Key Points
HLH-LTs after chimeric antigen receptor (CAR) T-cell therapy were independently associated with decreased overall and relapse-free survival.
Pre–CAR T-cell therapy variables associated with HLH-LTs included disease burden, inflammatory markers, thrombocytopenia, and neutropenia.
Abstract
Chimeric antigen receptor–associated hemophagocytic lymphohistiocytosis (HLH)–like toxicities (LTs) involving hyperferritinemia, multiorgan dysfunction, coagulopathy, and/or hemophagocytosis are described as occurring in a subset of patients with cytokine release syndrome (CRS). Case series report poor outcomes for those with B-cell acute lymphoblastic leukemia (B-ALL) who develop HLH-LTs, although larger outcomes analyses of children and young adults (CAYAs) with B-ALL who develop these toxicities after the administration of commercially available tisagenlecleucel are not described. Using a multi-institutional database of 185 CAYAs with B-ALL, we conducted a retrospective cohort study including groups that developed HLH-LTs, high-grade (HG) CRS without HLH-LTs, or no to low-grade (NLG) CRS without HLH-LTs. Primary objectives included characterizing the incidence, outcomes, and preinfusion factors associated with HLH-LTs. Among 185 CAYAs infused with tisagenlecleucel, 26 (14.1%) met the criteria for HLH-LTs. One-year overall survival and relapse-free survival were 25.7% and 4.7%, respectively, in those with HLH-LTs compared with 80.1% and 57.6%, respectively, in those without. In multivariable analysis for death, meeting criteria for HLH-LTs carried a hazard ratio of 4.61 (95% confidence interval, 2.41-8.83), controlling for disease burden, age, and sex. Patients who developed HLH-LTs had higher pretisagenlecleucel disease burden, ferritin, and C-reactive protein levels and lower platelet and absolute neutrophil counts than patients with HG- or NLG-CRS without HLH-LTs. Overall, CAYAs with B-ALL who developed HLH-LTs after tisagenlecleucel experienced high rates of relapse and nonrelapse mortality, indicating the urgent need for further investigations into prevention and optimal management of patients who develop HLH-LTs after tisagenlecleucel.
Introduction
Chimeric antigen receptor (CAR) T-cell therapies have transformed the treatment landscape for relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL).1,2 Impressive efficacy and safety of the CD19-directed CAR T-cell product tisagenlecleucel (Kymriah) in children and young adults (CAYAs)1,3 led to its Food and Drug Administration (FDA) approval, and it remains the only FDA-approved CAR T-cell therapy in children.
The most common severe toxicity of CAR T-cell therapy is cytokine release syndrome (CRS), a hyperinflammatory condition with symptoms ranging from isolated fevers to life-threatening multiorgan dysfunction. CRS shares features with hemophagocytic lymphohistiocytosis (HLH)4-6 including fever, splenomegaly, cytopenias, hypertriglyceridemia, hypofibrinogenemia, hyperferritinemia ≥500 ng/mL, and hypercytokinemia.5,7 Although patients with high-grade [HG]–CRS often meet the HLH 2004 criteria,7 the existence of a subgroup of patients with CRS developing extreme hyperferritinemia and organ dysfunction beyond that typically seen in CRS prompted criteria for HLH-like toxicities (LTs) occurring secondary to CAR T-cell therapy, or carHLH, to be developed. Proposed carHLH criteria require higher ferritin thresholds than the HLH 2004 criteria (≥10 000 or 100 000 ng/mL) and the presence of multiorgan dysfunction, coagulopathy, and/or cellular hemophagocytosis.6,8,9 However, there remains a lack of an agreed-upon definition for carHLH, which has motivated efforts by the American Society for Transplantation and Therapy (ASTCT) to establish consensus criteria, currently in development. To date, there are few reports on the outcomes of patients developing HLH-LTs; however, in the reports available, outcomes are poor. Four of 4 CAYAs with B-ALL developing HLH-LTs after CD19/4-1BB CAR T cells experienced mortality with a median overall survival (OS) of 44.5 days,10 5 of 6 adults with diffuse large B-cell lymphoma treated with CD19/CD28 CAR T cells who developed HLH toxicities experienced death with a median OS of 2 months,11 and 81 of 121 (66.9%) children and adults treated with CAR T-cell products with HLH-LTs reported in the FDA Adverse Events Reporting System experienced death.12
The incidence of HLH-LTs in patients with relapsed/refractory B-ALL has varied with different CAR T-cell products on clinical trials, ranging from 14.1% to 14.8% of CAYAs treated with CD19/4-1BB CAR T-cell products (tisagenlecleucel, SJCAR19, and Seattle CD19-CAR T)9,10 to 35.6% of CAYAs treated with a CD22/4-1BB CAR T-cell product.9 However, the incidence of HLH-LTs in CAYAs treated with tisagenlecleucel in the real-world setting has not been described, and large multi-institutional outcomes are not reported. Our objectives were to (1) characterize the incidence of HLH-LTs in CAYAs with B-ALL after real-world use of tisagenlecleucel, (2) identify preinfusion characteristics associated with HLH-LTs, and (3) describe outcomes of CAYAs that developed HLH-LTs.
Methods
Study design
We conducted a retrospective multi-institutional cohort study involving 15 pediatric centers that deliver tisagenlecleucel, participating in the Pediatric Real World CAR Consortium. Patients ≤26 years old with B-ALL who underwent tisagenlecleucel manufacturing and infusion between 30 August 2017 and 6 March 2020 were eligible for inclusion. Each center obtained institutional review board approval, and deidentified retrospective data were collected using REDCap electronic data capture tools hosted at Stanford University. The study was conducted in accordance with the Declaration of Helsinki.
Patients were categorized as having HLH-LTs using criteria adapted from definitions previously proposed by 2 groups: 1 focused on adults with non-Hodgkin lymphoma,6 and the second focused on CAYAs with B-ALL treated with a CD22-directed CAR T-cell product.8 Criteria used in this study included a peak ferritin of ≥10 000 ng/mL in a patient with CRS (active or resolved), in addition to ≥2 of the following: renal toxicity, hepatic toxicity, hypoxia, coagulopathy, and/or the presence of hemophagocytosis on bone marrow (BM) aspirate or biopsy. Renal toxicity, hepatic toxicity, and coagulopathy characterizations were inclusive of any Common Terminology Criteria for Adverse Events grade. Patients not meeting the criteria for HLH-LTs were grouped as ASTCT grade 3 to 5 CRS (HG-CRS) and ASTCT grade 0 to 2 CRS (no to low-grade [NLG]–CRS). Subgroup analyses were performed for patients with (1) HLH-LTs and peak ferritin ≥100 000 ng/mL or 10 000 to <100 000 ng/mL; (2) patients with grade 3 to 5 CRS (HG-CRS) and grade 1 to 2 CRS (LG-CRS), with and without HLH-LTs; and (3) patients with 5% to <50% and ≥50% preinfusion BM lymphoblasts, with and without HLH-LTs.
Variables
Primary analyses included comparing OS, relapse-free survival (RFS), and nonrelapse mortality (NRM), defined as death without recurrent or progressive leukemia, between groups with HLH-LTs, HG-CRS, and NLG-CRS. Additional variables included maximum CRS grade and immune effector cell–associated neurotoxicity (ICANS)/neurotoxicity, treatments of CRS and ICANS/neurotoxicity, postinfusion C-reactive protein (CRP) and ferritin, duration of cytopenias, infections within 28 days of tisagenlecleucel infusion, and cause of death in those who experienced mortality. Of note, as ICANS grading was published in 2019, and CAR T-cell toxicities reported in this study occurred between 2017 and 2020, neurotoxicity was graded via CAR T‑cell therapy–associated toxicity (CARTOX) criteria, National Cancer Institute, National Institutes of Health/Common Terminology Criteria for Adverse Events version 4.03 and version 5.0, or immune effector cell-associated neurotoxicity syndrome (ICANS) criteria. CRS grading was performed through standardized regrading assessed via the ASTCT consensus guidelines.13 Preinfusion variables included demographic information, B-ALL BM disease burden, CRP, ferritin, absolute neutrophil count (ANC), absolute lymphocyte count (ALC), platelet count, prior lines of treatment, receipt of optimal fludarabine exposure in lymphodepleting chemotherapy as described previously,14 and tisagenlecleucel parameters. High disease burden was defined as ≥5% BM lymphoblasts or the presence of extramedullary disease or peripheral lymphoblasts; low disease burden was defined as <5% BM lymphoblasts and the absence of extramedullary disease or peripheral lymphoblasts, as described previously.15
Statistical analysis
Statistical analyses were performed using R package version 4.1.2 (R Core Team 2021; Vienna, Austria) and GraphPad Prism, version 9.3.1 (San Diego, CA). Group comparisons were performed using Kruskal-Wallis tests for continuous variables and Fisher exact tests for categorical variables. For survival analyses, HLH-LTs and CRS status were treated as baseline values, as median CRS onset occurred 2.5, 3, and 5 days after infusion in the HLH-LT, HG-CRS, and NLG-CRS groups, respectively, and no events (relapse, death, or censoring) occurred before CRS onset in any patient. Kaplan-Meier curves for OS and RFS were compared using log-rank tests. To assess the effect of HLH-LT status on OS and RFS, univariate and multivariable Cox proportional hazards models were developed, including variables such as disease burden, age, and variables found to be associated with the development of HLH-LTs, including preinfusion ANC, platelets, ferritin, and CRP. Other covariates included sex, race/ethnicity, tocilizumab doses (≥2 vs <2), and days of steroids (≥6 vs <6). Hazard ratios (HRs) and their 95% confidence intervals (CIs) were determined. Significance level was set at 2-tailed α = 0.05.
Results
HLH-LT incidence and pretisagenlecleucel characteristics
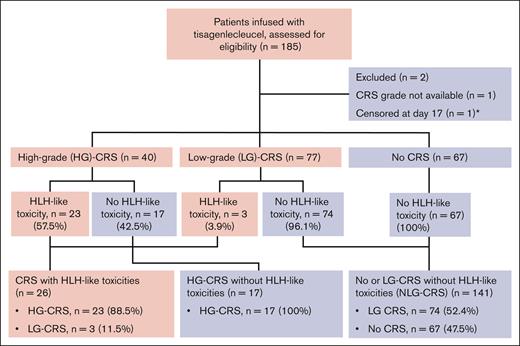
A total of 185 patients with B-ALL infused with commercially available tisagenlecleucel were included. One patient without CRS grade recorded was excluded, and 1 patient was excluded from the survival analysis because of censoring at day 17 after CAR T-cell infusion (Figure 1). Of those infused with tisagenlecleucel, 26 (14.1%) met the criteria for HLH-LTs (Figure 2A), and 13 of 185 (7.0%) met the criteria for HLH-LTs with peak ferritin ≥100 000 ng/mL (Table 1; supplemental Table 2). Preinfusion parameters were compared between the groups with HLH-LTs (n = 26) and the groups without HLH-LTs: HG-CRS (n = 17) and NLG-CRS (n = 141) (Table 1). Median preinfusion BM blasts were markedly higher in the HLH-LT group than in the HG-CRS and NLG-CRS groups (66.0% vs 8.0% vs 5.0%, P < .0001), as were the ferritin and CRP values. The HLH-LT group had lower preinfusion platelet and ANC values than the HG-CRS and NLG-CRS groups (Table 1). ALCs had a trend toward lower values in the HLH-LT group, but this did not reach statistical significance. There were no significant differences in age, sex, race/ethnicity, upfront leukemia genetics risk group, end of induction risk stratification, prior lines of therapy, central nervous system involvement, extramedullary disease, CAR T-cell viability, dose, transduction efficiency, or CAR expression between the groups. Comparing subgroups with HLH-LTs and peak ferritin ≥100 000 ng/mL vs peak ferritin 10 000 to <100 000 ng/mL, there were no significant differences in preinfusion disease burden, ferritin, CRP, ANC, ALC, or platelets (supplemental Figure 1). Other variables in these subgroups are shown in supplemental Tables 1 and 2. Evaluating preinfusion variables by peak CRS grade (HG-CRS, LG-CRS, and no CRS) irrespective of HLH-LTs, higher ferritin values, CRP values, and BM disease burden and lower ANC and platelet values were observed in the group with HG-CRS compared with groups with LG-CRS and no CRS (supplemental Table 3). However, when the HG-CRS group was subdivided into those with and without HLH-LTs, inflammatory markers and BM disease burden were significantly higher, and ANC and platelet values were significantly lower in the group with HG-CRS + HLH-LTs compared with the group with HG-CRS without HLH-LTs (supplemental Table 3). Notably, after infusion, HG-CRS was associated with the development of HLH-LTs, with 23 of 40 patients with HG-CRS developing HLH-like toxicities compared with 3 of 77 patients with LG-CRS (Fisher exact odds ratio, 33.4; 95% CI, 8.9-110.4; P < .0001).
CONSORT diagram. 185 patients treated with tisagenlecleucel were divided into groups based on peak CRS grade experienced: ASTCT grade 3 to 5 CRS (HG-CRS), ASTCT grade 1 to 2 CRS (LG-CRS), and no CRS. The proportion of patients within each CRS group that experienced HLH-LTs is shown. Patients were then grouped into those who experienced CRS and developed HLH-LTs, those with HG-CRS who did not experience HLH-LTs (HG-CRS), and those with no or LG-CRS who did not experience HLH-LTs (NLG-CRS). Shaded boxes indicate inclusion of patients with HLH-LTs. ∗1 patient excluded from survival analyses because of censoring without event at day 17 after CAR T-cell infusion.
CONSORT diagram. 185 patients treated with tisagenlecleucel were divided into groups based on peak CRS grade experienced: ASTCT grade 3 to 5 CRS (HG-CRS), ASTCT grade 1 to 2 CRS (LG-CRS), and no CRS. The proportion of patients within each CRS group that experienced HLH-LTs is shown. Patients were then grouped into those who experienced CRS and developed HLH-LTs, those with HG-CRS who did not experience HLH-LTs (HG-CRS), and those with no or LG-CRS who did not experience HLH-LTs (NLG-CRS). Shaded boxes indicate inclusion of patients with HLH-LTs. ∗1 patient excluded from survival analyses because of censoring without event at day 17 after CAR T-cell infusion.
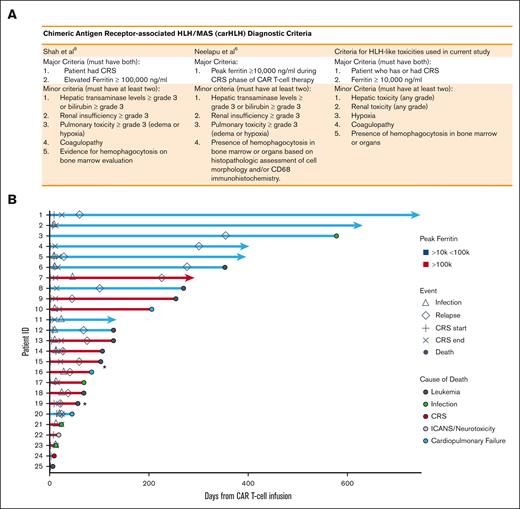
Diagnostic criteria for HLH-LT and swimmer plot of patients with HLH-LTs. Diagnostic criteria for carHLH described previously and criteria for HLH-LT used in the current study. (B) Swimmer’s plot depicting clinical course and outcomes of patients that met criteria for HLH-LT, with those having peak ferritins ≥100 000 ng/mL shown in red, those with peak ferritins 10 000 to 100 000 ng/mL shown in blue. ∗indicates that cause of death in 2 patients noted to be from respiratory failure as well as leukemia.
Diagnostic criteria for HLH-LT and swimmer plot of patients with HLH-LTs. Diagnostic criteria for carHLH described previously and criteria for HLH-LT used in the current study. (B) Swimmer’s plot depicting clinical course and outcomes of patients that met criteria for HLH-LT, with those having peak ferritins ≥100 000 ng/mL shown in red, those with peak ferritins 10 000 to 100 000 ng/mL shown in blue. ∗indicates that cause of death in 2 patients noted to be from respiratory failure as well as leukemia.
Baseline characteristics of patients with CRS + HLH-LTs, HG-CRS without HLH-LTs, and NLG-CRS without HLH toxicities
| . | CRS + HLH-LT, n = 26 . | HG-CRS without HLH-LT, n = 17 . | NLG-CRS without HLH-LT, n = 141 . | P value . |
|---|---|---|---|---|
| Age, median (IQR), y | 11.5 (4.5-16.75), n = 26 | 13 (10-18), n = 17 | 12 (8-18), n = 141 | .45 |
| Male, n (%) | 13/26 (50.0) | 10/17 (58.8) | 87/141 (61.6) | .53 |
| Race/ethnicity, n (%) | ||||
| Hispanic | 10/26 (38.5) | 7/17 (41.2) | 53/141 (37.6) | .93 |
| Non-Hispanic White | 14/26 (53.8) | 8/17 (47.1) | 67/141 (47.5) | |
| Other | 2/26 (7.7) | 2/17 (11.8) | 21/141 (14.9) | |
| Initial cytogenetic risk category, n (%) | ||||
| Favorable | 2/18 (11.1) | 3/13 (23.1) | 19/108 (17.6) | .55 |
| Intermediate | 7/18 (38.9) | 2/13 (15.4) | 40/108 (37.0) | |
| Unfavorable | 9/18 (50.0) | 8/13 (61.5) | 49/108 (45.4) | |
| Risk stratification at end of induction, n (%) | ||||
| Low risk | 0/23 (0.0) | 0/16 (0.0) | 6/116 (5.2) | .09 |
| Standard risk | 0/23 (0.0) | 1/16 (6.3) | 20/116 (17.2) | |
| High risk | 8/23 (34.8) | 5/16 (31.3) | 19/116 (16.4) | |
| Very high risk | 15/23 (65.2) | 10/16 (62.5) | 71/116 (61.2) | |
| Timing of initial B-ALL relapse, n (%) | ||||
| On therapy relapse | 17/26 (65.4) | 6/17 (35.3) | 39/142 (27.5) | .0008∗∗∗ |
| Early relapse | 8/26 (30.8) | 6/17 (35.3) | 32/142 (22.5) | .39 |
| Late relapse | 5/26 (19.2) | 5/17 (29.4) | 44/142 (30.9) | .48 |
| Lines of therapy before CAR, median (IQR) | 2 (1.25-4), n = 26 | 2 (1-3), n = 17 | 2 (1-3), n = 141 | .35 |
| Prior CD19-directed therapy, n (%) | 8/26 (30.8) | 4/17 (23.5) | 25/141 (17.7) | .28 |
| Optimal† lymphodepleting chemotherapy, n (%) | 11/20 (55) | 8/15 (53.3) | 82/116 (70.7) | .18 |
| BM disease burden %, median (IQR) | 66.0 (26.2-84.0), n = 25 | 8.0 (3.7-36.0), n = 13 | 5.0 (0.24-28.7), n = 80 | <.0001∗∗∗∗ |
| B-ALL disease burden, n (%) | ||||
| Low | 0 (0.0) | 6 (37.5) | 76 (55.1) | <.0001∗∗∗∗ |
| High∗ | 26 (100.0) | 10 (62.5) | 62 (44.9) | |
| Total (n) | 26 | 16 | 138 | |
| Timing of pre–CAR T-cell infusion BM assessment, median (IQR), wk | 2.5 (1.0-9.0), n = 26 | 2.0 (1.25-4.63), n = 16 | 2.0 (1.1-7.8), n = 138 | .87 |
| Peripheral blasts, % of white blood cells, median (IQR) | 25 (0-50), n = 22 | 0 (0-0), n = 15 | 0 (0-0), n = 127 | <.0001∗∗∗∗ |
| CNS disease pre–CAR T-cell infusion (CNS 2 + 3), n (%) | 2/19 (10.5) | 0/12 (0.0) | 10/118 (8.5) | .63 |
| Extramedullary disease pre–CAR T-cell infusion, n (%) | 3/26 (11.5) | 2/17 (11.8) | 10/141 (7.1) | .50 |
| Pre–CAR T-cell infusion ALC (cells per μL), median (IQR) | 30 (0.1-60), n = 21 | 50 (17.5-97.5), n = 16 | 70 (20-157.5), n = 134 | .09 |
| Pre–CAR T-cell infusion ANC (cells per μL), median (IQR) | 10 (0-160), n = 21 | 490 (197.5-1175), n = 16 | 530 (232.5-1040), n = 134 | <.0001∗∗∗∗ |
| Pre–CAR T-cell infusion platelet (×109/L), median (IQR) | 35.5 (20.25-92), n = 26 | 110.0 (46-148), n = 17 | 121.5 (56-206), n = 138 | <.0001∗∗∗∗ |
| Pre–CAR T-cell infusion ferritin (ng/mL), median (IQR) | 3745 (1291.8-6194.6), n = 25 | 831 (663.0-2174.5), n = 15 | 1364 (603.5-2660.4), n = 115 | .002∗∗ |
| Pre–CAR T-cell infusion CRP (mg/dL), median (IQR) | 3.165 (1.01-7.29), n = 22 | 0.559 (0.390-2.30), n = 14 | 0.600 (0.300-2.51), n = 111 | .006∗∗ |
| CAR viability (%), median (IQR) | 88.6 (87.6-93.3), n = 25 | 87.0 (85.3-95.5), n = 17 | 87.6 (83.2-92.2), n = 140 | .13 |
| CAR dose infused (CAR T cells × 106/kg), median (IQR) | 1.585 (1.23-2.48), n = 26 | 1.630 (1.28-2.00), n = 16 | 1.80 (1.33-2.45), n = 139 | .60 |
| Transduction efficiency (no. of copies per cell), median (IQR) | 0.315 (0.26-0.40), n = 24 | 0.400 (0.27-0.55), n = 16 | 0.34 (0.21-0.51), n = 130 | .32 |
| CAR expression by flow (% CAR+ viable cells), median (IQR) | 14.50 (10.50-18.80), n = 25 | 14.10 (12.20-16.30), n = 17 | 14.05 (9.80-19.10), n = 136 | .97 |
| . | CRS + HLH-LT, n = 26 . | HG-CRS without HLH-LT, n = 17 . | NLG-CRS without HLH-LT, n = 141 . | P value . |
|---|---|---|---|---|
| Age, median (IQR), y | 11.5 (4.5-16.75), n = 26 | 13 (10-18), n = 17 | 12 (8-18), n = 141 | .45 |
| Male, n (%) | 13/26 (50.0) | 10/17 (58.8) | 87/141 (61.6) | .53 |
| Race/ethnicity, n (%) | ||||
| Hispanic | 10/26 (38.5) | 7/17 (41.2) | 53/141 (37.6) | .93 |
| Non-Hispanic White | 14/26 (53.8) | 8/17 (47.1) | 67/141 (47.5) | |
| Other | 2/26 (7.7) | 2/17 (11.8) | 21/141 (14.9) | |
| Initial cytogenetic risk category, n (%) | ||||
| Favorable | 2/18 (11.1) | 3/13 (23.1) | 19/108 (17.6) | .55 |
| Intermediate | 7/18 (38.9) | 2/13 (15.4) | 40/108 (37.0) | |
| Unfavorable | 9/18 (50.0) | 8/13 (61.5) | 49/108 (45.4) | |
| Risk stratification at end of induction, n (%) | ||||
| Low risk | 0/23 (0.0) | 0/16 (0.0) | 6/116 (5.2) | .09 |
| Standard risk | 0/23 (0.0) | 1/16 (6.3) | 20/116 (17.2) | |
| High risk | 8/23 (34.8) | 5/16 (31.3) | 19/116 (16.4) | |
| Very high risk | 15/23 (65.2) | 10/16 (62.5) | 71/116 (61.2) | |
| Timing of initial B-ALL relapse, n (%) | ||||
| On therapy relapse | 17/26 (65.4) | 6/17 (35.3) | 39/142 (27.5) | .0008∗∗∗ |
| Early relapse | 8/26 (30.8) | 6/17 (35.3) | 32/142 (22.5) | .39 |
| Late relapse | 5/26 (19.2) | 5/17 (29.4) | 44/142 (30.9) | .48 |
| Lines of therapy before CAR, median (IQR) | 2 (1.25-4), n = 26 | 2 (1-3), n = 17 | 2 (1-3), n = 141 | .35 |
| Prior CD19-directed therapy, n (%) | 8/26 (30.8) | 4/17 (23.5) | 25/141 (17.7) | .28 |
| Optimal† lymphodepleting chemotherapy, n (%) | 11/20 (55) | 8/15 (53.3) | 82/116 (70.7) | .18 |
| BM disease burden %, median (IQR) | 66.0 (26.2-84.0), n = 25 | 8.0 (3.7-36.0), n = 13 | 5.0 (0.24-28.7), n = 80 | <.0001∗∗∗∗ |
| B-ALL disease burden, n (%) | ||||
| Low | 0 (0.0) | 6 (37.5) | 76 (55.1) | <.0001∗∗∗∗ |
| High∗ | 26 (100.0) | 10 (62.5) | 62 (44.9) | |
| Total (n) | 26 | 16 | 138 | |
| Timing of pre–CAR T-cell infusion BM assessment, median (IQR), wk | 2.5 (1.0-9.0), n = 26 | 2.0 (1.25-4.63), n = 16 | 2.0 (1.1-7.8), n = 138 | .87 |
| Peripheral blasts, % of white blood cells, median (IQR) | 25 (0-50), n = 22 | 0 (0-0), n = 15 | 0 (0-0), n = 127 | <.0001∗∗∗∗ |
| CNS disease pre–CAR T-cell infusion (CNS 2 + 3), n (%) | 2/19 (10.5) | 0/12 (0.0) | 10/118 (8.5) | .63 |
| Extramedullary disease pre–CAR T-cell infusion, n (%) | 3/26 (11.5) | 2/17 (11.8) | 10/141 (7.1) | .50 |
| Pre–CAR T-cell infusion ALC (cells per μL), median (IQR) | 30 (0.1-60), n = 21 | 50 (17.5-97.5), n = 16 | 70 (20-157.5), n = 134 | .09 |
| Pre–CAR T-cell infusion ANC (cells per μL), median (IQR) | 10 (0-160), n = 21 | 490 (197.5-1175), n = 16 | 530 (232.5-1040), n = 134 | <.0001∗∗∗∗ |
| Pre–CAR T-cell infusion platelet (×109/L), median (IQR) | 35.5 (20.25-92), n = 26 | 110.0 (46-148), n = 17 | 121.5 (56-206), n = 138 | <.0001∗∗∗∗ |
| Pre–CAR T-cell infusion ferritin (ng/mL), median (IQR) | 3745 (1291.8-6194.6), n = 25 | 831 (663.0-2174.5), n = 15 | 1364 (603.5-2660.4), n = 115 | .002∗∗ |
| Pre–CAR T-cell infusion CRP (mg/dL), median (IQR) | 3.165 (1.01-7.29), n = 22 | 0.559 (0.390-2.30), n = 14 | 0.600 (0.300-2.51), n = 111 | .006∗∗ |
| CAR viability (%), median (IQR) | 88.6 (87.6-93.3), n = 25 | 87.0 (85.3-95.5), n = 17 | 87.6 (83.2-92.2), n = 140 | .13 |
| CAR dose infused (CAR T cells × 106/kg), median (IQR) | 1.585 (1.23-2.48), n = 26 | 1.630 (1.28-2.00), n = 16 | 1.80 (1.33-2.45), n = 139 | .60 |
| Transduction efficiency (no. of copies per cell), median (IQR) | 0.315 (0.26-0.40), n = 24 | 0.400 (0.27-0.55), n = 16 | 0.34 (0.21-0.51), n = 130 | .32 |
| CAR expression by flow (% CAR+ viable cells), median (IQR) | 14.50 (10.50-18.80), n = 25 | 14.10 (12.20-16.30), n = 17 | 14.05 (9.80-19.10), n = 136 | .97 |
CNS, central nervous system; IQR, interquartile range.
∗High BM disease burden is defined as ≥5% lymphoblasts, the presence of peripheral blasts, and/or extramedullary disease.
Optimal lymphodepleting chemotherapy: fludarabine area under the curve of ≥13.8 mg × hr/L using a pharmacokinetic model. CNS indicates central nervous system; IQR, 25th to 75th percentile interquartile range; lactate dehydrogenase. P values: Kruskal-Wallis tests for comparison of continuous variables and Fisher exact tests for comparison of categorical variables with a 2-tailed significance level of .05.
Toxicities of tisagenlecleucel
Patients with HLH-LTs had the highest posttisagenlecleucel peak ferritin (92 204 vs 5467 vs 2035 ng/mL, P < .0001), change in ferritin (83 874 vs 4753 vs 325 ng/mL, P < .0001), peak CRP (20.93 vs 6.15 vs 2.85 mg/dL, P < .0001), and change in CRP (14.0 vs 5.1 vs 1.1 mg/dL, P < .0001) compared with those in HG-CRS and NLG-CRS groups (Table 2). Time to CRS onset was not significantly different between groups, but CRS duration was longest in the HLH-LT group (11 vs 5 vs 3 days, P < .0001) (Figure 2B). In the HLH-LT group, onset of HLH-LTs was available in 7 patients and occurred at a median of 12 days after tisagenlecleucel infusion (range, 4-21) with a median duration of 7 days (range, 4.0-25.0 days) (Table 2). There was overlap in CRS and HLH-LTs in 6 of 7 patients for whom data were available (supplemental Table 4). Tocilizumab doses and days of steroid use were not significantly different in the HLH-LT and HG-CRS groups (Table 2). Anakinra was used in 3 patients with HLH-LTs and in 0 patients with HG-CRS or NLG-CRS. Siltuximab was used in 1 patient in the NLG-CRS group as treatment for ICANS. Those with HLH-LTs had longer hospitalization and intensive care unit–level care than those with HG-CRS and NLG-CRS (Table 2). More patients with HLH-LTs required platelet transfusions after CAR T-cell infusion (95.8% vs 47.0% vs 26.1%, P < .0001), and the duration of platelet transfusion requirement was longest in the HLH-LT group (Table 2). Patients with HLH-LTs experienced comparable rates of grade 4 neutropenia to the HG-CRS and NLG-CRS groups, but the time to ANC recovery was longest in the HLH-LT group (34 vs 13 vs 13 days, P = .0081). Infections within 28 days of infusion occurred at more than triple the rate in the HLH-LT group than of that in the HG-CRS and NLG-CRS groups (63.6% vs 17.6% vs 13.9%, P < .0001) with earlier occurrence of first infection after tisagenlecleucel (13 days vs 73 days vs 50 days, P = .0434). Infections occurred after CRS onset in all but 1 patient, but 4 of 7 patients with available data on HLH-LT timing reported infections preceding HLH-LTs. The remaining 3 did not experience infections within the first 28 days (supplemental Table 4). Organ toxicities were expectedly more common in the HLH-LT group than in the HG-CRS or NLG-CRS groups (supplemental Figure 2).
Outcomes and toxicities in patients with CRS + HLH toxicities, HG-CRS without HLH toxicities, and NLG-CRS without HLH toxicities
| . | CRS + HLH-LT, n = 26 . | HG-CRS without HLH-LT, n = 17 . | NLG-CRS without HLH-LT, n = 141 . | P value . |
|---|---|---|---|---|
| Experienced CRS, n (%) | 26/26 (100) | 17/17 (100) | 75/141 (53.2) | <.0001∗∗∗∗ |
| ASTCT grade of CRS, median (IQR) | 4 (3.0-4.0), n = 25 | 3 (3-4), n = 17 | 1 (0-1), n = 141 | <.0001∗∗∗∗ |
| ASTCT grade of CRS, median (IQR) | 4 (3.0-4.0), n = 25 | 3 (3-4), n = 17 | .1696 | |
| ASTCT CRS grade, n (%) | ||||
| 0 | 0/25 (0) | 0/17 (0) | 67/141 (47.5) | |
| 1 | 0/25 (0) | 0/17 (0) | 45/141 (31.9) | |
| 2 | 3/25 (12) | 0/17 (0) | 29/141 (20.6) | |
| 3 | 7/25 (28) | 12/17 (70.6) | 0/141 (0) | |
| 4 | 14/25 (56) | 5/17 (29.4) | 0/141 (0) | |
| 5 | 1/25 (4.0) | 0/17 (0) | 0/141 (0) | |
| D between CAR T-cell infusion and CRS onset, median (IQR) | 2.5 (1-7.25), n = 24 | 3 (2-6), n = 17 | 5 (3-8), n = 67 | .10 |
| Duration of CRS, median (IQR), d | 11 (10-15), n = 19 | 5 (4-13), n = 15 | 3 (2-5), n = 65 | <.0001∗∗∗∗ |
| Experienced ICANS/neurotoxicity, n (%) | 13/24 (54.2) | 6/16 (37.5) | 20/140 (14.3) | <.0001∗∗∗∗ |
| Maximum grade ICANS/neurotoxicity∗, median (IQR) | 1.0 (0.0-2.0), n = 23 | 0.0 (0.0-2.5), n = 16 | 0.0 (0.0-0.0), n = 141 | <.0001∗∗∗∗ |
| Onset of HLH toxicities, median (range) | 12.0 (4.0-21.0), n = 7 | |||
| Duration of HLH toxicities, median (range) | 7.0 (4.0-25.0), n = 7 | |||
| Maximum ferritin (ng/mL), median (IQR) | 92 204 (38 305-221 638), n = 26 | 5 467 (2 186-14 614), n = 15 | 2 035 (855-4 048), n = 119 | <.0001∗∗∗∗ |
| Maximum ferritin (ng/mL), total (N), n (%) | 26 | 15 | 119 | |
| <10 000 | 0 (0.0) | 10 (66.7) | 103 (86.6) | <.0001∗∗∗∗ |
| ≥10 000 and <100 000 | 13 (50.0) | 4 (26.7) | 15 (12.6) | |
| ≥100 000 | 13 (50.0) | 1 (6.7) | 1 (0.8) | |
| Change in ferritin (ng/mL), median (IQR) | 83 874 (35 353-233 720), n = 25 | 4 753 (1 447-13 671), n = 13 | 325 (0-1 614), n = 106 | <.0001∗∗∗∗ |
| Maximum CRP (mg/dL), median (IQR) | 20.93 (11.61-30.53), n = 24 | 6.15 (4.02-16.78), n = 14 | 2.85 (0.87-10.83), n = 112 | <.0001∗∗∗∗ |
| Change in CRP (mg/dL), median (IQR) | 14.0 (7.8-20.5), n = 22 | 5.1 (3.3-9.2), n = 12 | 1.1 (0.2-9.5), n = 101 | <.0001∗∗∗∗ |
| Treatments received for CRS | ||||
| Doses of tocilizumab, median | 2 (1-2.75), n = 26 | 2 (1-2), n = 17 | 0 (0-0), n = 130 | <.0001∗∗∗∗ |
| Doses of tocilizumab, median | 2 (1-2.75), n = 26 | 2 (1-2), n = 17 | .50 | |
| D of steroid, median (IQR) | 4 (0-7), n = 25 | 0 (0-5.25), n = 16 | 0 (0-0), n = 127 | <.0001∗∗∗∗ |
| D of steroid, median (IQR) | 4 (0-7), n = 25 | 0 (0-5.75), n = 16 | .23 | |
| Other treatments, patients received, n | ||||
| Anakinra | 3 | 0 | 0 | |
| Siltuximab | 0 | 0 | 1 | |
| D hospitalized between CAR T-cell infusion and discharge, median (IQR) | 25 (17-38.5), n = 26 | 16 (13-23), n = 17 | 10 (2-17), n = 140 | <.0001∗∗∗∗ |
| D requiring intensive care unit–level care, median (IQR) | 10 (5-13), n = 26 | 5 (3-8), n = 17 | 0 (0-0), n = 139 | <.0001∗∗∗∗ |
| Experienced grade 4 neutropenia, n (%) | 21/24 (87.5) | 9/16 (56.3) | 88/134 (65.1) | .06 |
| Recovered from neutropenia, n (%) | 9/20 (45) | 7/9 (77.8) | 81/87 (93.1) | <.0001∗∗∗∗ |
| D to ANC recovery, median (IQR) | 34 (23-39), n = 9 | 13 (12-16.5), n = 7 | 13 (9-18), n = 88 | .008∗∗ |
| Required platelet transfusions after CAR T-cell infusion, n (%) | 23/24 (95.8) | 8/17 (47) | 35/134 (26.1) | <.0001∗∗∗∗ |
| Days after CAR T-cell infusion that last platelet transfusion administered, median (IQR) | 35 (25-50.5), n = 15 | 19 (9.5-25.75), n = 8 | 29 (21-49), n = 21 | .04∗ |
| Developed infection within 28 d of CAR T-cell infusion (%) | 14/22 (63.6%) | 3/17 (17.6%) | 19/137 (13.9%) | <.0001∗∗∗∗ |
| Onset of first infection (d from CAR T-cell infusion), median (IQR) | 13 (10-21.5), n = 15 | 73 (14.5-245.25), n = 8 | 50 (11-168.5), n = 47 | .04∗ |
| Refractory disease at d 28†,n (%) | 6/21 (28.6) | 3/17 (17.6) | 20/141 (14.2) | .20 |
| OS, median d | 128 | Undefined | Undefined | <.0001∗∗∗∗ |
| RFS, median d | 60 | Undefined | 701 | <.0001∗∗∗∗ |
| Relapse occurred, n (%) | 16/25 (64) | 5/17 (29.4) | 44/141 (31.2) | .007∗∗ |
| Time to relapse, median d | 60, n = 16 | 178, n = 5 | 101, n = 45 | .1033 |
| Death occurred, n (%) | 19/25 (76) | 3/17 (17.6) | 29/141 (20.6) | <.0001∗∗∗∗ |
| Time to death, median d | 85, n = 19 | 313, n = 3 | 221, n = 29 | .0261 |
| Relapse or death occurred, n (%) | 23/25 (92) | 7/17 (41.2) | 57/141 (40.4) | <.0001∗∗∗∗ |
| Causes of death, n (%) | ||||
| Leukemia | 12/19 (63.2) | 3/3 (100) | 23/29 (79.3) | |
| CRS | 1/19 (5.3) | 0/3 (0.0) | 1/29 (3.4) | |
| Neurotoxicity | 1/19 (5.3) | 0/3 (0.0) | 0/29 (0.0) | |
| Infection | 4/19 (21.1) | 0/3 (0.0) | 6/29 (20.7) | |
| Cardiopulmonary failure | 3/19 (15.8) | 0/3 (0.0) | 3/29 (10.3) | |
| Hematopoietic stem cell transplant-related complications | 0/19 (0.0) | 0/3 (0.0) | 3/29 (10.3) | |
| NRM‡, n (%) | 7/25 (28.0) | 0/17 (0.0) | 6/141 (4.3) | .0009∗∗∗ |
| CD19− relapse, n (%) | 10/14 (71.4) | 3/5 (60) | 13/39 (33.3) | .04∗ |
| Myeloid transformation of leukemia, n (%) | 3/16 (18.8) | 0/4 (0.0) | 1/41 (2.44) | .11 |
| . | CRS + HLH-LT, n = 26 . | HG-CRS without HLH-LT, n = 17 . | NLG-CRS without HLH-LT, n = 141 . | P value . |
|---|---|---|---|---|
| Experienced CRS, n (%) | 26/26 (100) | 17/17 (100) | 75/141 (53.2) | <.0001∗∗∗∗ |
| ASTCT grade of CRS, median (IQR) | 4 (3.0-4.0), n = 25 | 3 (3-4), n = 17 | 1 (0-1), n = 141 | <.0001∗∗∗∗ |
| ASTCT grade of CRS, median (IQR) | 4 (3.0-4.0), n = 25 | 3 (3-4), n = 17 | .1696 | |
| ASTCT CRS grade, n (%) | ||||
| 0 | 0/25 (0) | 0/17 (0) | 67/141 (47.5) | |
| 1 | 0/25 (0) | 0/17 (0) | 45/141 (31.9) | |
| 2 | 3/25 (12) | 0/17 (0) | 29/141 (20.6) | |
| 3 | 7/25 (28) | 12/17 (70.6) | 0/141 (0) | |
| 4 | 14/25 (56) | 5/17 (29.4) | 0/141 (0) | |
| 5 | 1/25 (4.0) | 0/17 (0) | 0/141 (0) | |
| D between CAR T-cell infusion and CRS onset, median (IQR) | 2.5 (1-7.25), n = 24 | 3 (2-6), n = 17 | 5 (3-8), n = 67 | .10 |
| Duration of CRS, median (IQR), d | 11 (10-15), n = 19 | 5 (4-13), n = 15 | 3 (2-5), n = 65 | <.0001∗∗∗∗ |
| Experienced ICANS/neurotoxicity, n (%) | 13/24 (54.2) | 6/16 (37.5) | 20/140 (14.3) | <.0001∗∗∗∗ |
| Maximum grade ICANS/neurotoxicity∗, median (IQR) | 1.0 (0.0-2.0), n = 23 | 0.0 (0.0-2.5), n = 16 | 0.0 (0.0-0.0), n = 141 | <.0001∗∗∗∗ |
| Onset of HLH toxicities, median (range) | 12.0 (4.0-21.0), n = 7 | |||
| Duration of HLH toxicities, median (range) | 7.0 (4.0-25.0), n = 7 | |||
| Maximum ferritin (ng/mL), median (IQR) | 92 204 (38 305-221 638), n = 26 | 5 467 (2 186-14 614), n = 15 | 2 035 (855-4 048), n = 119 | <.0001∗∗∗∗ |
| Maximum ferritin (ng/mL), total (N), n (%) | 26 | 15 | 119 | |
| <10 000 | 0 (0.0) | 10 (66.7) | 103 (86.6) | <.0001∗∗∗∗ |
| ≥10 000 and <100 000 | 13 (50.0) | 4 (26.7) | 15 (12.6) | |
| ≥100 000 | 13 (50.0) | 1 (6.7) | 1 (0.8) | |
| Change in ferritin (ng/mL), median (IQR) | 83 874 (35 353-233 720), n = 25 | 4 753 (1 447-13 671), n = 13 | 325 (0-1 614), n = 106 | <.0001∗∗∗∗ |
| Maximum CRP (mg/dL), median (IQR) | 20.93 (11.61-30.53), n = 24 | 6.15 (4.02-16.78), n = 14 | 2.85 (0.87-10.83), n = 112 | <.0001∗∗∗∗ |
| Change in CRP (mg/dL), median (IQR) | 14.0 (7.8-20.5), n = 22 | 5.1 (3.3-9.2), n = 12 | 1.1 (0.2-9.5), n = 101 | <.0001∗∗∗∗ |
| Treatments received for CRS | ||||
| Doses of tocilizumab, median | 2 (1-2.75), n = 26 | 2 (1-2), n = 17 | 0 (0-0), n = 130 | <.0001∗∗∗∗ |
| Doses of tocilizumab, median | 2 (1-2.75), n = 26 | 2 (1-2), n = 17 | .50 | |
| D of steroid, median (IQR) | 4 (0-7), n = 25 | 0 (0-5.25), n = 16 | 0 (0-0), n = 127 | <.0001∗∗∗∗ |
| D of steroid, median (IQR) | 4 (0-7), n = 25 | 0 (0-5.75), n = 16 | .23 | |
| Other treatments, patients received, n | ||||
| Anakinra | 3 | 0 | 0 | |
| Siltuximab | 0 | 0 | 1 | |
| D hospitalized between CAR T-cell infusion and discharge, median (IQR) | 25 (17-38.5), n = 26 | 16 (13-23), n = 17 | 10 (2-17), n = 140 | <.0001∗∗∗∗ |
| D requiring intensive care unit–level care, median (IQR) | 10 (5-13), n = 26 | 5 (3-8), n = 17 | 0 (0-0), n = 139 | <.0001∗∗∗∗ |
| Experienced grade 4 neutropenia, n (%) | 21/24 (87.5) | 9/16 (56.3) | 88/134 (65.1) | .06 |
| Recovered from neutropenia, n (%) | 9/20 (45) | 7/9 (77.8) | 81/87 (93.1) | <.0001∗∗∗∗ |
| D to ANC recovery, median (IQR) | 34 (23-39), n = 9 | 13 (12-16.5), n = 7 | 13 (9-18), n = 88 | .008∗∗ |
| Required platelet transfusions after CAR T-cell infusion, n (%) | 23/24 (95.8) | 8/17 (47) | 35/134 (26.1) | <.0001∗∗∗∗ |
| Days after CAR T-cell infusion that last platelet transfusion administered, median (IQR) | 35 (25-50.5), n = 15 | 19 (9.5-25.75), n = 8 | 29 (21-49), n = 21 | .04∗ |
| Developed infection within 28 d of CAR T-cell infusion (%) | 14/22 (63.6%) | 3/17 (17.6%) | 19/137 (13.9%) | <.0001∗∗∗∗ |
| Onset of first infection (d from CAR T-cell infusion), median (IQR) | 13 (10-21.5), n = 15 | 73 (14.5-245.25), n = 8 | 50 (11-168.5), n = 47 | .04∗ |
| Refractory disease at d 28†,n (%) | 6/21 (28.6) | 3/17 (17.6) | 20/141 (14.2) | .20 |
| OS, median d | 128 | Undefined | Undefined | <.0001∗∗∗∗ |
| RFS, median d | 60 | Undefined | 701 | <.0001∗∗∗∗ |
| Relapse occurred, n (%) | 16/25 (64) | 5/17 (29.4) | 44/141 (31.2) | .007∗∗ |
| Time to relapse, median d | 60, n = 16 | 178, n = 5 | 101, n = 45 | .1033 |
| Death occurred, n (%) | 19/25 (76) | 3/17 (17.6) | 29/141 (20.6) | <.0001∗∗∗∗ |
| Time to death, median d | 85, n = 19 | 313, n = 3 | 221, n = 29 | .0261 |
| Relapse or death occurred, n (%) | 23/25 (92) | 7/17 (41.2) | 57/141 (40.4) | <.0001∗∗∗∗ |
| Causes of death, n (%) | ||||
| Leukemia | 12/19 (63.2) | 3/3 (100) | 23/29 (79.3) | |
| CRS | 1/19 (5.3) | 0/3 (0.0) | 1/29 (3.4) | |
| Neurotoxicity | 1/19 (5.3) | 0/3 (0.0) | 0/29 (0.0) | |
| Infection | 4/19 (21.1) | 0/3 (0.0) | 6/29 (20.7) | |
| Cardiopulmonary failure | 3/19 (15.8) | 0/3 (0.0) | 3/29 (10.3) | |
| Hematopoietic stem cell transplant-related complications | 0/19 (0.0) | 0/3 (0.0) | 3/29 (10.3) | |
| NRM‡, n (%) | 7/25 (28.0) | 0/17 (0.0) | 6/141 (4.3) | .0009∗∗∗ |
| CD19− relapse, n (%) | 10/14 (71.4) | 3/5 (60) | 13/39 (33.3) | .04∗ |
| Myeloid transformation of leukemia, n (%) | 3/16 (18.8) | 0/4 (0.0) | 1/41 (2.44) | .11 |
ICANS/neurotoxicity grading was not standardized, but performed with ASTCT, CAR T‑cell therapy–associated toxicity neurological assessment, National Cancer Institute, National Institutes of Health/Common Terminology Criteria for Adverse Events version 4.03 and version 5.0 criteria.
Deaths from leukemia before day 28 and all with persistent disease at 1 month assessment after tisagenlecleucel counted as refractory disease.
NRM defined as death without recurrent or progressive disease. P values: comparisons between groups were via Kruskal-Wallis tests for continuous variables and Fisher exact tests for categorical variables, with a 2-tailed significance level of .05.
Relapse and mortality following tisagenlecleucel
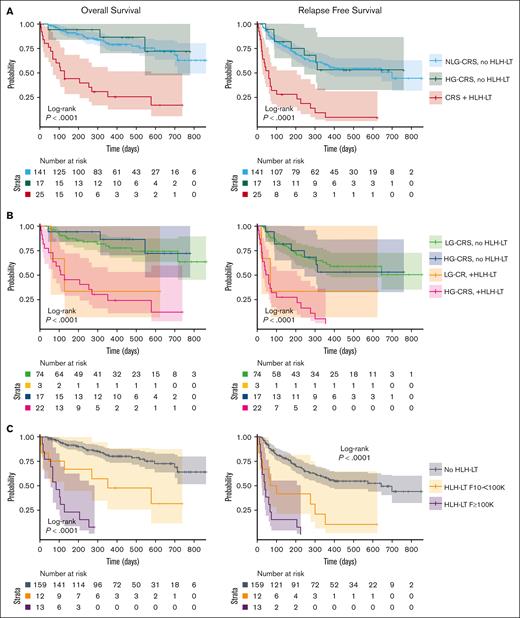
One hundred eighty-three patients were available for OS and RFS analysis. One with HLH-LTs was censored at day 17 after infusion and 1 without CRS grade recorded was excluded. Median OS was markedly reduced in the HLH-LT group compared with that in the HG-CRS and NLG-CRS groups (128 days vs not reached vs not reached, P < .0001), as was median RFS (60 days vs not reached vs 701 days, P < .0001) (Figure 3A). Twelve-month OS in the HLH-LT, HG-CRS, and NLG-CRS groups was 25.7% vs 86.3% vs 79.1%, respectively, whereas 12-month RFS was 4.7% vs 52.9% vs 57.9%, respectively (supplemental Table 5). Strikingly, 23 of 25 patients (92.0%) with HLH-LTs experienced relapse or death, compared with 7 of 17 (41.2%) with HG-CRS, and 57 of 141 (40.4%) with NLG-CRS, P < .0001, with a median follow-up of 128, 451, and 353, respectively (Table 2).
OS and RFS of HLH-LT and non–HLH-LT groups. (A) OS and RFS of patients with CRS (any grade) and HLH-LTs (CRS + HLH-LT), those with ASTCT grade 3 to 5 CRS (HG-CRS) without HLH-LTs (HG-CRS, no HLH-LT), and those with no CRS or ASTCT grade 1 to 2 CRS without HLH-LTs (NLG-CRS, no HLH-LT). (B) OS and RFS of patients with ASTCT grade 1 to 2 CRS (LG-CRS) and grade 3 to 5 CRS (HG-CRS), with and without HLH-LT. (C) OS and RFS of group without HLH-LT (no HLH-LT), those with HLH-LT and peak ferritin ≥10 000 and < 100 000 ng/mL (HLH-LT, 10 to <100 000), and those with HLH-LT with peak ferritin ≥100 000 ng/mL (HLH-LT, ≥100 000). Survival curves were generated with Kaplan-Meier method and compared using log-rank tests with significance level of P < .05.
OS and RFS of HLH-LT and non–HLH-LT groups. (A) OS and RFS of patients with CRS (any grade) and HLH-LTs (CRS + HLH-LT), those with ASTCT grade 3 to 5 CRS (HG-CRS) without HLH-LTs (HG-CRS, no HLH-LT), and those with no CRS or ASTCT grade 1 to 2 CRS without HLH-LTs (NLG-CRS, no HLH-LT). (B) OS and RFS of patients with ASTCT grade 1 to 2 CRS (LG-CRS) and grade 3 to 5 CRS (HG-CRS), with and without HLH-LT. (C) OS and RFS of group without HLH-LT (no HLH-LT), those with HLH-LT and peak ferritin ≥10 000 and < 100 000 ng/mL (HLH-LT, 10 to <100 000), and those with HLH-LT with peak ferritin ≥100 000 ng/mL (HLH-LT, ≥100 000). Survival curves were generated with Kaplan-Meier method and compared using log-rank tests with significance level of P < .05.
The outcomes of patients with HG-CRS and LG-CRS, with and without HLH-LTs, were also compared (Figure 3B). Patients with HG-CRS + HLH-LTs (median OS, 118 days; median RFS, 52.5 days) and LG-CRS + HLH-LTs (median OS, 128 days; median RFS, 69 days) had shorter OS/RFS when compared with patients with HG-CRS or LG-CRS without HLH-LTs for whom group median OS/RFS were not reached (Figure 3B; supplemental Table 6). The median OS and RFS were also evaluated by individual CRS grade (grades 4-5, 3, and 2) (supplemental Table 7). As expected, higher grades of CRS correlated with worse OS/RFS. However, both OS and RFS were diminished among patients with HLH-LTs within each evaluated CRS grade strata (supplemental Table 7; supplemental Figure 3).
To evaluate how peak ferritin affected OS and RFS within the HLH-LT group, we stratified the HLH-LT group by maximum ferritin ≥100 000 ng/mL and 10 000 to 100 000 ng/mL and compared with all without HLH-LTs. The group with HLH-LTs and peak ferritin ≥100 000 ng/mL had the shortest median OS and RFS (median OS, 85 days vs 353 days vs not reached, P < .0001; median RFS, 41 vs 85 vs 645, P < .0001) (Figure 3C). Twelve of 13 (92.3%) patients with HLH-LTs and peak ferritin ≥100 000 ng/mL died, and the 1 who survived experienced relapse (Figure 3C).
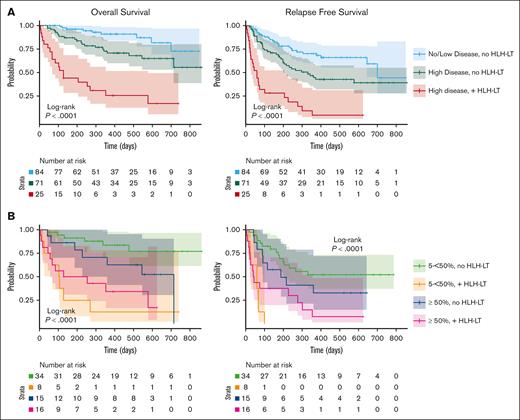
Previously, our consortium and others demonstrated that high disease burden pre–CAR T-cell infusion is associated with inferior OS and RFS.15-17 We compared groups with high disease burden that did and did not develop HLH-LT and the group with low disease burden, all of whom did not develop HLH-LT. The group with high disease burden and HLH-LT had substantially shortened median OS (128 days vs not reached vs not reached, P < .0001) and median RFS (60 vs 332 vs 701 days, P < .0001), compared with groups without high and low disease burden without HLH-LTs (Figure 4A). We next evaluated high disease burden groups with 5% to <50% and ≥50% preinfusion BM lymphoblasts to determine who did and did not develop HLH-LTs (Figure 4B). At both the 5% to <50% and ≥50% preinfusion disease burden strata, OS/RFS were significantly reduced in those who developed HLH-LTs.
Outcomes of patients with high and low disease burden, with and without HLH-LTs. (A) OS and RFS for group with no or low pre–CAR T-cell infusion disease burden without HLH-LTs (no/low disease, no HLH-LT), high disease burden without HLH-LTs (high disease, no HLH-LT), and high disease with HLH-LTs (high disease, with HLH-LT). High pre–CAR T-cell infusion disease burden is defined as ≥ 5% BM blasts, the presence of extramedullary disease, or evidence of leukemia in the peripheral blood. Low disease burden is detectable BM disease <5% without high disease burden criteria. No patients with no disease or low disease burden developed HLH-LTs. (B) OS for patients with 5% to <50% BM blasts (5% to <50%), and ≥50% BM blasts (≥50%), with and without HLH-LTs. Survival curves were generated with the Kaplan-Meier method and compared using log-rang tests with significance level of P< .05.
Outcomes of patients with high and low disease burden, with and without HLH-LTs. (A) OS and RFS for group with no or low pre–CAR T-cell infusion disease burden without HLH-LTs (no/low disease, no HLH-LT), high disease burden without HLH-LTs (high disease, no HLH-LT), and high disease with HLH-LTs (high disease, with HLH-LT). High pre–CAR T-cell infusion disease burden is defined as ≥ 5% BM blasts, the presence of extramedullary disease, or evidence of leukemia in the peripheral blood. Low disease burden is detectable BM disease <5% without high disease burden criteria. No patients with no disease or low disease burden developed HLH-LTs. (B) OS for patients with 5% to <50% BM blasts (5% to <50%), and ≥50% BM blasts (≥50%), with and without HLH-LTs. Survival curves were generated with the Kaplan-Meier method and compared using log-rang tests with significance level of P< .05.
Leukemia was the cause of death in most patients who experienced mortality in the HLH-LT, HG-CRS, and NLG-CRS groups (63.2% vs 100% vs 79.3%) (Table 2). NRM occurred most frequently in those with HLH-LTs (7/25, 28%) compared with 0 of 17 (0%) with HG-CRS and 6 of 141 (4.3%) with NLG-CRS (P = .0009). Of the 7 with NRM in the HLH-LT group, the cause of death was infection in 4, CRS in 1, ICANS in 1, and cardiac in 1. Of those experiencing relapse, there was a higher incidence of CD19− disease at relapse in the HLH-LT and HG-CRS groups compared with the NLG-CRS group (71.4% vs 60.0% vs 33.3%, P = .04).
To evaluate the contribution of HLH-LT to the risk of relapse or death, a multivariable Cox proportional hazard model was created, using covariates found to be associated with relapse or death in univariate analysis (supplemental Figure 4), as well as key demographic variables of age and sex. HLH-LT had a HR of 3.68 (95% CI, 2.15-6.32) while adjusting for high disease burden (HR, 1.94; 95% CI, 1.16-3.25) and demographic variables (Figure 5A). In a second model for relapse or death that included HLH-LT subgroups with peak ferritin ≥100 000 ng/mL and 10 000 to <100 000 ng/mL, HLH-LT with peak ferritin ≥100 000 ng/mL had a HR of 6.51 (95% CI, 3.32-12.74) and HLH-LT with peak ferritin 10 000 to <100 000 ng/mL had a HR of 2.59 (95% CI, 1.28-5.23), adjusting for high disease burden (HR, 1.90; 95% CI, 1.14-3.16) and demographic variables (Figure 5B).
Multivariable Cox regression analyses of OS and RFS. Multivariable analyses of OS and RFS were performed for covariates of age, sex, disease burden, and HLH-LT status in a model including all patients that met criteria for HLH-LTs (A) and with HLH-LT group separated into peak ferritin groups (10-100 000 ng/mL and ≥100 000ng/mL) (B). HRs with 95% CIs are shown. High disease burden was defined as BM disease burden ≥5%, the presence of extramedullary disease, or evidence of leukemia in the peripheral blood. No/low disease burden was defined as BM disease burden <5% and the absence of extramedullary disease or peripheral lymphoblasts. P values: 2-tailed significance level was set at .05.
Multivariable Cox regression analyses of OS and RFS. Multivariable analyses of OS and RFS were performed for covariates of age, sex, disease burden, and HLH-LT status in a model including all patients that met criteria for HLH-LTs (A) and with HLH-LT group separated into peak ferritin groups (10-100 000 ng/mL and ≥100 000ng/mL) (B). HRs with 95% CIs are shown. High disease burden was defined as BM disease burden ≥5%, the presence of extramedullary disease, or evidence of leukemia in the peripheral blood. No/low disease burden was defined as BM disease burden <5% and the absence of extramedullary disease or peripheral lymphoblasts. P values: 2-tailed significance level was set at .05.
In a multivariable model for death, after adjusting for key demographic variables, HLH-LT status had a HR of 4.61 (95% CI, 2.41-8.83) (Figure 5A). High disease burden carried a HR of 2.58 (95% CI, 1.17-5.68), and male sex was protective (HR, 0.54; 95% CI, 0.30-0.97). Notably, high disease burden was present in 61% of females vs 50% of males in this cohort. Tocilizumab and steroid use, which were associated with death in univariate analysis (supplemental Figure 4), were no longer significant and therefore removed from the final model. In a second multivariable model including HLH-LT subgroups by peak ferritin, those with HLH-LTs and peak ferritin ≥100 000 ng/mL had a HR of 11.36 (95% CI, 5.18-24.91), those with HLH-LTs and peak ferritin 10 000 to <100 000 ng/mL had a HR of 2.75 (95% CI, 1.15-6.59), and high disease burden status had a HR of 2.43 (95% CI, 1.11-5.33) (Figure 5B).
As preinfusion ANC, platelets, disease burden, ferritin, and CRP were associated with HLH-LTs, we evaluated the association of these preinfusion variables with OS and RFS with a univariate Cox regression analysis. Lower platelet counts and increased disease burden, ferritin, and CRP were associated with death (supplemental Table 8). Similarly, variables associated with relapse or death included lower platelet counts, high disease burden, greater CRP, and female sex. However, low platelet counts, high ferritins, and high CRP were all correlated with a high preinfusion disease burden and therefore not included in the final multivariable models for OS/RFS.
Discussion
This study represents the largest outcome analysis of HLH-LTs in CAYAs with B-ALL treated with commercially available tisagenlecleucel to date. A consensus definition for HLH-LTs is lacking, and to identify thresholds encompassing a group with meaningfully distinct outcomes, we used the ferritin cutoff of ≥10 000 ng/mL, shown previously to be sensitive and specific for the diagnosis of HLH in children18 and proposed in previous carHLH criteria,6 and used more inclusive organ toxicity definitions. Using these more inclusive parameters, we found a similar HLH-LT incidence to prior reports involving CD19-directed/4-1BB CAR T-cell products,9,10 and identified a group with distinct preinfusion laboratory parameters and significantly worse outcomes when compared with groups with HG-CRS or NLG-CRS without HLH-LTs.
Preinfusion disease burden, inflammation, neutropenia, and thrombocytopenia were associated with the development of both HG-CRS and HLH-LTs. The overlap in preinfusion risk factors for HLH-LTs and HG-CRS is expected, as most patients who developed HLH-LTs also had HG-CRS, and in the limited number of patients for whom timing of HLH-LT onset was available, the onset of HLH-LTs most often occurred while CRS was ongoing. However, 42.5% of patients with HG-CRS did not experience HLH-LTs, and compared with this group, patients with HG-CRS complicated by HLH-LTs had distinctly higher preinfusion inflammatory markers and disease burdens and lower ANC and platelets. This suggests that the magnitude of these preinfusion variables is important in predicting risk for HLH-LT development and that HLH-LT is most often preceded by HG-CRS in CAYAs treated with tisagenlecleucel. HLH-LT occurs rarely in patients with LG-CRS and never in patients without CRS. This is in contrast to the lack of association between HG-CRS and HLH-LTs with CD22-directed CAR T-cell use reported previously.9 The very high preinfusion disease burden in the group that developed HLH-LTs is a notable risk factor. High disease burden is associated with increased CAR T-cell proliferation.19 As T cells are considered key drivers of HLH pathophysiology,20 and increased CAR T-cell proliferation has been associated with HLH-LTs previously,9 a plausible theory is that greater CD19-antigen load in patients with a higher disease burden drives CAR T-cell proliferation, which leads to exaggerated CRS meeting the HLH-LT definition. Previously, those developing HLH-LTs after treatment with CD22/4-1BB CAR T cells had associated natural killer lymphopenia and monocytopenia.9 Although we did not evaluate natural killer cells or monocytes, the lower ANC and trend toward lower ALC in the group developing HLH-LTs may be indicative of a reduced capacity for downregulation of CAR T-cell activity and merits further study. Preinfusion inflammation, observed in the group that developed HLH-LTs, has been associated with increased toxicity and poor outcomes in at least 2 prior reports: an adult cohort with diffuse large B-cell lymphoma and increased ferritin, interleukin 6 (IL-6), and intratumoral M1-macrophage pre–CD19-directed/CD28 CAR T cells,21 and a pediatric B-ALL cohort with increased IL-18/interferon gamma (IFN-γ) pre–CD19-directed/4-1BB CAR T cells.22 These findings raise questions about how preexisting inflammation affects CAR T-cell efficacy and toxicities. It is possible that primed macrophages, a major source of ferritin, IL-1β, IL-6, IL-10, IL-18, and tumor necrosis factor α, cytokines elevated in CRS5 and HLH,9 have a lower threshold for activation, although this requires further study. Genetic risk factors for HLH are well described,23 but not evaluated here. Future studies may evaluate strategies to reduce preinfusion inflammation and examine the contribution of genetic variations to CAR-associated HLH-LTs.
Patients with HLH-LTs experienced greater duration of severe cytopenias and earlier infections than those without HLH-LTs. Chronic inflammation in malignancies has been associated with impaired hematopoietic stem cell fitness.24 In addition, IL-18, which has been associated with delayed recovery of cytopenias in stem cell transplant recipients,25 was also elevated in patients treated with CD22-directed CAR T cells that developed HLH-LTs.9 It is likely that hypercytokinemia, high BM disease burden, and frequent infections contribute to prolonged cytopenias in the group with HLH-LTs. Importantly, infections occurred >3× as commonly in the HLH-LT group than in the HG-CRS and NLG-CRS groups within 28 days of tisagenlecleucel. Similar exposure to tocilizumab and steroids in the HLH-LT and HG-CRS cohorts suggests that the profound cytopenias in those with HLH-LTs contributed much of the infection susceptibility. Notably, all except 1 infection in the HLH-LT group occurred after the onset of CRS, suggesting that infection was typically associated with but not a driver of CRS. Infections may, however, contribute to worsening inflammation and HLH-LTs, as infections preceded the onset of HLH-LTs in 4 of 7 patients with known timing of HLH-LTs.
The main finding of this study was that OS and RFS were dramatically shortened in the group with CRS and HLH-LTs, whereas those with HG-CRS not meeting the HLH-LT definition had favorable survival outcomes overlapping with those with NLG-CRS. HLH-LTs were independently associated with increased risk of relapse and death, controlling for disease burden, age, and sex. This risk was heightened in those with peak ferritin ≥100 000 ng/mL, despite no significant difference in disease burden between groups with HLH-LTs and peak ferritin of 10 000 to <100 000 ng/mL and ≥100 000 ng/mL. No patients with HLH-LTs and peak ferritin ≥100 000 ng/mL, and strikingly few with HLH-LTs and peak ferritin 10 000 to <100 000 ng/mL, survived without relapse.
Given the inferior outcomes of those with CRS complicated by HLH-LTs, it will be important to determine whether these toxicities are indicative of a distinct pathophysiology from CRS, or remain within the spectrum of CRS, but offer additional prognostic information beyond the current ASTCT CRS grading criteria. Notably, the most prevalent cause of death was leukemia in all groups (HLH-LT, HG-CRS, and NLG-CRS), but the relapse rate in the HLH-LT group was double that of the HG-CRS and NLG-CRS groups, indicating a lack of sustained leukemia clearance in this group. Patients who developed HLH-LTs also experienced a high rate of CD19− disease at relapse, may be secondary to the increased likelihood of CD19− clone selection with high disease burden.26 Notably, there was no difference in prior CD19-directed therapy exposure between groups to explain this finding. Furthermore, the high rate of NRM, which occurred in nearly 30% of those with HLH-LTs, contributed to poor outcomes in the group with HLH-LTs. Importantly, in addition to leukemia-related mortality, over a quarter of the HLH-LT group experienced NRM, indicating inflammatory toxicities and infections pose a substantial risk in those with HLH-LTs.
Optimal management of HLH-LTs remains unclear. Leukemia, infections, organ dysfunction, CRS, and neurotoxicity/ICANS are the predominant causes of death in those with HLH-LTs in current and prior reports.10,11 Given elevations of IL-1β, IL-6, IL-18, and IFN-γ seen in those with HLH-LTs after CAR T-cell infusion9,10 and those with grade 4 to 5 CRS,5 treatment with CRS-directed therapies of tocilizumab with or without steroids, if not already started, should be considered. Other potential treatment options include the use of anakinra or ruxolitinib, which have been used previously in patients with HLH-LTs after CAR T-cell infusion.9,10 As IFN-γ is elevated in CRS and is considered to have a central role in primary HLH, use of emapalumab in life-threatening circumstances has rationale, and has been described after CAR T-cell therapy in preclinical models27 and case reports.28
Given the poor outcomes of the HLH-LT group, prevention strategies for those at high risk of HLH-LTs warrant investigation. Success with mitigating severe CAR T-cell–associated inflammatory toxicities has been demonstrated with the use of preemptive tocilizumab with first sustained postinfusion fever in patients with B-ALL and ≥40% BM lymphoblasts,19 and with the prophylactic use of anakinra in adult patients with lymphoma.29 Although anakinra has had mixed results when used for treating severe CRS/ICANS,30 prophylactic use may have greater benefit, and more study is needed in CAYAs. To use preventive strategies for HLH-LTs, knowing which patients are at greatest risk is necessary, and validation of a predictive model for HLH-LTs will be essential for the prospective study of inflammation mitigation strategies. Finally, investigations into relapse prevention for those with HLH-LTs are necessary. The high CD19− relapse rate in this cohort reinforces the need for close disease surveillance, including next-generation sequencing.31 Considerations such as consolidative stem cell transplant and sequential antigen targeting should be explored.
Our study was limited by several factors. The study’s retrospective design introduces concerns for recall bias. In addition, the timing of preinfusion disease assessment was not standardized across sites and timing of HLH-LTs was recorded in only a subset of the patients. Therefore, no conclusions regarding the temporal relationship of tisagenlecleucel infusion, development of HLH-LTs, and other complications, such as infections can be drawn. Strengths of this analysis are its size, multi-institutional involvement, and measurement of potential confounders influencing outcomes, such as age, leukemia genetics, end of induction risk group, disease burden, and CRS treatments.
In conclusion, this study demonstrates that patients with HLH-LTs after tisagenlecleucel experienced more frequent relapse, substantially shortened survival, and increased toxicities compared with those without HLH-LTs, including those with HG-CRS. We describe an association of high preinfusion disease burden, inflammatory markers, and severe cytopenias with HLH-LTs. Given the dismal outcomes in this group, validation of these findings and prospective studies targeting questions of prevention and optimal management are urgently needed.
Acknowledgments
The authors acknowledge the following individuals for their roles in supporting the successful institution of this study with administrative support from Anika Dove and Daisy Torres and the clinical investigators, Eugenia H. Cho and Benjamin R. Oshrine participated in technical editing of the manuscript. The authors acknowledge the Stanford REDCap platform developed and operated by Stanford Medicine Research information technology team. The REDCap platform services at Stanford are subsidized by Stanford School of Medicine Research Office and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant UL1 TR001085.
This work was supported by a grant from the St. Baldrick’s/Stand Up 2 Cancer Pediatric Dream Team Translational Cancer Research (C.L.M.). Stand Up 2 Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. C.L.M. is a member of the Parker Institute for Cancer Immunotherapy, which supports the Stanford University Cancer Immunotherapy Program. The work was also supported by the Virginia and D.K. Ludwig Fund for Cancer Research (C.L.M.) and the V Foundation (K.O.M). K.I. and J.J.C. are partially supported by a grant from the NIH (U54MD007601 [Ola HAWAII]). S.P. is partially supported by a grant from the Food and Drug Administration (grant number U01 FD004979/U01 FD005978), which supports the UCSF-Stanford Center of Excellence in Regulatory Sciences and Innovation.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, HHS, or Food and Drug Administration.
Authorship
Contribution: K.O.M. and S.S.L. performed study design, conceptualization, data analysis, data curation, data interpretation, writing, and literature search; K.I. and J.J.C. performed data analysis, data interpretation, data curation, and methodology; A.D. and A.V. performed conceptualization, writing, data interpretation, and literature search; C.B., S.M., and E.E. provided data curation, project administration, and resources; S.P., H.L.P., C.P., C.L.B., J.R., H.E.S., J.-A.T., A.M., M.V., D.M., N.A.K., P.B., M.H., P.B., A.K.K., S.B., V.A.F., V.C., M.Q., P.S., C.K., and K.J.C. performed review, editing and data curation; C.L.M., T.W.L., and L.M.S. performed conceptualization, administration, supervision, writing, review, and editing.
Conflict-of-interest disclosure: C.L.M. is a coinventor on numerous patents related to chimeric antigen receptor (CAR) therapies; is a cofounder of Lyell Immunopharma, Syncopation Life Sciences, and Link Cell Therapies, which are developing CAR-based therapies; and consults for Lyell, NeoImmune Tech, Apricity, Nektar, Immatics, Ensoma, Mammoth, Glaxo Smith Kline, and Bristol Myers Squibb. J.-A.T. serves on the advisory board for Novartis. V.A.F. has served as a consultant at Adaptimmune. K.J.C. has served on the advisory board for Novartis and Atara Biotherapeutics and has received research funding from Novartis, Atara Biotherapeutics, Cellectis, and Bristol Myers Squibb. C.L.B. has received research funding from Merck, Sharp, and Dohme, Inc., Bristol Myers Squibb, and Kiadis Pharma and has pending patent applications in the field of engineered cellular therapy. T.W.L. has held consulting or advisory roles for Novartis, Bayer, Cellectis, Aptitude Health, Clinical Education Alliance, Deciphera, Juno Health, Massive Bio, Med Learning Group, Medscape, Physicians’ Education Resource, Y-mAbs Therapeutics, Advanced Microbubbles, AI Therapeutics, Jazz Pharaceuticals, GentiBio, Menarini, and Pyramid Biosciences. L.M.S. has served on the advisory board for Novartis. The remaining authors declare no competing financial interests.
Correspondence: Kevin O. McNerney, Cancer and Blood Disorders Institute, Johns Hopkins All Children’s All Children’s Hospital, 501 6th Ave S, Suite 302, St. Petersburg, FL 33701; e-mail: kmcnern1@jhmi.edu.
References
Author notes
∗K.O.M. and S.S.L. contributed equally to this study.
Presented in abstract form at the 2022 annual meeting of Transplant and Cellular Therapy Tandem Meetings, 26 April 2022, Salt Lake City, UT,; and the 2022 Histiocyte Society Meeting, 20 September 2022, Stockholm, SE.
Data are available on request from the corresponding author, Kevin O. McNerney (kmcnern1@jhmi.edu).
The full-text version of this article contains a data supplement.