TO THE EDITOR:
Children with severe mucopolysaccharidosis type I or Hurler syndrome (MPS-IH) have a severe phenotype due to a deficiency in the lysosomal enzyme α-l-iduronidase, responsible for the catabolism of glycosaminoglycans (GAGs). The resulting effect is an accumulation of heparan sulfate and dermatan sulfate, leading to the clinical manifestations of the disease: short stature, progressive intellectual disability, dysostosis multiplex, cardiac complications, hepatosplenomegaly, and other organ system involvement.1 MPS-IH–affected individuals undergo hematopoietic cell transplant (HCT) and enzyme replacement therapy (ERT) as treatments to provide a source of the missing enzyme.2,3
The standard treatment approach for MPS-IH has been to expediently proceed to HCT with the best available donor. There has been debate on whether umbilical cord blood (UCB) is a superior stem cell source compared with bone marrow (BM) for patients with Hurler syndrome in terms of absolute enzyme activity, which may be plausible given the biologic distinctness of each cell source,4 in addition, prior analyses have been confounded by testing being performed at different laboratories and by different methods.5,6 We have rigorously measured leukocyte and plasma iduronidase (IDUA) activity as well as urine GAG-related biomarkers in patients with MPS-IH after HCT in a single institution–single laboratory analysis.
We performed biomarker analysis on long-term surviving patients with MPS-IH who had received UCB (n = 33) or BM (n = 8) grafts after myeloablative conditioning from 1990 to 2019 (supplemental Table 1). The median age at HCT was nearly identical between both groups at 1.1 years and 1.2 years for UCB and BM, respectively. The era of transplant for the BM recipients had a greater range, as BM as a stem cell source predated the widespread use of UCB as a donor source. Subsequently, the time to most recent follow-up was different at 6.6 years for UCB and 16.5 years for BM recipients, respectively. Given the possible effect of age on urine biomarker excretion, we determined the median age at the most recent follow-up for UCB recipients at 8.1 years and 18.1 years for BM recipients (see supplemental Material for more details). In all cases, informed written consent from a parent or guardian was obtained from all patients on Institutional Review Board-approved protocols at the University of Minnesota.
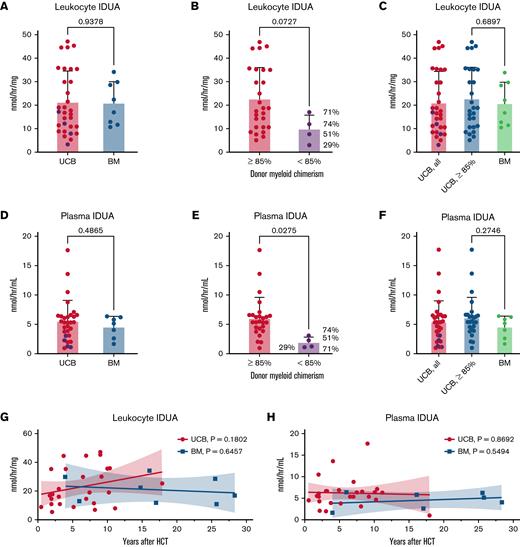
At the most recent follow-up, we found no difference in leukocyte IDUA levels between UCB and BM recipients (P = .9378) (Figure 1A). There were 4 UCB recipients that did not achieve complete myeloid engraftment (defined as >85% donor in CD15+ or CD33+ cells). Leukocyte IDUA tended to be lower in those with mixed myeloid chimerism (P = .0727) (Figure 1B), and excluding them did not significantly alter the comparison between groups (P = .6897) (Figure 1C). We found no difference in plasma IDUA activity between UCB and BM recipients (P = .4865) (Figure 1D). Like leukocyte IDUA, plasma IDUA also was lower in those with mixed myeloid chimerism (P = .0275) (Figure 1E), and excluding them did not significantly alter the comparison of the means of plasma IDUA activity (P = .2746) (Figure 1F). Several reports suggest UCB recipients engraft “better” vs BM recipients.6-9 An analysis from Boelens and colleagues showed that stem cell source did not predict graft failure, though UCB recipients had higher levels of complete (>95%) donor engraftment.7 The patients with mixed chimerism in our study had 5/6 to 6/6 level matching, so other factors likely contributed to their mixed chimerism (we speculate on viable cell dose, antibody formation, and microenvironment as considerations). In addition, the number of BM recipients in these analyses is relatively small as a possible contributor. Finally, we observed stable long-term iduronidase activity over several decades (Figure 1G-H).
Long-term assessment of iduronidase activity in patients with MPS-IH after HCT. (A-C) Leukocyte IDUA activity in UCB and BM recipients. Black dots represent individuals with myeloid chimerism <85%, and the actual myeloid chimerism percentages are shown in parentheses. Note, of the 4 UCB patients with <85% myeloid chimerism, 2 were 6/6 HLA-matched, and 2 were 5/6 HLA-matched. (D-F) Plasma IDUA activity in UCB and BM recipients. Black dots represent individuals with myeloid chimerism <85%. Shown are the means and standard deviation; P values are derived from a Student’s t test. (G,H) IDUA levels over time from HCT with P values from linear regression analyses for each cohort.
Long-term assessment of iduronidase activity in patients with MPS-IH after HCT. (A-C) Leukocyte IDUA activity in UCB and BM recipients. Black dots represent individuals with myeloid chimerism <85%, and the actual myeloid chimerism percentages are shown in parentheses. Note, of the 4 UCB patients with <85% myeloid chimerism, 2 were 6/6 HLA-matched, and 2 were 5/6 HLA-matched. (D-F) Plasma IDUA activity in UCB and BM recipients. Black dots represent individuals with myeloid chimerism <85%. Shown are the means and standard deviation; P values are derived from a Student’s t test. (G,H) IDUA levels over time from HCT with P values from linear regression analyses for each cohort.
Urine heparan sulfate-derived nonreducing ends (NREs) have been previously shown to be a sensitive marker of GAG storage.10-12 The NREs uI0S0 and I0S6 were not different between UCB and BM recipients (P = .2337 and P = .2453, respectively) (Figure 2A-B). Assessment of urine heparan sulfate (uHS) and urine total GAG (uGAG) content found them to be lower in BM recipients. Specifically, uHS were 1.06 and 0.78 mg/mmol Cr in UCB and BM recipients, respectively (P = .0413) (Figure 2C). Total uGAG were 20.58 and 9.85 mg/mmol Cr, respectively (P = .0059) (Figure 2D).
Long-term assessment of urinary glycosaminoglycan-related biomarkers in patients with MPS-IH after HCT according to age or time of follow-up. (A-D) Urine glycosaminoglycan-related biomarkers. I0S0 and I0S6 are nonreducing ends. uHS represents total heparin sulfate calculated from the addition of the internal disaccharides, D0A0 + D0S0. Total uGAG is the total glycosaminoglycan determined in the urine. Urine samples were normalized to creatinine. Shown are the means and standard deviation; P values are derived from a Student’s t test. (E-H) Urinary biomarkers over time. Only patients with myeloid chimerism >85% were included in analyses. The P values derived from ordinary least squares linear regression analyses are shown in each panel, and dotted lines show 95% confidence intervals. The black lines and R2 are derived from a curve fitting a logarithmic function (log [Y]) across the entire cohort. The yellow boxes show the normal range for age. (E) uHS normal range: age 1 to 10 years: 0.048 to 0.624 mg/mmol Cr; >10 years: 0.007 to 0.422 mg/mmol Cr. (F) The normal ranges are: age 1 to 2 years: 5.4 to 30.8 mg/mmol Cr; 3 to 6 years: 5.2 to 16.7 mg/mmol Cr; 7 to 13 years: 2.4 to 10.2 mg/mmol Cr; ≥14 years: 0.0 to 7.1 mg/mmol Cr. (G) The normal ranges are: age 1 to 10 years: ≤0.004 mg/mmol Cr; >10 years: ≤0.002 mg/mmol Cr. (H) The normal ranges are: age 1 to 10 years: ≤0.02 mg/mmol Cr; >10 years: ≤0.01 mg/mmol/Cr. (I) shows the multivariate correlation matrix and corresponding scatterplots for MPS biomarkers; the color scale shows the strength of the correlation coefficient. (J) shows the P values associated with the multivariate correlation table. (K) shows the linear regression between leukocyte IDUA and uHS. R2 = 0.1818. P = .0237.
Long-term assessment of urinary glycosaminoglycan-related biomarkers in patients with MPS-IH after HCT according to age or time of follow-up. (A-D) Urine glycosaminoglycan-related biomarkers. I0S0 and I0S6 are nonreducing ends. uHS represents total heparin sulfate calculated from the addition of the internal disaccharides, D0A0 + D0S0. Total uGAG is the total glycosaminoglycan determined in the urine. Urine samples were normalized to creatinine. Shown are the means and standard deviation; P values are derived from a Student’s t test. (E-H) Urinary biomarkers over time. Only patients with myeloid chimerism >85% were included in analyses. The P values derived from ordinary least squares linear regression analyses are shown in each panel, and dotted lines show 95% confidence intervals. The black lines and R2 are derived from a curve fitting a logarithmic function (log [Y]) across the entire cohort. The yellow boxes show the normal range for age. (E) uHS normal range: age 1 to 10 years: 0.048 to 0.624 mg/mmol Cr; >10 years: 0.007 to 0.422 mg/mmol Cr. (F) The normal ranges are: age 1 to 2 years: 5.4 to 30.8 mg/mmol Cr; 3 to 6 years: 5.2 to 16.7 mg/mmol Cr; 7 to 13 years: 2.4 to 10.2 mg/mmol Cr; ≥14 years: 0.0 to 7.1 mg/mmol Cr. (G) The normal ranges are: age 1 to 10 years: ≤0.004 mg/mmol Cr; >10 years: ≤0.002 mg/mmol Cr. (H) The normal ranges are: age 1 to 10 years: ≤0.02 mg/mmol Cr; >10 years: ≤0.01 mg/mmol/Cr. (I) shows the multivariate correlation matrix and corresponding scatterplots for MPS biomarkers; the color scale shows the strength of the correlation coefficient. (J) shows the P values associated with the multivariate correlation table. (K) shows the linear regression between leukocyte IDUA and uHS. R2 = 0.1818. P = .0237.
Given that urine glycosaminoglycan excretion decreases with age, and the BM recipients were further out from transplant, we plotted the biomarkers by age after transplant as a surrogate for follow-up time after transplant along with the range for unaffected individuals Figure 2E-G. Although uHS showed a continuous decline by age, no patient was in the normal (unaffected) range. For total uGAG, only 3 patients were found to be within the normal range for age after transplant. Overall, there was an inverse correlation between age and uHS and total uGAG for the entire cohort (r, −0.3879; P = .0342; and r, −0.7188; P < .0001, respectively). Evaluation of uI0S0 and uI0S6 showed similar results, and no patient reached the normal range for either of these NREs (Figure 2G-H).
“Early” decreases in urine GAG-based biomarkers (within the first year after HCT) likely reflect a reduction in storage material due to successful donor cell engraftment. While age and follow-up time are colinear variables, our data suggest urine GAG-related biomarkers may continue to decrease long after HCT. Wynn and colleagues also reported a continued decrease in urine dermatan sulfate/chondroitin sulfate going out to 3.5 years after HCT.13,14 Although the long-term decreases in urine biomarkers need to be considered with the age-related reduction in uGAG that occurs naturally, which are likely a result of growth, kidney function, and metabolism,15-18 it should be noted that MPS-IH children have very little growth after 5 years of age, which may confound the issue.19 Additionally, there perhaps are “hard to reach” areas in terms of donor engraftment/enzyme delivery, which includes the bones and joints that could contribute to an overall long and slow process of GAG clearance in patients with MPS-IH after HCT, perhaps even occurring over the lifetime of a patient.
Multivariate correlation analyses showed very positive correlations among urine biomarkers, as one might expect (Figure 2I-J). Of note, leukocyte IDUA had a stronger negative correlation with urine biomarkers (r, −0.22 to −0.47) than did plasma IDUA (r, −0.01 to −0.27). Interestingly, the strongest (negative) correlation was between leukocyte IDUA and uHS with a significant linear association, as shown in Figure 2K (P = .0237).
Higher IDUA linked to lower uHS, along with IDUA being higher in fully engrafted patients, suggests that patients with mixed chimerism may not clear GAG as well as fully engrafted patients. Furthermore, even in the setting of full engraftment, very few patients achieved a reduction in urine biomarkers into the unaffected range. One could speculate that it may be possible for urine GAG-related biomarkers to be driven lower through IDUA overexpression or supplemental ERT, but the clinical utility of this is yet unknown.
In conclusion, our data support that the choice of stem cell source for patients with MPS-IH should depend on the level of human leukocyte antigen-matching, cell dose, the timing of the transplant, and donor availability. Additionally, the biomarkers presented here represent novel tools that can be explored in relation to clinical outcomes in future studies.
Contribution: P.J.O., A.O.G., J.B.E., L.E.P., and E.B. provided critical editing of the manuscript; L.M.P. and M.P. provided interpretation of the biomarker data; and T.C.L. collected the data and authored the manuscript.
Conflict-of-interest disclosure: P.J.O. receives honoraria and consulting fees from Immusoft, Orchard Therapeutics, Avrobio, and Neurogene; and clinical trial support from Bluebird Bio. J.B.E. receives research support from Lysogene, Orchard Therapeutics, Sobi, and Takeda; and adboard honoraria and/or consulting from Amicus, Bluebird Bio, Denali Therapeutics, EcoR1, Orchard Therapeutics, JCR Pharmaceuticals, Regenxbio, Sanofi Genzyme, and Takeda. L.E.P. receives research support from BioMarin, Takeda, and Sobi; is a recipient of honoraria and consulting fees from BioMarin and Lysogene; and speaker’s fees for educational events for Sanofi Genzyme and BioMarin. L.M.P. is the Lead Director, Biochemical Genetics Laboratory at Greenwood Genetics. E.B. is a speaker for Sanofi/Genzyme and receives research support from BioMarin and Bluebird Bio. M.P. is the Section Chief of Biochemical Genetics at ARUP Laboratories. T.C.L. is a recipient of honoraria and consulting fees from Immusoft, Orchard Therapeutics, and Neurogene; and receives clinical trial support from Bluebird Bio. The remaining authors declare no competing financial interests.
Correspondence: Troy Lund, University of Minnesota, Pediatric Blood and Marrow Transplant Division, Metabolic Program, Stem Cell Institute, Global Pediatrics, MMC 366, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: lundx072@umn.edu.
References
Author notes
Primary data are available from the corresponding author: lundx072@umn.edu.

![Long-term assessment of urinary glycosaminoglycan-related biomarkers in patients with MPS-IH after HCT according to age or time of follow-up. (A-D) Urine glycosaminoglycan-related biomarkers. I0S0 and I0S6 are nonreducing ends. uHS represents total heparin sulfate calculated from the addition of the internal disaccharides, D0A0 + D0S0. Total uGAG is the total glycosaminoglycan determined in the urine. Urine samples were normalized to creatinine. Shown are the means and standard deviation; P values are derived from a Student’s t test. (E-H) Urinary biomarkers over time. Only patients with myeloid chimerism >85% were included in analyses. The P values derived from ordinary least squares linear regression analyses are shown in each panel, and dotted lines show 95% confidence intervals. The black lines and R2 are derived from a curve fitting a logarithmic function (log [Y]) across the entire cohort. The yellow boxes show the normal range for age. (E) uHS normal range: age 1 to 10 years: 0.048 to 0.624 mg/mmol Cr; >10 years: 0.007 to 0.422 mg/mmol Cr. (F) The normal ranges are: age 1 to 2 years: 5.4 to 30.8 mg/mmol Cr; 3 to 6 years: 5.2 to 16.7 mg/mmol Cr; 7 to 13 years: 2.4 to 10.2 mg/mmol Cr; ≥14 years: 0.0 to 7.1 mg/mmol Cr. (G) The normal ranges are: age 1 to 10 years: ≤0.004 mg/mmol Cr; >10 years: ≤0.002 mg/mmol Cr. (H) The normal ranges are: age 1 to 10 years: ≤0.02 mg/mmol Cr; >10 years: ≤0.01 mg/mmol/Cr. (I) shows the multivariate correlation matrix and corresponding scatterplots for MPS biomarkers; the color scale shows the strength of the correlation coefficient. (J) shows the P values associated with the multivariate correlation table. (K) shows the linear regression between leukocyte IDUA and uHS. R2 = 0.1818. P = .0237.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/23/10.1182_bloodadvances.2022007212/5/m_blooda_adv-2022-007212-gr2.jpeg?Expires=1767847191&Signature=OPdzJ1qFS1OPwXDAD-Q5NSDPMxKpYLMnKj~29sh~jdq5dGi3iBcn3OI9uYc3RjQVjFYKaIFfwWr5Ojx5XsNRp8M0nuShPUTdQMvxm5GEyFyjXxwnW4ovpyv2FUIoLjjWUn42hbBbH1ZOm-Z2so3xN365uw48kzAPsW~5ZrNedylTdU6tWctsVTU9iCmu0WgdBlbrEJ6oya-ujeDvdHPR04-t5ZrHpECAReO96TL21bTkmumX3aBb9d8xyP5be7dTR3ATTjgsAlweK~NK1324Hi2Oz~jbDn9zThv2L6s9YGC1U0elixhiZIxIQ6ULnUCl2TST9f2QTLu4KytCzQWgow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)