and subclincal HHV-6 reactivation (n = 130, supplemental Figure 3A), a significant difference was found, indicating that the enrichment of HHV-6–specific T cells can be at least partially explained by the higher antigen exposure. Despite this observation, no significant association was found between the viremia in peripheral blood and high or low levels of HHV-6–specific T cells based on the identified threshold of 18 specific SFCs per 105 PBMCs (supplemental Figure 3), probably, because the viral load in tissues can also affect HHV-6–specific T-cell responses. Indeed, some patients had classical or possible EODs despite low viremia in the peripheral blood in our cohort.
These data indicate the clinical utility of evaluating HHV-6–specific T-cell responses at HHV-6 reactivation diagnosis to assess the individual with risk of developing subsequent severe symptoms.
Discussion
HHV-6, the etiological agent for roseola infantum, remains in a latent state after primary infection. Viral reactivation can be life-threatening for patients who are immunocompromised. Furthermore, the high prevalence of latent infection and asymptomatic reactivation, the difficulties in obtaining an accurate diagnosis, controversial results on the clinical efficacy of antiviral therapies,8 and their associated side effects (ie, myelotoxicity and renal toxicity) complicate the treatment.
Therefore, it is important to distinguish between HHV-6 reactivation, which is merely defined as a newly detected HHV-6 DNA in the peripheral blood, and HHV-6–related EODs. In this study, we described our center policy in monitoring HHV-6 viremia during early posttransplant phase, in line with centrer experience,4,20,27 adopting a multidisciplinary model based on different tools to identify as clinically relevant HHV-6 infection, both classical HHV-6–related EODs according to ECIL-consensus and possible HHV-6–related EODs. Although aware that we have arbitrarily defined the entity of clinically relevant HHV-6 infection going beyond ECIL-consensus definitions, we are confident that this model may be helpful in providing new insights in the management of HHV-6 morbidity, given the controversial and unclear role of this viral infection in the context of HSCT.
Over the last decade, very few studies have analyzed the value of HHV-6–specific T cells in transplant recipients. In the pediatric transplant settings, de Pagter et al18 showed how HHV-6–specific CD8+ T–cell proliferative capacity was increased at 6 months after HHV-6 reactivation compared with that in patients in whom HHV-6 did not reactivate. Nonetheless, to this day, no laboratory assessments for viral-specific immunoreconstitution have been used as standard in clinical practice.
We performed a first study on HHV-6 infection in the context of haploidentical transplant,20 reporting a correlation between T-cell immune reconstitution (CD3+ cell count ≥200/μL) and favorable transplant outcome in the presence of viral reactivation. More recently, we have reported a higher occurrence of viral infections, including HHV-6, with the use of PT-Cy.4
In this prospective observational study, we assessed the incidence and clinical implications of HHV-6 viremia, paralleled by the analysis of HHV-6–specific T cells via an IFN-γ ELISpot assay. HHV-6 reactivation occurred in 63% of patients at 100 days, with a median time of 25 days after HSCT. However, only 40% of patients in whom HHV-6 reactivated presented HHV-6 positivity receiving antiviral treatment, for the presence of HHV-6–related clinical manifestations and/or HHV-6 disease.
The clinical impact of HHV-6 viremia remains often difficult to distinguish at diagnosis, because some patients had EOD despite low viremia. Indeed, a multidisciplinary effort with a focus on patient global picture is warranted to properly identify patients who are at risk for virus-related clinically relevant infection and would benefit from antiviral treatment.
Controversy regarding the clinical impact of HHV-6 at diagnosis and therapy8,32,33 remains because of the high prevalence of latent infection and asymptomatic reactivation and the discrepancy in viral loads measured in peripheral blood and tissue samples.
We identified predicting factors for the occurrence of clinically relevant infections, such as the use of PT-Cy and steroid treatment, together with low CD3+ cell counts. This finding is in line with recent literature reporting high viral infection rates among PT-Cy recipients,4,5,34,35 as compared with anti T–lymphocyte globulin–based GVHD prophylaxis,36 and may contribute to the infectious risk stratification for HSCT recipients if it is further validated in larger and different patient populations.
We observed a significant association between the development of clinically relevant HHV-6 infection and acute GVHD, both for grades 2, 3, or 4 and 3 or 4, as already reported.37 The OS and TRM were not affected by clinically relevant HHV-6 infection. Disease status during the transplant remained a strong determinant for a good transplant outcome, because HSCT recipients in complete remission showed the best OS, better PFS, and low relapse rates (43% of patients who had active disease during transplant and 14% of patients in complete remission experienced relapse). Patients in our cohort mainly (87% of the cases) received unmanipulated peripheral blood stem cells.
A second issue addressed in this study relates to the dynamics of HHV-6–specific T-cell responses. As described in literature,13 we observed limited immunological responses to HHV-6 in the PBMCs of HDs. Nonetheless, after in vitro stimulation with viral antigens, the frequencies of HHV-6–reacting T cells in HD increased at levels detectable via ELISpot, underlining the immunogenicity of the tegument protein, U5415 and the adequate sensitivity of the assay.
We reported data from 117 adult patients, including 49 controls in whom HHV-6 did not reactivate controls and 68 patients in whom HHV-6 reactivated. The number of IFN-γ–producing HHV-6–specific T cells were significantly higher in patients in whom HHV-6 reactivated than in whom HHV-6 did not reactivate. To evaluate HHV-6 and CMV cross reactivity, patient samples were stimulated parallelly in the presence of CMV-specific peptides. There was no association between the 2 specificities, indicating that the expansion of HHV-6–specific T-cell responses during viral reactivations was independent of CMV-specific immunity.
We observed that in patients in whom HHV-6 reactivated, the informative time point for the ELISpot assay was at least 4 days from the first day of viral DNAemia. Interestingly, we found that reaching the threshold of 18 SFCs per 105 PBMC from the fourth day after HHV-6 DNAemia predicted a clinically relevant infection with higher specificity and sensitivity than that of polyclonal CD3+ cell counts. This information might be potentially useful in clinical routine, given the timeframe of at least 15 days from the first HHV-6 DNA PCR positivity in the peripheral blood to the development of clinical symptoms. Notably, the frequency of HHV-6–specific T cells was higher in patients with clinically relevant HHV-6 infection than in patients with subclinical reactivation. Intriguingly, T-cell responses were superimposable in patients with classical HHV-6 EODs (proven encephalitis and delayed engraftment or myelosuppression) and other possible EODs. Even if higher CD3+ counts were independently associated to a lower risk of developing clinically relevant reactivations in multivariate analysis, total CD3+ cell counts were less reliable than HHV-6–specific T-cell responses as a predictive biomarker.
Overall, these results highlight IFN-γ ELISpot as an innovative and informative assay for confirming the diagnosis of clinically relevant HHV-6 infections, supporting the value of the immunological findings particularly for nonconventional possible EODs. Confirmatory studies are needed to foster its implementation in a real-life HSCT setting.
The positive correlation between the frequency of HHV-6–specific T cells and the clinical manifestations is counterintuitive and opposite to the correlation described with other posttransplant viral reactivation events, such as CMV reactivation. There are important differences in the frequencies and functional phenotype of HHV-6 and CMV-specific T cells in HDs and in patients in whom HHV-6 did not reactivate. Although the high rate of viral reactivation in immune suppressed participants indicates that long-term immunity is elicited by primary infection, HHV-6–specific T cells are present at very low frequencies in healthy participants, which is in sharp contrast to the strong responses observed for CMV that correlate with a lower rate of CMV reactivations and disease in HSCT context.38-41 Although CMV-specific T cells tend to accumulate with age, a phenomenon known as memory inflation,42 HHV-6–specific T cells circulate at very low frequency in HDs13,16 or in patients in whom HHV-6 did not reactivate, probably because of the induction of a higher frequency of immunomodulatory, virus-specific regulatory T cells.43 Accordingly, HHV-6–specific T cells displayed a less differentiated phenotype than CMV-specific T lymphocytes. The low frequencies of HHV-6–specific T cells might also be related to the lymphotropism of the virus and to its immunosuppressive properties. HHV-6 infection inhibits interleukin-2 synthesis and T-cell proliferation, induces CD4 T–cell apoptosis, cell-cycle arrest, and major histocompatibility complex and T-cell receptor downmodulation.44
Thus, the emergence of HHV-6–specific T cells at frequencies detectable by ELISpot in patients who underwent transplant is likely the effect of a new wave of antigen exposure, able to boost the otherwise scanty immune response. Interestingly, the level of T-cell response directly correlates with clinical symptoms, being low but detectable in subclinical cases and higher in HHV-6–related EOD.
In our study, ∼90% of the evaluable patients in whom HHV-6 reactivated within 60 days after HSCT could be successfully screened for HHV-6–specific immune response. Besides the availability of viable cryopreserved samples, some patients failed to reach the threshold of 25 CD3+ cells per μL at the time of sampling, a threshold required for an informative T-cell test. This limitation should be taken into account in the design of future trials that test the role of HHV-6–specific T-cell responses in the management of viral reactivations and should prompt the comparison of IFN-γ ELISpot with alternative immunomonitoring approaches, that is, dextramer or tetramer–based approaches, potentially more informative during lymphopenia.41
Overall, our results provide a complete overview on HHV-6 reactivation after allo-HSCT, describing clinically relevant infections, risk factors, and details on posttransplant HHV-6–specific immune reconstitution. The decision to treat HHV-6 infection in HSCT recipients may be taken by combining virological and immunological findings. Notably, immunological findings may represent innovative and additional criteria in the diagnosis of HHV-6–related EOD, particularly in case of nonconventionally possible EODs. We are convinced that a deeper knowledge on HHV-6–specific immunity could pave the way for studies on donor-derived and off-the-shelf virus-specific T cells therapies as well as on timing for treatment strategies.
Acknowledgments
The authors thank the San Raffaele URC (clinical trial office), the participating patients and their families, and all nurses and data managers who contributed to this study.
This study was partially financially supported by the HHV-6 Foundation, with no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: R.G., C.B., and F.C. designed the study; M.N., V.B., and V.V. performed the immunological monitoring; S.R. and R.D. performed viral monitoring; M.N., F.L., E.X., and R.G. interpreted the data; F.L. and M.N. performed statistical analysis; G.F. and E.C. prepared the tables; R.G. and C.B. provided scientific advice and supervision; M.N., F.L., E.X., R.G., F.C., and C.B. wrote the manuscript; E.T. revised the manuscript; and all authors contributed to sample and data collection and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fabio Ciceri, Hematology and Bone Marrow Transplantation Unit, San Raffaele Scientific Institute, Via Olgettina 60, 20132 Milan, Italy; e-mail: ciceri.fabio@hsr.it; and Raffaella Greco, Hematology and Bone Marrow Transplantation Unit, San Raffaele Scientific Institute, Via Olgettina 60, 20132 Milan, Italy; e-mail: greco.raffaella@hsr.it.
References
Author notes
∗M.N. and F.L. contributed equally to this study.
†C.B. and R.G. contributed equally to this study.
The datasets generated for this study are available on request from the corresponding author, Raffaella Greco (greco.raffaella@hsr.it).
The full-text version of this article contains a data supplement.