TO THE EDITOR:
The optimal graft composition and cell dose for haploidentical allogeneic peripheral blood stem cell transplantation (PBSCT) using posttransplantation cyclophosphamide (PTCy) and the impact of these characteristics on transplantation outcomes remain as unsettled questions. In general, studies examining the impact of CD34+ cell dose in matched donor PBSCT have demonstrated improved survival with higher doses, with the drawback of possibly increased rates of chronic graft-versus-host disease (GVHD).1-5 Whether these findings can be extended to transplantations performed using haploidentical donors with PTCy has not yet been demonstrated. Nonetheless, early studies of haploidentical unmanipulated PBSC allografts using PTCy were commonly performed with the restriction of CD34+ doses at 5 × 106/kg recipient weight to minimize the risk of GVHD.6 This caution was sensible early on, given that mobilized PBSC grafts, with ∼10-fold increase in T-cell content, exert higher rates of chronic GVHD.7,8 Mussetti et al reported the influence of graft composition (CD3+ cell count specifically) on chronic GVHD development after haploidentical PBSCT with PTCy.8 However, recent studies have questioned the practice of capping CD34+ cell doses in haploidentical PBSCT. Findings from these studies demonstrated the protective effect of CD34+ graft content on nonrelapse mortality (NRM), with no association with GVHD.8-10
Im et al recently performed a registry-based analysis of recipients of PTCy-based haploidentical transplantations, using outcomes reported to the Center for International Blood and Marrow Transplant Research.11 These investigators found that donor age and graft source were the risk factors for acute and chronic GVHD, respectively. The frequency of missing CD34+ cell dose was 6.7% of the cohort. The Center for International Blood and Marrow Transplant Research typically omits data that are <95% complete from the final analysis; therefore, the CD34+ dose was not considered as a predictor variable in the final analysis of this study. We hypothesized that this variable, despite missing 6.7% of the cohort values, retains interpretable values in the context of this study. Using the publicly available data set from this study,11,12 we sought to examine whether the CD34+ cell dose was predictive of the clinical outcomes of interest, considering patients with missing variables as an independent group. Because marrow-derived products typically deliver a lower total CD34+ cell dose, we restricted our analysis to patients who received PBSCT grafts.
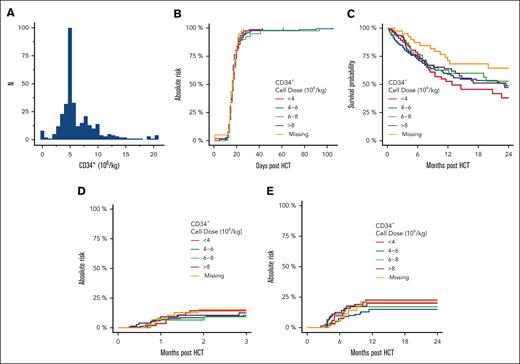
The median age of this population (N = 375) was 55 years (range, 18-76). The patient, disease, and transplantation characteristics are summarized in Table 1. We found an evident bias toward the use of ∼5 × 106 CD34+ cells/kg recipient weight in the data set, suggesting that dose capping was widely used within the cohort (Figure 1A).
Baseline characteristics and transplantation outcomes based on the CD34+ cell dose
| Characteristic∗ . | <4†, n = 56 . | 4-6†, n = 157 . | ≥6-8†, n = 46 . | ≥8†, n = 77 . | Missing, n = 39 . | P value . |
|---|---|---|---|---|---|---|
| Recipient age at transplant, y | 52 (38, 61) | 54 (37, 64) | 58 (42, 65) | 58 (46, 66) | 51 (35, 60) | .11 |
| Donor age at transplant, y | 36 (25, 50) | 40 (29, 51) | 38 (32, 50) | 36 (27, 45) | 38 (31, 54) | .4 |
| Recipient sex, female | 22 (39%) | 61 (39%) | 20 (43%) | 35 (45%) | 15 (38%) | .9 |
| Conditioning regimen | < .0001 | |||||
| Bu/Cy | 11 (20%) | 43 (27%) | 6 (13%) | 19 (25%) | 1 (2.6%) | |
| TBI ± Flu/Cy, MAC | 17 (30%) | 40 (25%) | 4 (8.7%) | 14 (18%) | 25 (64%) | |
| TBI ± Flu/Cy, RIC | 23 (41%) | 60 (38%) | 32 (70%) | 42 (55%) | 10 (26%) | |
| Flu/Mel | 4 (7.1%) | 11 (7.0%) | 1 (2.2%) | 1 (1.3%) | 2 (5.1%) | |
| Other | 1 (1.8%) | 3 (1.9%) | 3 (6.5%) | 1 (1.3%) | 1 (2.6%) | |
| HCT-CI | .78 | |||||
| 0 | 9 (16%) | 22 (14%) | 4 (8.7%) | 12 (16%) | 1 (2.6%) | |
| 1-2 | 15 (27%) | 40 (25%) | 17 (37%) | 23 (30%) | 12 (31%) | |
| 3+ | 32 (57%) | 91 (58%) | 24 (52%) | 42 (55%) | 25 (64%) | |
| Missing | 0 (0%) | 4 (2.5%) | 1 (2.2%) | 0 (0%) | 1 (2.6%) | |
| DRI | .99 | |||||
| Low | 4 (7.1%) | 12 (7.6%) | 2 (4.3%) | 6 (7.8%) | 4 (10%) | |
| Intermediate | 28 (50%) | 78 (50%) | 25 (54%) | 34 (44%) | 18 (46%) | |
| High/very high | 22 (39%) | 60 (38%) | 17 (37%) | 35 (45%) | 15 (38%) | |
| Missing | 2 (3.6%) | 7 (4.5%) | 2 (4.3%) | 2 (2.6%) | 2 (5.1%) | |
| KPS | .02 | |||||
| 90-100 | 24 (43%) | 83 (53%) | 24 (52%) | 34 (44%) | 7 (18%) | |
| ≤80 | 31 (55%) | 71 (45%) | 21 (46%) | 42 (55%) | 32 (82%) | |
| Missing | 1 (1.8%) | 3 (1.9%) | 1 (2.2%) | 1 (1.3%) | 0 (0%) |
| Characteristic∗ . | <4†, n = 56 . | 4-6†, n = 157 . | ≥6-8†, n = 46 . | ≥8†, n = 77 . | Missing, n = 39 . | P value . |
|---|---|---|---|---|---|---|
| Recipient age at transplant, y | 52 (38, 61) | 54 (37, 64) | 58 (42, 65) | 58 (46, 66) | 51 (35, 60) | .11 |
| Donor age at transplant, y | 36 (25, 50) | 40 (29, 51) | 38 (32, 50) | 36 (27, 45) | 38 (31, 54) | .4 |
| Recipient sex, female | 22 (39%) | 61 (39%) | 20 (43%) | 35 (45%) | 15 (38%) | .9 |
| Conditioning regimen | < .0001 | |||||
| Bu/Cy | 11 (20%) | 43 (27%) | 6 (13%) | 19 (25%) | 1 (2.6%) | |
| TBI ± Flu/Cy, MAC | 17 (30%) | 40 (25%) | 4 (8.7%) | 14 (18%) | 25 (64%) | |
| TBI ± Flu/Cy, RIC | 23 (41%) | 60 (38%) | 32 (70%) | 42 (55%) | 10 (26%) | |
| Flu/Mel | 4 (7.1%) | 11 (7.0%) | 1 (2.2%) | 1 (1.3%) | 2 (5.1%) | |
| Other | 1 (1.8%) | 3 (1.9%) | 3 (6.5%) | 1 (1.3%) | 1 (2.6%) | |
| HCT-CI | .78 | |||||
| 0 | 9 (16%) | 22 (14%) | 4 (8.7%) | 12 (16%) | 1 (2.6%) | |
| 1-2 | 15 (27%) | 40 (25%) | 17 (37%) | 23 (30%) | 12 (31%) | |
| 3+ | 32 (57%) | 91 (58%) | 24 (52%) | 42 (55%) | 25 (64%) | |
| Missing | 0 (0%) | 4 (2.5%) | 1 (2.2%) | 0 (0%) | 1 (2.6%) | |
| DRI | .99 | |||||
| Low | 4 (7.1%) | 12 (7.6%) | 2 (4.3%) | 6 (7.8%) | 4 (10%) | |
| Intermediate | 28 (50%) | 78 (50%) | 25 (54%) | 34 (44%) | 18 (46%) | |
| High/very high | 22 (39%) | 60 (38%) | 17 (37%) | 35 (45%) | 15 (38%) | |
| Missing | 2 (3.6%) | 7 (4.5%) | 2 (4.3%) | 2 (2.6%) | 2 (5.1%) | |
| KPS | .02 | |||||
| 90-100 | 24 (43%) | 83 (53%) | 24 (52%) | 34 (44%) | 7 (18%) | |
| ≤80 | 31 (55%) | 71 (45%) | 21 (46%) | 42 (55%) | 32 (82%) | |
| Missing | 1 (1.8%) | 3 (1.9%) | 1 (2.2%) | 1 (1.3%) | 0 (0%) |
Bu, busulfan; cy, cyclophosphamide; DRI, disease risk index; flu, fludarabine; GRFS, GVHD relapse-free survival; HCT-CI, hematopoietic cellular transplantation comorbidity index; KPS, Karnofsky performance status; MAC, myeloablative conditioning; mel, melphalan; OS, overall survival; RIC, reduced intensity conditioning; TBI, total body irradiation.
n (%); median (interquartile range).
All cell doses listed as ×106 CD34+ cells per kg recipient weight.
Outcomes in patients who underwent mobilized blood–derived related haploidentical allogeneic HCT. (A) Frequency plot of CD34+ stem cell dose per recipient weight (kg). There is a strong bias toward the use of ∼5 × 106 CD34+ cells per kg. (B) Cumulative incidence of primary neutrophil engraftment based on the CD34+ cell dose per kg. (C) Overall survival. (D) Cumulative incidence of grade 3 or 4 acute GVHD. (E) Cumulative incidence of moderate-to-severe chronic GVHD.
Outcomes in patients who underwent mobilized blood–derived related haploidentical allogeneic HCT. (A) Frequency plot of CD34+ stem cell dose per recipient weight (kg). There is a strong bias toward the use of ∼5 × 106 CD34+ cells per kg. (B) Cumulative incidence of primary neutrophil engraftment based on the CD34+ cell dose per kg. (C) Overall survival. (D) Cumulative incidence of grade 3 or 4 acute GVHD. (E) Cumulative incidence of moderate-to-severe chronic GVHD.
We first sought to determine whether CD34+ cell dose was predictive of clinical outcomes using a multivariable Cox proportional hazards model. Contributing covariates were evaluated in a univariable framework, retaining variables that significantly contributed to outcomes in the multivariable model (two-sided α error, 0.05). When analyzed as a continuous variable, there was no association between the CD34+ cell dose and 1-year overall survival (hazard ratio [HR], 0.96; 95% confidence interval [95% CI], 0.91-1.004), grade 3 or 4 acute GVHD (HR, 1.01; 95% CI, 0.92-1.11), moderate-to-severe chronic GVHD (HR, 1.03; 95% CI, 0.96-1.10), NRM (HR, 0.97; 95% CI, 0.90-1.04), or primary neutrophil engraftment by day 100 (HR, 0.92; 95% CI, 0.82-1.04). Similarly, CD3+ cell dose was not predictive of the outcomes (data not shown). CD34+ and CD3+ cell doses were weakly correlated (Kendall τ, 0.10; P < .001). The missing rate for CD3+ cell dose was 25.9%. The total nucleated cell dose was not available.
We then sought to determine whether the CD34+ cell dose cutoff points commonly used in clinical decision-making were predictive of categorical variables. We grouped the cohort based on the CD34+ cell dose as follows: <4, from 4 to 6, from ≥6 to 8, and ≥8 × 106 CD34+/kg recipient weight to conform with common clinically relevant cutoff points for stem cell dose. The clinical outcomes are summarized in Table 1 and illustrated in Figure 1B-D. Again, we found no association between CD34+ cell dose and major transplantation outcomes.
To our knowledge, we present the largest analysis, to date, investigating the impact of CD34+ cell dose on major transplantation outcomes in haploidentical PBSCT. Similar to previous studies in this setting,8-10 we found no detrimental impact of increasing the CD34+ cell dose on outcomes, including the development of grade 3 or 4 acute GVHD or moderate-to-severe chronic GVHD. In addition, we found that CD3+ dose did not negatively influence outcomes. No optimal dose emerged from our analysis, with a lack of difference in major outcomes across commonly used CD34+ cell dose cutoff points. Collectively, these results call into question the common practice of capping CD34+ cell dose in haploidentical PBSCT.
In contrast to 2 recent studies in this setting, we did not observe a protective effect of an increased CD34+ cell dose against NRM.9,10 We also did not observe inferior survival outcomes in patients who received cell doses <5 × 106 CD34+/kg or any impact of CD34+ cell dose on neutrophil engraftment. In recent reports from Elmariah et al and a registry analysis from the European Society for Blood and Marrow Transplantation, both groups advocated targeting cell doses >5 × 106 CD34+/kg based on the increased risk of NRM driven by infection observed with lower doses. In contrast, Mussetti et al found that the CD34+ cell dose was only protective in the setting of bone marrow grafts but not when peripheral blood stem cells were used in haploidentical transplantations performed with PTCy. The reason for this discrepancy across studies is unclear and may be related to differences in the populations studied. Our failure to detect differences in outcomes with low cell doses may be related to the fact that only a minority of patients received doses of <5 × 106 CD34+ cells/kg. Because of the small number of patients who received a very low CD34+ cell dose, the results might have limited the power so should be interpreted with caution.
The limitations of our study include heterogeneity of the data set with regard to conditioning intensity, underlying disease, donor characteristics, and specific GVHD prophylaxis agents used alongside PTCy, which were not controlled for. In addition, 6.7% of the cohort had missing data regarding cell dose, which may have introduced bias, although we did not find differences in outcomes in this proportion of patients. The total nucleated cell dose was not available and could not be investigated as a potential contributor to the clinically relevant outcomes. The rates of cytokine release syndrome were also not available for analysis; studies have shown mixed findings regarding the impact of CD3+ or CD34+ cell dose on cytokine release syndrome incidence and severity.13-16 Finally, as noted, cell doses were heavily clustered at ∼5 × 106 CD34+ cells per kg, which might have affected the statistical power to detect differences in outcomes outside this range.
We conclude that restricting the CD34+ cell dose to 5 × 106 CD34+ cells per kg recipient weight, although common practice, is not warranted based on these data. Prospective studies may consider omitting strict cell dose restrictions from the treatment plan. Larger sets are needed to determine whether cell doses of <4 million CD34+ cells per kg are safe and to better elucidate the relationship between cell dose and NRM in haploidentical PBSCT.
Contribution: M.T.N., A.I., and B.S. contributed to the study proposal and design; B.S. contributed to data acquisition, data analysis, and interpretation; M.T.N. drafted the manuscript; and all authors critically revised and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mariam T. Nawas, Hematopoietic Cellular Therapy Program, Department of Medicine, University of Chicago Medicine, 5841 S Maryland Ave, Chicago, IL 60637; e-mail: nawasm@bsd.uchicago.edu.
References
Author notes
The original data set is publicly available in Publicly Available Data sets 2020 at https://cibmtr.org/CIBMTR/Resources/Publicly-Available-Data sets#.