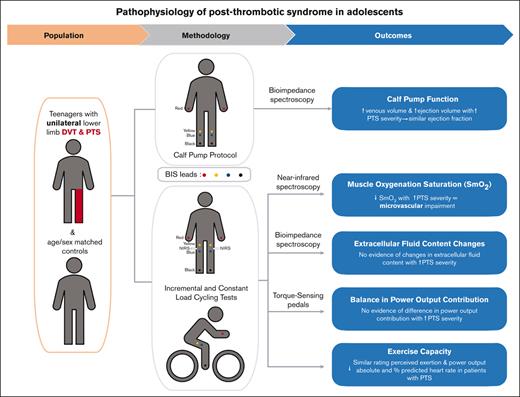
Key Points
We investigated the response to exercise in children with unilateral lower-limb PTS, and in age- and sex-matched controls.
We found compensated calf pump performance but impaired microvascular function, which worsened with increasing PTS severity.
Abstract
A better understanding of the pathophysiology of pediatric postthrombotic syndrome (PTS) is needed to develop strategies to treat this condition. We investigated calf pump function, exercise capacity, balance in power output, and changes in limb muscle oxygen saturation (SmO2) and fluid content during exercise in 10 pediatric patients with unilateral lower-limb PTS, and in age- and sex-matched controls (1:1-1:2 ratio). Outcomes were investigated using bioimpedance spectroscopy, torque-sensing pedals, and near-infrared spectroscopy during incremental- and constant-load cycling tests. The median age at participation was 17 years (25th-75th percentile, 15-18 years); 68% of participants were females. The median CAPTSure score in the affected leg of affected participants was 35 points (25th-75th percentile, 24-46 points), indicating moderate/severe PTS; 20% of patients had a history of central venous catheter–related thrombosis. Increasing PTS severity was associated with higher calf pump venous volume and higher ejection volume, leading to compensated calf pump performance. We found no evidence of PTS impact on exercise capacity. Leg contribution to power output was similar in affected and unaffected legs. However, the PTS-affected legs showed lower SmO2 during active cycling and recovery with increasing PTS severity, indicating impaired microvascular function in the muscle. These findings suggest that PTS severity is associated with impaired blood flow, presumably from elevated venous pressure during and after exercise. The fact that microvascular function is impaired in young patients with PTS underscores the relevance of developing strategies to mitigate the effects of this chronic vascular disease to minimize its deleterious effects as children grow older.
Introduction
Postthrombotic syndrome (PTS) is the most common chronic complication of deep vein thrombosis (DVT) in children, affecting 1 in every 2 to 4 children who sustain a DVT in their limbs.1-3 In view of the increasing frequency of DVT in the pediatric population, which was reported to increase by 300% between 2001 and 2019,4,5 the frequency of PTS is also expected to increase.
As we reported in previous works, limb tiredness, impaired limb endurance, and local pain are the most common clinical findings of pediatric PTS.6 Impaired endurance and pain are also among the most important clinical features of PTS in children and indicate higher disease severity.7 In turn, increasing PTS severity is associated with lower global functioning and happiness scores8 and with parental dissatisfaction.7 Given the negative impact of PTS in children throughout their lifespan, it is relevant to improve the management of this condition. However, there is no curative treatment for PTS, and management strategies in pediatrics are limited.9,10 Advances in the management of pediatric PTS require a better understanding of the underlying mechanism of disease. In fact, present knowledge of PTS pathophysiology is solely based on studies conducted in animal models or in adult patients.
We sought to investigate the pathophysiology of PTS in children by using acute exercise to detect and quantify underlying functional deficits. More specifically, we examined calf pump function, exercise capacity, balance in power output, and changes in limb muscle oxygen saturation and fluid content during exercise in pediatric patients with unilateral lower-limb DVT and PTS, and age- and sex-matched controls. Our main hypothesis was that during exercise at the same relative intensity, PTS-affected limbs had lower muscle oxygen saturation and higher extracellular fluid content than non-PTS–affected limbs. In addition, we hypothesized that calf muscle-vein pump function and power output were compromised in PTS-affected limbs, and that during aerobic exercise testing, the ventilatory threshold occurred at a lower power output in patients with PTS as compared with controls.
Methods
In total, 10 patients aged from 12 to 21 years at the time of study participation with a history of unilateral lower-limb DVT and PTS (ie, scores of >10 points, as measured by the index for clinical assessment of PTS in children, CAPTSure), and 12 healthy sex- and age-matched controls were prospectively recruited in a 1:1 to 1:2 ratio in this single-center case-control study. Patients with PTS were identified during thrombosis preclinic meetings and from existing thrombosis databases, and controls were recruited through postings. The Get Active questionnaire11 was used to screen for contraindications to performing an exercise protocol. Written informed consent was obtained before study participation. Approval from the research ethics boards of The Hospital for Sick Children (SickKids) and the University of Toronto were obtained before study initiation. All participants were tested within a single 2.5-hour experimental visit to the Exercise Medicine Laboratory at the Clinical Research Centre, SickKids, by 3 study investigators, including a certified exercise physiologist (J.E.S.).
The main outcomes of the study were: (1) calf pump function determined by the calf venous volume, ejection volume, and ejection fraction during light exercise (supplemental Appendix 1),12-15 measured using bioimpedance spectroscopy (BIS); (2) exercise capacity, as determined by the heart rate, percentage of maximum predicted heart rate, power output, and rating of perceived exertion at the ventilatory threshold during an incremental cycling test; (3) balance in power output contribution of each leg during incremental and constant-load cycling, measured using 2 torque-sensing pedals; (4) muscle oxygen saturation (SmO2) changes in each leg during incremental and constant-load cycling tests, measured using near-infrared spectroscopy (NIRS); and (5) changes in extracellular fluid content in each leg during incremental and constant-load cycling tests, determined using BIS.
Both legs of participants with and without PTS were tested simultaneously. The procedures and measurement techniques are detailed in hereafter.
The main predictor was PTS severity, measured using CAPTSure, an index for diagnosis and severity rating of pediatric PTS developed using expert consensus7,16; the scoring system is based on the relative importance of each sign and symptom to patients, parents, and clinicians. The tool has proven reliability17 and validity.7,8 The final score ranges from 0 (no PTS) to 100 points (worst possible PTS). The minimum detectable change is 11 points.17
Other collected variables included limb dominance, height, and weight. Limb dominance was determined using the Waterloo Footedness Questionnaire–Revised.18 Height and weight were used to calculate the participants’ body mass index (BMI). Leisure-time physical activity level was assessed using the Godin leisure-time exercise questionnaire (GLTEQ), through which respondents self-report the number of times they engage in mild, moderate, and strenuous physical activity for at least 15 minutes during a typical 7-day period. The reported value is multiplied by the metabolic equivalent of task value corresponding to each intensity to obtain a leisure activity score.19,20 Following the developers’ recommendations, the score was estimated for moderate to strenuous intensity only.19
The experimental consultation was partitioned into 4 discrete assessments: anthropometrics, calf pump function, incremental cycling, and constant-load submaximal cycling, the protocols of which are outlined hereafter.
Anthropometrics
Height and weight were measured to the nearest 0.1 cm and 0.1 kg, respectively, using calibrated instruments.
Calf muscle-vein pump function
Calf pump function of each leg was assessed by following a standard protocol12,13 (supplemental Appendix 1) and using BIS, a noninvasive technique that has been used for this purpose in adult patients.15 The principle underlying BIS is that the opposition of biological tissues to the flow of imperceptible electrical current at a suitable frequency is inversely proportional to the fluid volume of the tissue. Changes in impedance at low frequency reflect changes in extracellular fluid, including blood, lymph, and interstitial fluid, of which blood and lymph are the most dynamic components.21 BIS assessments were performed in the calves using SFB7 (Impedimed Ltd) devices specifically modified by the manufacturer to record resistance and reactance, at a fixed frequency, every millisecond for a total of 10 seconds. The frequency selected for the study was 5 kHz because impedance at this frequency reflects fluid content of the extracellular compartment. Segmental impedance values were determined per the principle of equipotentials.22 The coefficient of variation for impedance at low frequency in children ranges between 0.33% and 0.46%.23 Voltage-sensing electrodes were placed on the gastrocnemius muscle, 6 cm above (yellow) and 6 cm below (blue) a line on the upper-third of the calf (supplemental Appendix 2). Drive electrodes were placed at the intermalleolar line (black) and interstyloid line (red) ipsilateral to the limb being measured. Resistance values were measured in ohms.
Incremental cycling
Next, participants underwent an incremental cycling test during which exercise capacity, changes in SmO2 and extracellular fluid content in each limb, and power contribution of each limb were assessed. Participants pedaled on a Lode Corival ergometer fitted with 2 torque-sensing pedals (Garmin Vector 3), which measure the percentage of power contribution (balance) of the right vs the left leg. The participants began pedaling at a minimal power output of 25 watts, which was augmented by 15 watts every 2 minutes until reaching the ventilatory threshold, the participant asked to stop, or any of the American College of Sports Medicine stopping criteria were met.24 The ventilatory threshold is the preferred noninvasive test to measure exercise capacity in children and youths, because it does not require maximal effort.25 It was identified using the talk test, a valid and reliable method26,27 that is used to identify the ventilatory threshold by asking the participant to read a set prose passage report and assessing whether they can speak comfortably26,28; the talk test was considered positive if the participant answered “no” or “not sure.” After reaching the ventilatory threshold (active phase), participants completed a 2-to-3–minute active recovery at 25 watts (recovery phase). Heart rate, rating of perceived exertion, and power output were documented throughout the incremental cycling test. Heart rate was monitored with a Polar heart rate monitor (Polar H10Polar, Kempele, Finland), and was used to determine the percentage of the predicted maximum heart rate, as per the Tanaka method,29 at the ventilatory threshold. Perceived subjective effort during exercise was measured with the Borg 6-to-20 Rating of Perceived Exertion scale.30 The tool is appropriate for the age range of our participants; scores range from 6 (no exertion at all) to 20 points (maximal exertion). In addition to the aforementioned parameters, SmO2 and extracellular fluid content were monitored throughout the incremental cycling test using NIRS and BIS, respectively. NIRS is a noninvasive technique that determines in vivo SmO2 based on the difference in the light absorption of oxygenated vs deoxygenated hemoglobin (+myoglobin) at specific wavelengths.31 Moxy sensors (Fortiori Design, Hutchinson, MN) were used to measure SmO2, which was expressed as the percentage of oxygenated hemoglobin. The coefficient of variation for SmO2 during cycling exercise is 4% at a power output of 100 watts.32 The Moxy devices were placed anteriorly on the upper vastus lateralis. PerfPRO Studio (Hartware Technologies, Rockford, MI) software was used to track changes in SmO2 content in real time; SmO2 levels were recorded at 2 Hz. BIS was used to monitor extracellular fluid content during cycling; electrodes were placed 6 cm above (yellow) and 6 cm below (blue) the vastus lateralis (supplemental Appendix 2). Drive electrodes were placed as mentioned earlier. Impedance was measured every 2 minutes.
Constant submaximal-load cycling
After a 20-minute rest and questionnaire period, a constant submaximal-load cycling exercise assessment was conducted. Participants pedaled for 4minutes, starting at a power output equivalent to 25% of the watts at the ventilatory threshold. Power output was incrementally increased by 25% every minute to a power output equivalent to 90% of that recorded at the ventilatory threshold. The participants then pedaled for 5 minutes at this 90% wattage. The active phase of the constant-load cycling was followed by 2 to 3 minutes of recovery, during which participants cycled at a minimal power output (25 watts). Power output balance, SmO2, and extracellular fluid content were assessed throughout the test. NIRS and BIS sensors were placed on the thighs, as described earlier. BIS measurements were taken every minute. Heart rate and rating of perceived exertion were monitored throughout the testing.
Statistical analysis
Appropriate measures of central tendency and dispersion were used to describe the distribution of continuous variables; categorical variables were summarized using percentages or ratios. For inferential statistics, prespecified analyses were carried out, as described hereafter.
General characteristics of participants
GLTEQ scores and BMIs were compared between participants with and without PTS using the nonparametric Wilcoxon rank-sum test.
Calf muscle-vein pump function
The resistance obtained during BIS was digitally filtered using the Butterworth filter to estimate parameters of calf pump function. Minimum venous volume was defined as the mean resistance at the end of recumbency (supplemental Appendix 1, phase II); maximum venous volume was defined as the mean resistance at the end of the standing (supplemental Appendix 1, phase III). The difference between these 2 values was calculated to estimate functional venous volume. Ejection volume was estimated as the mean resistance during light exercise (supplemental Appendix 1, phase V). Ejection fraction was calculated using the following equation: ejection fraction = (ejection volume/venous volume) × 100.12-15 In view of the repeated observations per participant, given that both legs were assessed, generalized estimating equations (GEE) with an exchangeable correlation structure were used to analyze the association between each outcome (ejection volume, venous volume, and ejection fraction) and PTS severity, adjusted for leg dominance (ie, whether the assessed leg was dominant or not).
Exercise capacity
Markers of exercise capacity at the ventilatory threshold, including absolute heart rate (in beats per minute), percentage of age-predicted maximum heart rate, rating of perceived exertion, and power output (in watts), were compared between participants with and without PTS using the Wilcoxon rank-sum test.
Balance in power output
The trapezoidal rule was used to estimate the area under the curve (AUC) for the percentage of power exerted by each leg over time during the active segments of the incremental and constant-load cycling tests. To obtain comparable results, AUCs were estimated up to the shortest recorded time on each test. GEE with an exchangeable correlation structure was used to analyze the association between power AUC and PTS severity, adjusted for leg dominance. In addition, linear mixed models with a random intercept for legs within subjects were fit to analyze changes in power balance over time during active cycling. Models with and without a random slope for time were compared; the final model was selected using the Bayesian information criterion (BIC). Covariates of the models included PTS severity, time, and leg dominance.
Muscle oxygenation during exercise
Muscle oxygenation during cycling was estimated by assessing (1) the single lowest SmO2 value documented at any point during active cycling; (2) the SmO2 peak at 10 seconds during recovery, which is a marker of microvascular responsiveness33; and (3) the AUC for the SmO2 peak during recovery phase of incremental and constant-load cycling. To obtain comparable results, the AUCs were estimated up to the shortest recorded recovery time. The association between these SmO2 parameters and PTS was analyzed using GEE with an exchangeable correlation structure. Linear mixed models with a random intercept for legs within subjects were fit to analyze changes in SmO2 over time during the active and recovery phases of incremental and constant-load cycling. Models with and without a random slope for time were compared; the final model was selected using the BIC. Covariates in the models included PTS severity, time, and dominance, as described earlier.
Extracellular fluid content during exercise
Linear mixed models with random intercepts for legs within subjects were fit to analyze changes in resistance during the active phase of incremental and constant-load cycling; covariates in the models included PTS severity, time, and leg dominance. As described earlier, models with and without a random slope for time were compared; the final model was selected based on the BIC.
Results
The 22 participants (10 patients with unilateral PTS, 12 controls) were recruited between March 2021 and August 2022. Median age of the participants at the time of the study visit was 17 years (25th-75th percentile, 15-18 years). In total, 68% of participants were females (15 of 22); right-leg dominance was more common (17 of 22, 77%).
The GLTEQ moderate to vigorous activity score was similar in participants with and without PTS (median of 38 points; 25th-75th percentile, 22-54 vs 38 points; 25th-75th percentile 37-52 points; respectively; P = .71). BMI was also similar in participants with and without PTS (median of 18; 25th-75th percentile, 16-24 vs 17; 25th-75th percentile, 15-19; respectively; P = .18).
The median time between DVT diagnosis and study participation in the 10 participants with PTS was 3.8 years (25th-75th percentile, 2.0-9.6 years). Five patients had DVT in their left leg, and 5 in their right leg (5 of 10; 50%, respectively); 2 (2 of 10, 20%) had central venous catheter–related DVT. The median CAPTSure score at the time of study participation was 35 points (25th-75th percentile, 24-46 points), indicating moderate to severe PTS.8 Three (3 of 10, 30%) patients had complete DVT resolution, 3 (3 of 10, 30%) had no resolution, and 4 had partial DVT resolution (4 of 10, 40%). Additional details regarding the thrombotic events can be found in Table 1.
Clinical characteristics of patients with lower-limb PTS (n = 10)
| Variable . | Value∗ . |
|---|---|
| Location of index lower-limb DVT | |
| Ilio-femoral | 6 (60%) |
| Femoro-popliteal | 3 (30%) |
| Infrapopliteal | 1 (10%) |
| Age at diagnosis of DVT in y | 13 (12-15) |
| Age at study participation in y | 17 (15-18) |
| Time since DVT diagnosis in y | 3 (1.6-10.5) |
| DVT risk factors† | |
| Antiphospholipid syndrome | 3 (30%) |
| May-Thurner syndrome | 3 (30%) |
| Central venous catheter | 2 (20%) |
| Oral contraceptives | 1 (10%) |
| Management strategy | |
| Anticoagulation alone | 9 (90%) |
| Thrombolysis/thrombectomy | 1 (10%) |
| Anticoagulation length in mo | 18 (5-53) |
| Variable . | Value∗ . |
|---|---|
| Location of index lower-limb DVT | |
| Ilio-femoral | 6 (60%) |
| Femoro-popliteal | 3 (30%) |
| Infrapopliteal | 1 (10%) |
| Age at diagnosis of DVT in y | 13 (12-15) |
| Age at study participation in y | 17 (15-18) |
| Time since DVT diagnosis in y | 3 (1.6-10.5) |
| DVT risk factors† | |
| Antiphospholipid syndrome | 3 (30%) |
| May-Thurner syndrome | 3 (30%) |
| Central venous catheter | 2 (20%) |
| Oral contraceptives | 1 (10%) |
| Management strategy | |
| Anticoagulation alone | 9 (90%) |
| Thrombolysis/thrombectomy | 1 (10%) |
| Anticoagulation length in mo | 18 (5-53) |
Median (25th-75th percentile); n (%).
No risk factors identified in 2 patients.
Calf muscle-vein pump function
Calf function parameters are shown in Table 2. Ejection volume, venous volume, and ejection fraction were lower in the PTS-affected legs as compared with the legs without PTS. Because resistance is inversely proportional to fluid content, the lower resistance indicates higher intracellular fluid content in PTS-affected legs. Adjusted for leg dominance, multivariable analysis showed that both venous volume and ejection volume increased with increasing PTS severity, compensating the ejection fraction (Table 3).
Distribution of outcomes in legs with and without PTS (dichotomous variable)
| Outcome . | Legs with PTS, median (25th-75th percentile) . | Legs without PTS, median (25th-75th percentile) . | P value∗,† . | |
|---|---|---|---|---|
| Calf function | Ejection volume (ohms) | 3.28 (2.87-3.28) | 3.87 (2.89-4.62) | .06 |
| Venous volume (ohms) | 7.93 (4.50-8.62) | 8.70 (7.10-10.40) | .43 | |
| Ejection fraction (%) | 36 (29-56) | 45 (33-59) | .29 | |
| AUC for power during active cycling | Incremental cycling test | 20 768 (19 032-22 810) | 23 388 (17 494-26 822) | .86 |
| Constant-load cycling test | 12 698 (12 369-13 869) | 13 592 (12 611-14 511) | .19 | |
| Lowest SmO2 during active cycling (%) | Incremental cycling test | 25.9 (21.6-33.0) | 31.7 (24.2-39.6) | .13 |
| Constant-load cycling test | 46.5 (36.9-51.3) | 49.4 (42.4-60.5) | .002 | |
| SmO2 at 10 seconds during recovery (%) | Incremental cycling test | 39.9 (30.9-42.4) | 39.6 (29.4-52.7) | .02 |
| Constant-load cycling test | 48.7 (46.6-56.3) | 56.8 (45.4-74.6) | <.001 | |
| AUC for SmO2 during recovery | Incremental cycling test | 1 763 (1 133-1 918) | 1 768 (1 590-2 341) | .004 |
| Constant-load cycling test | 505 (477-566) | 566 (499-751) | <.001 | |
| Outcome . | Legs with PTS, median (25th-75th percentile) . | Legs without PTS, median (25th-75th percentile) . | P value∗,† . | |
|---|---|---|---|---|
| Calf function | Ejection volume (ohms) | 3.28 (2.87-3.28) | 3.87 (2.89-4.62) | .06 |
| Venous volume (ohms) | 7.93 (4.50-8.62) | 8.70 (7.10-10.40) | .43 | |
| Ejection fraction (%) | 36 (29-56) | 45 (33-59) | .29 | |
| AUC for power during active cycling | Incremental cycling test | 20 768 (19 032-22 810) | 23 388 (17 494-26 822) | .86 |
| Constant-load cycling test | 12 698 (12 369-13 869) | 13 592 (12 611-14 511) | .19 | |
| Lowest SmO2 during active cycling (%) | Incremental cycling test | 25.9 (21.6-33.0) | 31.7 (24.2-39.6) | .13 |
| Constant-load cycling test | 46.5 (36.9-51.3) | 49.4 (42.4-60.5) | .002 | |
| SmO2 at 10 seconds during recovery (%) | Incremental cycling test | 39.9 (30.9-42.4) | 39.6 (29.4-52.7) | .02 |
| Constant-load cycling test | 48.7 (46.6-56.3) | 56.8 (45.4-74.6) | <.001 | |
| AUC for SmO2 during recovery | Incremental cycling test | 1 763 (1 133-1 918) | 1 768 (1 590-2 341) | .004 |
| Constant-load cycling test | 505 (477-566) | 566 (499-751) | <.001 | |
Using GEE
Bolded values indicate statistical signficance
Results of multivariable GEE analysis for each outcome and PTS severity (continuous variable)
| Outcome . | PTS coefficient∗ . | Standard error . | P value† . | |
|---|---|---|---|---|
| Calf function | Ejection volume | −0.01 | 0.01 | .03 |
| Venous volume | −0.04 | 0.02 | .01 | |
| Ejection fraction | −0.17 | 0.40 | .67 | |
| AUC for power during active cycling | Incremental cycling test | 3.87 | 16.93 | .82 |
| Constant-load cycling test | −8.28 | 5.88 | .16 | |
| Lowest SmO2 during active cycling | Incremental cycling test | −0.10 | 0.04 | .009 |
| Constant-load cycling test | −0.23 | 0.06 | <.001 | |
| SmO2 at 10 seconds of recovery | Incremental cycling test | −0.26 | 0.06 | <.001 |
| Constant-load cycling test | −0.20 | 0.04 | <.001 | |
| AUC for SmO2 during recovery | Incremental cycling test | −11.37 | 1.82 | <.001 |
| Constant-load cycling test | −2.11 | 0.44 | <.001 | |
| Outcome . | PTS coefficient∗ . | Standard error . | P value† . | |
|---|---|---|---|---|
| Calf function | Ejection volume | −0.01 | 0.01 | .03 |
| Venous volume | −0.04 | 0.02 | .01 | |
| Ejection fraction | −0.17 | 0.40 | .67 | |
| AUC for power during active cycling | Incremental cycling test | 3.87 | 16.93 | .82 |
| Constant-load cycling test | −8.28 | 5.88 | .16 | |
| Lowest SmO2 during active cycling | Incremental cycling test | −0.10 | 0.04 | .009 |
| Constant-load cycling test | −0.23 | 0.06 | <.001 | |
| SmO2 at 10 seconds of recovery | Incremental cycling test | −0.26 | 0.06 | <.001 |
| Constant-load cycling test | −0.20 | 0.04 | <.001 | |
| AUC for SmO2 during recovery | Incremental cycling test | −11.37 | 1.82 | <.001 |
| Constant-load cycling test | −2.11 | 0.44 | <.001 | |
Change in outcome for every point increase in CAPTSure score, adjusted for limb dominance.
Bolded values indicate statistical significance
Exercise capacity
The markers of exercise capacity at the ventilatory threshold in participants with and without PTS are shown in Table 4. Although power output and rating of perceived exertion were similar in patients and controls, the absolute and percent predicted heart rate were lower in patients with PTS.
Markers of exercise capacity at the ventilatory threshold in participants with and without PTS (dichotomous variable)
| Characteristics . | Cases, n = 10∗ . | Controls, n = 12∗ . | P value‡,† . |
|---|---|---|---|
| Heart rate, in bpm | 144 (136-162) | 164 (151-173) | .02 |
| Percent of predicted maximum HR | 74 (70-82) | 83 (77-88) | .02 |
| Power output (watts) | 108 (89-115) | 100 (96-100) | .56 |
| Rating of perceived exertion score | 12 (11-13) | 12 (12-14) | .61 |
| Characteristics . | Cases, n = 10∗ . | Controls, n = 12∗ . | P value‡,† . |
|---|---|---|---|
| Heart rate, in bpm | 144 (136-162) | 164 (151-173) | .02 |
| Percent of predicted maximum HR | 74 (70-82) | 83 (77-88) | .02 |
| Power output (watts) | 108 (89-115) | 100 (96-100) | .56 |
| Rating of perceived exertion score | 12 (11-13) | 12 (12-14) | .61 |
bpm, beats per minute; HR, heart rate; IQR, interquartile range.
Median (25th-75th percentile).
Wilcoxon rank sum test, Wilcoxon rank sum exact test.
Bolded values indicate statistical significance
Balance in power output
AUC for power output during active cycling is shown in Table 2. Multivariable analysis showed no significant association between PTS severity and AUC for power contribution during active cycling (Table 3). Linear mixed models with random intercepts for legs within subjects, and a random slope for time, showed no significant association between PTS severity and the percentage of power exerted by each leg during active cycling (Table 5).
Results of multivariable linear mixed models for each outcome and PTS severity (continuous variable)
| Outcome . | PTS coefficient∗ . | Standard error . | P value† . |
|---|---|---|---|
| Power during active incremental test | −0.04 | 0.05 | .49 |
| Power during active constant-load test | −0.03 | 0.03 | .34 |
| SmO2 during active incremental cycling | −0.18 | 0.04 | <.001 |
| SmO2 during incremental cycling recovery | −0.32 | 0.05 | <.001 |
| SmO2 during active constant-load cycling | −0.17 | 0.06 | .01 |
| SmO2 during constant-load cycling recovery | −0.25 | 0.08 | .001 |
| Bioimpedance during active incremental cycling | 0.002 | 0.02 | .92 |
| Bioimpedance during active constant-load cycling | −0.01 | 0.02 | .48 |
| Outcome . | PTS coefficient∗ . | Standard error . | P value† . |
|---|---|---|---|
| Power during active incremental test | −0.04 | 0.05 | .49 |
| Power during active constant-load test | −0.03 | 0.03 | .34 |
| SmO2 during active incremental cycling | −0.18 | 0.04 | <.001 |
| SmO2 during incremental cycling recovery | −0.32 | 0.05 | <.001 |
| SmO2 during active constant-load cycling | −0.17 | 0.06 | .01 |
| SmO2 during constant-load cycling recovery | −0.25 | 0.08 | .001 |
| Bioimpedance during active incremental cycling | 0.002 | 0.02 | .92 |
| Bioimpedance during active constant-load cycling | −0.01 | 0.02 | .48 |
Change in outcome for every point increase in CAPTSure score, adjusted for time and limb dominance.
Bolded values indicate statistical significance
Muscle oxygenation during exercise
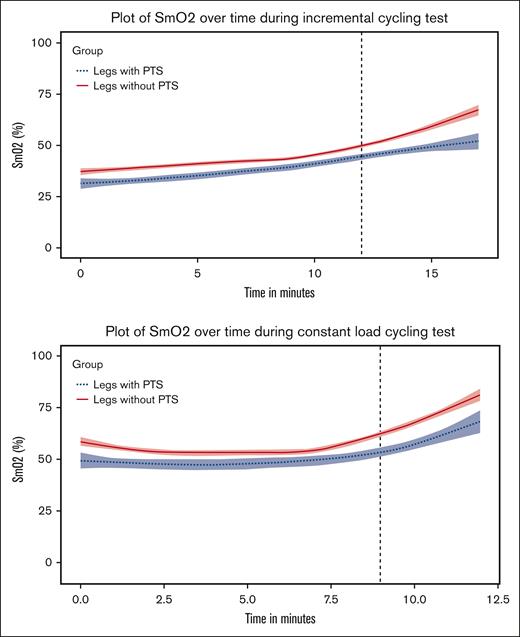
Figure 1 shows the smoothed curves for SmO2 over time for legs with and without PTS during the cycling tests, fitted by locally weighted regression. The single lowest SmO2 value during active cycling, the SmO2 at 10 seconds of recovery, and the AUC for SmO2 at recovery are shown in Table 2. All values were numerically lower in the PTS-affected legs. Adjusted for leg dominance, increasing PTS severity was associated with (1) lower SmO2 values during active cycling, (2) lower SmO2 at 10 seconds of recovery, and (3) lower AUC for SmO2 at recovery (Table 3). Adjusted for time and limb dominance, random intercepts random slopes linear mixed models showed that increasing PTS severity was associated with lower SmO2 during incremental and constant-load cycling in the active and recovery phase (Table 5).
Smoothed curves for SmO2 over time for legs with and without PTS fitted by locally weighted regression. Note: the black vertical dashed line indicates the median time at which exercise recovery starts.
Smoothed curves for SmO2 over time for legs with and without PTS fitted by locally weighted regression. Note: the black vertical dashed line indicates the median time at which exercise recovery starts.
Extracellular fluid content during exercise
Random intercept linear mixed models showed no evidence of significant association between PTS severity and tissue resistance during active cycling (Table 5).
Discussion
In this study, we aimed to better understand the pathophysiology of pediatric PTS by analyzing the response to a submaximal exercise challenge in patients with unilateral lower-limb DVT and PTS in comparison with the contralateral unaffected leg and to those of age- and sex-matched controls.
Our results demonstrated that higher PTS severity in children was associated with higher venous volume in the calf, which was offset by a higher ejection volume, leading to compensated calf pump performance, as assessed by ejection fraction. It has been shown that ejection fraction is impaired in adult patients with chronic venous diseases.34-37 Furthermore, calf pump function impairment correlates with disease severity,34,38 and is more pronounced in adults with history of DVT (ie, PTS) as compared with adults with primary chronic venous disease.35 Venous volume also increases with PTS progression in adult patients as a consequence of the larger venous capacitance that results from vein obstruction and reflux.35 Importantly, aging has multiple deleterious effects on the venous system, from endothelial cells and connective tissue to venous valves.39 Therefore, despite the still-compensated calf pump function in children with PTS, the ensuing age-related decline of calf pump efficiency is expected to lead to more severe PTS with age in these children. It is therefore relevant to support lifestyle strategies such as physical activity to promote healthy vein aging39 and delay age-related calf pump deterioration in affected children.
When analyzing exercise capacity, we found that the rating of perceived exertion and power output at the ventilatory threshold were similar in participants with and without PTS, indicating a similar degree of perceived physical strain and effort.40 However, heart rate and relative peak heart rate at the ventilatory threshold were lower in participants with PTS than in those without PTS. Importantly, when compared with normative pediatric data,41 heart rate at the ventilatory threshold was slightly lower in participants with PTS. In contrast, heart rate at the ventilatory threshold was slightly higher in participants without PTS as compared with normative data.41 These findings could be explained by some degree of deconditioning in controls and better fitness in participants with PTS.
We found no evidence that higher PTS severity was associated with a lower contribution of the affected leg to the power output. This is possibly because of the multiple factors at the muscular, cardiovascular, and neurological levels that interact to determine power output.42 In addition, exercise intensity was low to moderate for most of the active cycling and, therefore, participants may not have spent enough time in a range where the difference in leg output was relevant.
NIRS offers a dynamic view of muscle oxygen delivery and utilization during exercise.43 Under normal circumstances, the SmO2 signal increases rapidly after a challenge such as ischemia or exercise, and this SmO2 reperfusion slope is a sensitive measure of microvascular responsiveness.44-46 Our results indicate that delivery of oxygen to the muscle worsens with higher PTS severity during and after an exercise challenge. The lower SmO2 in the affected leg during active cycling suggests impaired delivery, and the lower SmO2 during exercise recovery further suggests an impairment of microvascular function that compromises the ability to perfuse the muscle. Reduced oxygen delivery relative to the oxygen demand of the muscle negatively affects the bioenergetic pathways of the skeletal muscle, leading to impaired muscle performance with faster onset of muscle fatigue,47 which could explain the poor limb endurance typically seen in children with PTS.6 Taken together, our results indicate that PTS severity is associated with impaired blood flow at the microvascular level, which could arise from elevated venous pressure during and after exercise, compromising the pressure gradient for flow across the vascular bed at the active muscle.
For several decades, NIRS has been used to study muscle oxygenation in adults with peripheral artery disease,43 and has shown lower oxygen reperfusion rates and longer muscle oxygen recovery time after arterial occlusion or an exercise challenge in affected limbs.48-52 In addition, NIRS has also been applied to assess calf pump function in adults with venous diseases, including PTS.53-58 Yamaki et al55 showed shorter time for oxygenated hemoglobin increase after standing in patients with PTS, suggesting impairment in venoarteriolar reflex in these patients. The venoarteriolar reflex is triggered by venular distension secondary to postural changes and results in arteriolar vasoconstriction, decrease in capillary pressure, and protection of dependent tissues against edema and hypertension.59,60 Capillary changes and microangiopathy have long been recognized in chronic venous diseases.61-64 Backflow and turbulent flow caused by structural changes in the venous wall and increased venous pressure transmitted retrograde to the capillary bed lead to microcirculatory and microlymphatic damage and dysfunction61,65-68 and to chronic changes in shear stress with activation of endothelial cells,69 all of which ultimately results in chronic inflammatory injury.69-71 Although microcirculatory dysfunction is more common in severe forms of venous disease, more recent studies show that the inflammatory process in the microcirculation starts early in the course of the disease. Virgini-Magalhaes et al67 studied patients with C1-C5 venous disease, classified in accordance with the clinical, etiological, anatomical, and pathophysiological classification system.72 The authors found that the density of functional capillaries decreases, the diameter of the dermal papilla and capillaries increases, and the capillary morphology changes early on, becoming progressively tortuous, bulky, and glomerulus-like as the disease progresses.67 Moreover, studies involving patients with C0S (adults with symptoms but no signs of venous disease) show changes in flow pattern, with bidirectional flow in the small veins73 and changes in capillary morphology, including enlargement of the dermal papilla,74 as compared with asymptomatic participants (C0A). In line with the microcirculatory problems detected in adult patients with C0S, our results indicate the occurrence of microcirculatory impairment at the muscular level in a group of younger patients that would also be largely classified in the C0S category of the clinical, etiological, anatomical, and pathophysiological classification system.
Lastly, we found no changes in tissue resistance during cycling, suggesting no extracellular fluid accumulation using BIS, which aligns with an effective calf pump function.
There are limitations to this study. Because of limitations to exercise testing during the COVID-19 pandemic, we were not able to determine the ventilatory threshold using respiratory gas exchange as initially planned and we used the talk test instead. However, as mentioned in the Methods section, the talk test is regarded as a reliable and valid method for determining the ventilatory threshold.26,27,75 In addition, the sample size is relatively small and likely underpowered to detect changes over time in the main outcomes, and therefore our findings should be interpreted as a starting point for larger studies.
In summary, we found larger calf venous volume, compensated calf pump performance, and impaired microvascular function in children with lower-limb PTS, as compared with their nonaffected limb and to the limbs of matched controls with no PTS. Although severe forms of PTS are less frequent in pediatric patients1 than in adults,76 the fact that we documented altered hemodynamic and microvascular parameters so early in life underscores the relevance of developing strategies to mitigate the effects of this chronic vascular disease and minimize its deleterious effects as these children grow older and age.
Acknowledgments
The authors thank the participants and their families for their time and support to research activities; and Nour Amiri, Riddhita De, and Jennifer Vincelli for their support during study conduction.
This study was supported by a research grant from the Physicians’ Services Incorporated Foundation.
Authorship
Contribution: M.L.A. designed the study, performed laboratory testing, analyzed data, and wrote the manuscript; R.F.B. supported data interpretation and critically reviewed the manuscript; D.B. recruited patients, performed laboratory testing, collected and entered data, and critically reviewed the manuscript; L.R.B., S.S., and L.W. supported study design and critically reviewed the manuscript; J.E.S. performed laboratory testing and critically reviewed the manuscript; G.W. performed laboratory testing and critically reviewed the manuscript; K.L. supported statistical analysis and critically reviewed the manuscript; and S.T. designed the study, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: L.W. provides consultancy services to ImpedIMed Ltd. The remaining authors declare no competing financial interests.
Correspondence: M. Laura Avila, Division of Paediatric Haematology/Oncology, The Hospital for Sick Children, 555 University Ave, Toronto, ON M5G 1X8, Canada; e-mail: laura.avila@sickkids.ca.
References
Author notes
Data are available on request from pts.research@sickkids.ca.
The full-text version of this article contains a data supplement.