TO THE EDITOR:
In recent years, there has been increasing use of the term autoimmune heparin-induced thrombocytopenia (aHIT) to describe patients whose blood samples activate platelets in the absence of exogenous heparin (ie, in the no-heparin/buffer-control serotonin release assay [SRA]).1-8 This term has been proposed to include not only syndromes in which patients develop anti–PF4-mediated thrombotic thrombocytopenia without proximate heparin exposure, such as spontaneous HIT and vaccine-induced immune thrombotic thrombocytopenia, but also several clinically severe HIT subtypes that occur after heparin exposure. These additional entities include delayed-onset HIT, persistent HIT, heparin flush–induced HIT, and severe HIT with a platelet count <20 000/μL with associated disseminated intravascular coagulation.1,2 Although limited data appear to support the correlation between reactivity in no-heparin SRA and disease severity in patients with aHIT who were exposed to herapin, rigorous studies to confirm this association are lacking.2,9 It has also been proposed that the incidence of aHIT is underrecognized because most laboratories (including ours) do not perform SRA in the absence of heparin for diagnostic purposes.1
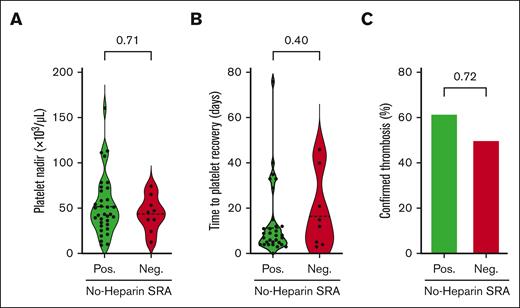
Herein, we evaluated the clinical utility of the aHIT test (no-heparin SRA) for predicting disease severity and assessed the technical validity of the assay. Details of the methods used are provided in the supplemental Materials. Three well-accepted measures of clinical severity: platelet nadir, time to platelet recovery, and the development of objectively confirmed thrombosis were evaluated in a cohort of 43 HIT samples, on which both conventional SRA (using 0.1 U/mL of heparin) and no-heparin SRA were performed. All 43 patients tested positive in the conventional SRA (≥20% serotonin release) and were inhibited (serotonin release inhibited by ≥50%) in the presence of high concentrations of unfractionated heparin (100 U/mL), confirming the presence of platelet-activating HIT antibodies. The cohort was divided into 2 groups: aHIT (n = 34) and classical HIT (n = 9), characterized by serotonin release of >50% and ≤50%, respectively, in the no-heparin SRA. As shown in Figure 1A, no differences were noted in platelet nadirs (mean, 52 × 103/μL vs 44 × 103/μL; P = .71), time to platelet recovery (mean, 13.5 days vs 18.3 days; P = .40), and rates of confirmed thrombosis (ie, thrombosis score of 2 in 4Ts scoring)10 (62% vs 50%; P = .72) between patient groups with aHIT and those with classical HIT. Based on these results, we hypothesized that residual heparin present in diagnostic HIT samples might result in false-positive reactions in no-heparin SRA, accounting for its lack of association with disease severity.
Indicators of clinical severity did not differ between patients with aHIT and those with classical HIT. A cohort of 43 patients with suspected HIT were qualitatively subgrouped as aHIT-positive (>50% in no-heparin SRA; n = 34) or aHIT-negative/classical (≤ 50% in no-heparin SRA; n = 9). (A) Platelet count nadir and (B) time to platelet recovery are displayed using violin plots with means displayed (dashed line). aHIT and classical HIT subgroups were compared using a nonparametric, unpaired t test (Mann-Whitney test). The time to platelet recovery was calculated based on the number of days between heparin cessation and the day the platelet count returned to >150 × 109/L. Four patients died or were discharged before achieving a normal platelet count and were excluded from the analysis presented in panel B. (C) The number of patients with objectively confirmed thromboses (4Ts thrombosis score of 2) was compared between the aHIT and classical HIT subgroups using Fisher's exact test, and the percentage of patients with confirmed thromboses is displayed.
Indicators of clinical severity did not differ between patients with aHIT and those with classical HIT. A cohort of 43 patients with suspected HIT were qualitatively subgrouped as aHIT-positive (>50% in no-heparin SRA; n = 34) or aHIT-negative/classical (≤ 50% in no-heparin SRA; n = 9). (A) Platelet count nadir and (B) time to platelet recovery are displayed using violin plots with means displayed (dashed line). aHIT and classical HIT subgroups were compared using a nonparametric, unpaired t test (Mann-Whitney test). The time to platelet recovery was calculated based on the number of days between heparin cessation and the day the platelet count returned to >150 × 109/L. Four patients died or were discharged before achieving a normal platelet count and were excluded from the analysis presented in panel B. (C) The number of patients with objectively confirmed thromboses (4Ts thrombosis score of 2) was compared between the aHIT and classical HIT subgroups using Fisher's exact test, and the percentage of patients with confirmed thromboses is displayed.
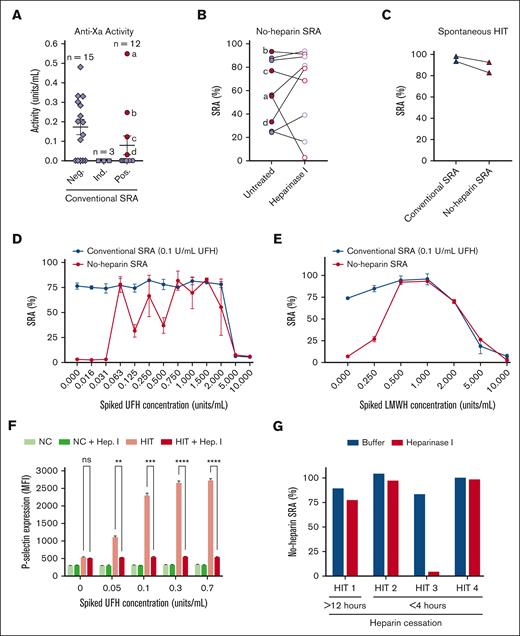
To assess the prevalence of residual heparin in samples submitted for HIT testing, an anti-Xa activity assay was performed on a separate cohort of 30 diagnostic samples with adequate citrated plasma volumes available from a recent HIT study.11 Of these diagnostic samples, 12, 3, and 15 were positive, indeterminate (inhibited by <50% with high concentrations of heparin, 100 U/mL), and negative, respectively, in the conventional SRA. As shown in Figure 2A, 14 of the 30 samples (47%) had detectable levels of heparin and in some cases, in significant concentrations (Figure 2A). Anti-Xa activity was detected in 12 samples from patients treated with unfractionated heparin (UFH) and in 2 samples from patients treated with low molecular-weight heparin (LMWH). A trend toward higher anti-Xa activity levels in the group that showed SRA-negative results relative to those with SRA-positive results was noted (P = .053, two-way analysis of variance). Nine conventional SRA-positive samples (solid circles) were further tested in no-heparin SRA with or without heparinase I treatment (Figure 2B). Notably, one sample demonstrated a significant decrease in its ability to activate platelets in the no-heparin SRA after heparinase I digestion (from 56% to 3% serotonin release) and happened to have the highest anti-Xa levels in our study cohort (>0.5 U/mL; point “a” in Figure 2A,B). In addition, two spontaneous HIT samples were compared in the no-heparin and conventional SRA, with both spontaneous HIT samples testing strongly positive in both assays (Figure 2C). Next, a known HIT sample with remote heparin therapy (and confirmed to have no anti-Xa activity) was spiked with varying concentrations of UFH (Figure 2D) or enoxaparin (Figure 2E) and evaluated using the conventional (black line) and no-heparin (aHIT test; red line) SRAs. Concentrations as low as 0.06 U/mL of UFH induced serotonin release by this sample in the aHIT test, whereas there was no impact on the conventional SRA until supratherapeutic concentrations of heparin were spiked into the sample (5 U/mL; Figure 2D). Similarly, concentrations of enoxaparin as low as 0.25 U/mL caused serotonin release in the aHIT test, whereas the impact on the conventional SRA was noted only at high levels, which were not expected to be attained in a patient (5 U/mL; Figure 2E). To investigate whether the removal of heparin from patient samples could abrogate platelet activation in no-heparin functional testing, a HIT sample devoid of heparin was spiked with UFH and incubated with heparinase I (or buffer) before performing a platelet activation assay using P-selectin expression as the activation endpoint (Figure 2F). As seen in Figure 2F, a broad range of concentrations of UFH (0.05-0.7 U/mL) spiked in the patient sample induced platelet activation in a dose-dependent manner, whereas heparinase I treatment completely abrogated this effect (Figure 2F).
A significant number of diagnostic HIT samples contain remnant heparin, and treatment with heparinase I can change the diagnosis from “aHIT” to “conventional” HIT. (A) Anti-Xa activity in diagnostic samples was quantified in citrated plasma samples obtained from 30 patients with suspected HIT and tested in the conventional SRA (0.1 U/mL unfractionated heparin). Diamonds represent SRA-negative samples (<20% serotonin release in the conventional SRA), squares represent SRA-indeterminate samples (≥20% in the conventional SRA with <50% inhibition with a high concentration of heparin (100 U/mL), and circles depict SRA-positive samples (≥20% in the conventional SRA with >50% inhibition with 100 U/mL heparin). SRA-positive samples with detectable anti-Xa activity are depicted by solid red circles, whereas those without are shown as solid purple circles. (B) No-heparin SRA testing was performed on nine conventional SRA-positive samples. For each sample, untreated (solid circles) and heparinase I-treated (open circles) no-heparin SRA results are depicted and connected by a line. (C) Two spontaneous HIT patient samples were tested in the conventional SRA (0.1 units/mL unfractionated heparin; open triangles) or in the no-heparin SRA (closed triangles). (D) Unfractionated heparin or (E) Enoxaparin (LMWH) was spiked into a classical HIT sample (conventional SRA-positive; no-heparin SRA-negative) at concentrations indicated on the x-axes, and conventional SRA (0.1 U/mL UFH; blue line) or no-heparin SRA (red line) testing was performed. (F) P-selectin expression was quantified via flow cytometry after washed platelets were incubated with a healthy donor (NC, normal control) or classical HIT sample (HIT) spiked with the indicated concentration of unfractionated heparin (indicated on the X-axis) with or without heparinase I treatment. P-selectin expression results were compared using 2-way analysis of variance. (G) Serum samples drawn from a patient with HIT >12 hours after heparin cessation, or three other patients with HIT <4 hours after heparin cessation were treated with buffer (blue) or heparinase I (red) and evaluated in the no-heparin SRA.
A significant number of diagnostic HIT samples contain remnant heparin, and treatment with heparinase I can change the diagnosis from “aHIT” to “conventional” HIT. (A) Anti-Xa activity in diagnostic samples was quantified in citrated plasma samples obtained from 30 patients with suspected HIT and tested in the conventional SRA (0.1 U/mL unfractionated heparin). Diamonds represent SRA-negative samples (<20% serotonin release in the conventional SRA), squares represent SRA-indeterminate samples (≥20% in the conventional SRA with <50% inhibition with a high concentration of heparin (100 U/mL), and circles depict SRA-positive samples (≥20% in the conventional SRA with >50% inhibition with 100 U/mL heparin). SRA-positive samples with detectable anti-Xa activity are depicted by solid red circles, whereas those without are shown as solid purple circles. (B) No-heparin SRA testing was performed on nine conventional SRA-positive samples. For each sample, untreated (solid circles) and heparinase I-treated (open circles) no-heparin SRA results are depicted and connected by a line. (C) Two spontaneous HIT patient samples were tested in the conventional SRA (0.1 units/mL unfractionated heparin; open triangles) or in the no-heparin SRA (closed triangles). (D) Unfractionated heparin or (E) Enoxaparin (LMWH) was spiked into a classical HIT sample (conventional SRA-positive; no-heparin SRA-negative) at concentrations indicated on the x-axes, and conventional SRA (0.1 U/mL UFH; blue line) or no-heparin SRA (red line) testing was performed. (F) P-selectin expression was quantified via flow cytometry after washed platelets were incubated with a healthy donor (NC, normal control) or classical HIT sample (HIT) spiked with the indicated concentration of unfractionated heparin (indicated on the X-axis) with or without heparinase I treatment. P-selectin expression results were compared using 2-way analysis of variance. (G) Serum samples drawn from a patient with HIT >12 hours after heparin cessation, or three other patients with HIT <4 hours after heparin cessation were treated with buffer (blue) or heparinase I (red) and evaluated in the no-heparin SRA.
To evaluate the effect of heparinase treatment in the no-heparin SRA, the test most frequently used to classify patients as having "autoimmune HIT," 4 diagnostic serum samples available in adequate volumes were selected such that they were drawn either after remote (>12 hours; n = 1) or recent (<4 hours; n = 3) heparin administration as a means to stratify samples into those that were expected to have no remnant heparin (>12 hours) and those likely to have remnant heparin (<4 hours), due to heparin’s half-life of 60 to 90 minutes.12 Direct anti-Xa levels could not be assayed in these patients because of the lack of paired citrated plasma samples. The results shown in Figure 2G demonstrate that the diagnosis of one of the three patients with recent heparin administration changed from "autoimmune" to "classical" HIT after heparinase I digestion. In contrast, heparinase I digestion did not affect platelet activation induced by the patient with remote heparin administration. Together, the data in Figure 2B,G suggest that although some samples, including those from patients with spontaneous HIT, exhibit true autoimmune HIT characteristics, a subset of samples from patients with recent heparin administration may produce false-positive results in the aHIT test (no-heparin SRA) because of remnant heparin in the patient’s diagnostic blood sample. This study also demonstrates that in contrast to the no-heparin SRA, levels of heparin expected in diagnostic HIT samples are very unlikely to affect results in the conventional SRA (Figure 2D,E) and, thus, support the robustness of the conventional SRA for HIT diagnostic testing.
Our observation that heparin contamination was noted in approximately half of all diagnostic HIT samples is consistent with published findings that heparin administration continued in 54% of patients while HIT testing results were pending.13 In a recent study, anti-Xa activity testing in a cohort of diagnostic HIT samples demonstrated that contaminating heparin (anti-Xa activity ≥ 0.1 IU/mL) was detected in 21 of 34 (62%) samples.14 In that study, a comparison of outcome severity in confirmed heparin-negative samples with an aHIT profile (ie, activation in the no-heparin SRA) vs classical HIT demonstrated no differences in platelet nadir or persistence of thrombocytopenia. Incidence of thrombosis, however, just attained statistical significance in that limited study (aHIT, n=13 vs. Classical HIT, n=68; p=0.04).
Autoimmune HIT antibodies in diagnostic samples are currently serologically defined by positivity in no-heparin SRA,1 however, our data suggest that there is a risk of overestimating the incidence of aHIT due to the high frequency of heparin contamination in diagnostic samples. Because of the lack of a robust association between reactivity in the no-heparin SRA and HIT disease severity, likely a consequence of residual heparin in some patient samples, aHIT testing of diagnostic samples should not be used to risk stratify patients with HIT. Whether no-heparin SRA-positive results associated with diagnostic samples confirmed to be devoid of heparin can predict more severe disease outcomes is an open question and requires further investigation. The feasibility of performing such studies on a routine diagnostic basis (eg, subjecting HIT diagnostic samples to heparinase I digestion or performing anti-Xa activity assays to exclude heparin-contaminated samples) should be considered.
Acknowledgment: This work was supported, in part, by a National Institutes of Health grant HL158932 (A.P.).
Contribution: A.J.K., R.K.P., and A.P. conceived the study; A.J.K. and R.R.L. performed the experiments; R.S. oversaw mass spectrometric measurements of serotonin; J.A. evaluated patient charts and documented patient outcomes and laboratory parameters; A.S., N.M.H., D.C., and D.A.G. provided helpful input and critique; A.J.K. designed the figures; A.P. authored the first draft of the manuscript; and all authors provided input and approved the final version.
Conflict-of-interest disclosure: D.A.G. reports grants from Incyte and personal fees from Abbott. R.K.P. has received honoraria for attending advisory board meetings of CSL Behring, Genentech Inc, Bayer Healthcare AG, HEMA Biologics, Instrumentation Laboratory, and Biomarin and has consulted for Merck. A.P. reports pending/issued patents (Mayo Clinic, Retham Technologies, and Versiti Blood Center of Wisconsin); has equity ownership in and serves as an officer of Retham Technologies; and is member of the advisory board of Veralox Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Anand Padmanabhan, Department of Laboratory Medicine & Pathology, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; e-mail: padmanabhan.anand@mayo.edu; and Rajiv K. Pruthi, Department of Medicine, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; e-mail: pruthi.rajiv@mayo.edu.
References
Author notes
Data are available upon reasonable request to the corresponding author, Anand Padmanabhan (padmanabhan.anand@mayo.edu).
The full-text version of this article contains a data supplement.