Key Points
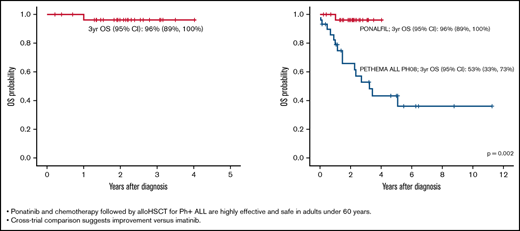
Ponatinib and chemotherapy followed by alloHSCT for Ph+ ALL are effective and safe in adults aged <60 years.
Cross-trial comparison suggests improvement vs imatinib.
Abstract
Promising results have been shown with the combination of ponatinib and chemotherapy in adults with Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL). The PONALFIL (Ponatinib With Chemotherapy for Young Adults Ph Positive Acute Lymphoblastic Leukemia) trial combined ponatinib (30 mg/d) with standard induction and consolidation chemotherapy followed by allogeneic hematopoietic stem cell transplant (alloHSCT) in newly diagnosed Ph+ ALL patients aged 18 to 60 years. Ponatinib was only given pre-emptively after alloHSCT. Primary end points were hematologic and molecular response before alloHSCT and event-free survival (EFS), including molecular relapse as event. Thirty patients (median age, 49 years; range, 19-59 years) entered the trial. All exhibited hematologic response, and alloHSCT was performed in 26 patients (20 in complete molecular response and 6 in major molecular response). Only 1 patient died (of graft-versus-host disease), and 5 patients exhibited molecular relapse after alloHSCT. No tyrosine kinase inhibitor was given after HSCT in 18 of 26 patients. Twenty-nine patients are alive (median follow-up, 2.1 years; range, 0.2-4.0 years), with 3-year EFS and overall survival (OS) of 70% (95% confidence interval, 51-89) and 96% (95% confidence interval, 89-100), respectively. Comparison of the PONALFIL and the ALLPh08 (Chemotherapy and Imatinib in Young Adults With Acute Lymphoblastic Leukemia Ph [BCR-ABL] Positive; same schedule, using imatinib as the tyrosine kinase inhibitor) trials by propensity score showed significant improvement in OS for patients in PONALFIL (3-year OS, 96% vs 53%; P = .002). The most frequent grade 3 to 4 adverse events were hematologic (42%), infectious (17%), and hepatic (22%), with only one vascular occlusive event. The combination of chemotherapy with ponatinib followed by alloHSCT is well tolerated, with encouraging EFS in adults with newly diagnosed Ph+ ALL. Cross-trial comparison suggests improvement vs imatinib (clinicaltrials.gov identifier #NCT02776605).
Introduction
The Philadelphia (Ph) chromosome or BCR::ABL1 rearrangement is the most frequent genetic aberration in adults with acute lymphoblastic leukemia (ALL), with an incidence of 25% to 30% in young adults and 40% to 50% in older adults and elderly patients.1 The introduction of tyrosine kinase inhibitors (TKIs) initially combined with standard-dose chemotherapy and later with attenuated or minimal chemotherapy followed by allogeneic hematopoietic stem cell transplant (alloHSCT) in fit patients significantly improved outcomes compared with historical controls.2-8 Imatinib was the first TKI used in trials, but similar or slightly better results have been observed with the use of dasatinib.7-9 Although not approved for regular use, trials with nilotinib showed promising results.10 Two recent clinical trials have shown impressive short-term results with the combination of a second- or third-generation TKI (dasatinib or ponatinib, respectively) with blinatumomab and without induction and consolidation chemotherapy.11,12 These results question the need for intensive chemotherapy and alloHSCT in all patients with Ph-positive (Ph+) ALL.
Ponatinib is a third-generation TKI with a wide spectrum of kinase inhibition.13 It is active against most known ABL1 mutations and is the only TKI with activity against Ph+ ALL subclones with the T315I mutation.14 Ponatinib has shown clinical activity as a single drug in relapsed or refractory Ph+ ALL.15 A phase 2 clinical trial combining ponatinib with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) in newly diagnosed Ph+ ALL patients showed promising results, with polymerase chain reaction (PCR)-based complete molecular response (CMR) of 73% after 3 months of therapy and a 3-year event-free survival (EFS) of 70%.16 In a propensity score–matched analysis, ponatinib in combination with hyper-CVAD chemotherapy showed a significantly better prognosis compared with hyper-CVAD and dasatinib.17 Moreover, a phase 2 clinical trial with ponatinib plus prednisone in patients aged ≥60 years or unfit for intensive chemotherapy and alloHSCT reported CMR at 24 weeks in 40.9% of patients, a median duration of CMR of 11.6 months, and a median EFS of 14.3 months.18 The results of these studies suggest a beneficial role for ponatinib in adults with newly diagnosed Ph+ ALL. A phase 3 trial comparing imatinib vs ponatinib combined with attenuated chemotherapy in adults with de novo Ph+ ALL is ongoing (clinicaltrials.gov identifier #NCT03589326).
In the phase 2 PONALFIL trial (Ponatinib With Chemotherapy for Young Adults Ph Positive Acute Lymphoblastic Leukemia; Clinicaltrials.gov identifier #NCT02776605), we investigated the efficacy and safety of first-line ponatinib plus standard induction and consolidation chemotherapy followed by alloHSCT in patients aged 18 to 60 years with newly diagnosed Ph+ ALL. A propensity score–matched analysis was also performed comparing outcomes with those of the ALLPh08 Trial (Chemotherapy and Imatinib in Young Adults With Acute Lymphoblastic Leukemia Ph [BCR-ABL] Positive; clinicaltrials.gov identifier #NCT01491763)19 that had the same design and schedule and used imatinib as the TKI.
Patients and methods
Study design and participants
The PONALFIL trial was a phase 2, open-label, single-arm trial performed in 30 adult patients at 9 centers in Spain. Eligible patients were aged 18 to 60 years with newly diagnosed Ph+ ALL and an Eastern Cooperative Oncology Group (ECOG) score ≤2, normal cardiac function (defined by an ejection fraction above 50%), and adequate organ function (serum bilirubin ≤3.0 mg/dL and serum creatinine ≤3.0 mg/dL, unless higher levels were believed to be due to ALL). Key exclusion criteria included: active hepatitis infection; any active infection not controlled by antibiotics; history of acute pancreatitis within 1 year or history of chronic pancreatitis; history of alcohol abuse; triglyceride levels >450 mg/dL; any clinically significant uncontrolled or active cardiovascular condition, including clinical evidence of grade 3 to 4 heart failure as defined by the New York Heart Association functional criteria, uncontrolled hypertension, arrhythmias, ischemic cardiovascular or neurologic events, or deep venous or pulmonary embolism; any impairment in gastrointestinal absorption of ponatinib; active second malignancy; or history of treatment with ponatinib.
The study protocol was reviewed and approved by the institutional review board or an independent ethics committee at all participating centers. All patients provided written informed consent.
Procedures
Patients received a 7-day steroid pretreatment during which the presence of the BCR-ABL1 transcript was centrally confirmed. Table 1 presents the chemotherapy schedule. Induction chemotherapy included vincristine, daunorubicin, and prednisone along 4 weeks. Consolidation chemotherapy consisted of high-dose methotrexate, high-dose cytarabine, mercaptopurine, and etoposide for 2 months. Patients received oral ponatinib 30 mg/d from diagnosis to 1 week before alloHSCT. Intrathecal therapy (IT) with methotrexate, cytarabine, and hydrocortisone was given at pre-phase (1 dose), during induction (2 doses), consolidation (3 doses), during the conditioning regimen of alloHSCT (1 dose), and after alloHSCT (5 doses, every 2 months) for a total of 12 doses. For patients presenting with active central nervous system (CNS) disease, the IT regimen was repeated twice weekly until the cerebrospinal fluid became clear of leukemic cells, and the cell count normalized. Regular cerebrospinal fluid prophylaxis was then administered.
Chemotherapy schedule of the PONALFIL trial
| Phase . | Route . | Dose . | Days . |
|---|---|---|---|
| Induction* | |||
| Ponatinib | By mouth | 30 mg | Until consolidation |
| Vincristine (maximum 2 mg) | IV | 1.5 mg/m2 | 1, 8, 15, 22 |
| Daunorubicin | IV | 45 mg/m2 | 1, 8, 15, 22 |
| Prednisone | IV | 60 mg/m2 | 1-14 |
| IV | 30 mg/m2 | 15-21 | |
| IV | 15 mg/m2 | 22-28 | |
| Triple IT† | IT | 1, 28 | |
| Consolidation | |||
| Ponatinib | By mouth | 30 mg | Until HSCT |
| Methotrexate | IV | 1.5 g/m2 | 1, 28, 56 |
| Mercaptopurine | By mouth | 50 mg/m2 | 1-7, 28-35, 56-63 |
| Etoposide | IV | 100 mg/m2 | 14, 42 |
| Cytarabine | IV | 1000 mg/m2/12h | 14, 15, 42, 43 |
| Triple IT† | IT | 1, 28, 56 | |
| Maintenance | |||
| Ponatinib‡ | By mouth | 30 mg (first year) 15 mg (second year) | Daily |
| Phase . | Route . | Dose . | Days . |
|---|---|---|---|
| Induction* | |||
| Ponatinib | By mouth | 30 mg | Until consolidation |
| Vincristine (maximum 2 mg) | IV | 1.5 mg/m2 | 1, 8, 15, 22 |
| Daunorubicin | IV | 45 mg/m2 | 1, 8, 15, 22 |
| Prednisone | IV | 60 mg/m2 | 1-14 |
| IV | 30 mg/m2 | 15-21 | |
| IV | 15 mg/m2 | 22-28 | |
| Triple IT† | IT | 1, 28 | |
| Consolidation | |||
| Ponatinib | By mouth | 30 mg | Until HSCT |
| Methotrexate | IV | 1.5 g/m2 | 1, 28, 56 |
| Mercaptopurine | By mouth | 50 mg/m2 | 1-7, 28-35, 56-63 |
| Etoposide | IV | 100 mg/m2 | 14, 42 |
| Cytarabine | IV | 1000 mg/m2/12h | 14, 15, 42, 43 |
| Triple IT† | IT | 1, 28, 56 | |
| Maintenance | |||
| Ponatinib‡ | By mouth | 30 mg (first year) 15 mg (second year) | Daily |
Pre-phase with prednisone 60 mg/m2 and triple IT was given for a maximum of 1 week, while ALL was fully characterized.
Triple IT with methotrexate (15 mg), cytarabine (30 mg), and hydrocortisone (20 mg).
After alloHSCT, only if molecular disease persisted or reappeared.
alloHSCT was scheduled in all patients after consolidation regardless of molecular response. Patients received a standard conditioning schedule of cyclophosphamide (60 mg/kg IV, on days −6 and −5) and fractioned total body irradiation of 13 Gy (days −4 to –1). For patients undergoing umbilical cord blood HSCT, the recommended conditioning regimen included thiotepa (5 mg/kg per day IV, on days –7 and –6; maximum dose of 10 mg/kg), fludarabine (50 mg/m2 per day IV over 1 hour, on days –5, –4, and –3), busulfan (3.2 mg/kg per day IV over 3 hours, on days –5, –4, and –3), and thymoglobulin (2 mg/kg per day over 8 hours, on days –4, –3, and −2). Non-myeloablative conditioning was only used in patients who were not candidates for a myeloablative regimen, and the regimen consisted of fludarabine (30 mg/m2 IV, days −8 to –4) and melphalan (70 mg/m2 IV, days –3 and –2). The first-choice donor was an HLA-identical sibling. If not available, a 10/10 HLA–matched unrelated donor was selected. If an unrelated donor with these characteristics was not found, the third option was a good umbilical cord blood donor (with 4/6 HLA compatibility and adequate cellularity) or a haploidentical donor. Autologous HSCT was only scheduled if there was absolute contraindication to any type of alloHSCT, with the conditioning regimen being the same as that used in the myeloablative alloHSCT. Management of HSCT was conducted according to the guidelines of each participating center.
After alloHSCT, frequent monitoring of molecular response was performed (every month) using quantitative reverse polymerase chain reaction (RQ-PCR) for BCR::ABL1. If the measurable residual disease (MRD) level was <0.01%, no further treatment was prescribed. If the MRD level was ≥0.01%, ponatinib at a dose of 30 mg/d by mouth was given for the first year and 15 mg/d during the second year. In case of autologous HSCT, ponatinib (30 mg/d, by mouth), mercaptopurine (40 mg/m2 per day, by mouth), and methotrexate (15 mg/m2 per week, intramuscularly) were scheduled during the first year after HSCT, and the ponatinib dose was reduced to 15 mg/d in the second year in patients with sustained CMR.
Ponatinib dose reduction (to 15 mg) or suspension was planned in case of serious nonhematologic or hematologic toxicities. In the case of arterial or venous occlusive events, ponatinib treatment was not resumed unless the potential benefits outweighed the risk of recurrent events, or the patient had no other treatment options. For serious nonhematologic adverse events (AEs) other than arterial or venous occlusions, ponatinib treatment was resumed only after resolution of the AE or when the potential benefit of resuming therapy was judged to outweigh the risks. In case of any hematologic AE, dose reduction or therapy suspension was allowed only after confirmed complete hematologic response (CHR). Supportive care measures (ie, antimicrobial prophylaxis or treatment, tumor lysis prophylaxis or therapy, transfusion of blood products) were implemented according to the standard procedures of each participating center.
Molecular studies
Bone marrow and peripheral blood samples from all patients at baseline were evaluated in a central facility. A fluorescence in situ hybridization study on myeloid precursors was not performed at diagnosis. Samples were processed according to the standardized consensus guidelines for MRD assessment in Ph+ ALL by real-time quantitative reverse transcriptase of the EuroMRD group [https://doi.org/10.1016/S2352-3026(18)30176-5]. MRD assessments by immunoglobulin heavy chain clonality studies or by multiparameter flow cytometry were not performed.
BCR::ABL1 kinase domain (KD) mutational testing was performed by direct Sanger sequencing and allele-specific PCR for T315I mutation at baseline and at molecular relapse in a centralized and certified laboratory. An exploratory copy number abnormality (CNA) analysis was performed at diagnosis using a 750 000 single nucleotide polymorphism array from Affymetrix (Thermo Fisher Scientific, Santa Clara, CA). Hematologic and cytogenetic responses were assessed in local laboratories.
Outcomes
The coprimary end points were the achievement of response (CHR, major molecular response [MMR], and CMR) after induction and before HSCT and the EFS. The secondary end points included the rate of patients receiving HSCT in first CMR, the transplant-related mortality (TRM), the overall survival (OS), the type and frequency of AE and severe AE (SAE), and the matched analysis comparing outcomes with those of the ALLPh08 trial,19 which included the same approach except for the use of imatinib (600 mg/d) instead of ponatinib.
CHR was defined as bone marrow blasts <5%, peripheral blood differential without blasts (neutrophils ≥1.5 × 109/L, platelets ≥100 × 109/L), and no evidence of extramedullary involvement by leukemia. CMR and MMR were defined as the BCR::ABL1/ABL1 ratio <0.01% and <0.10%, respectively, assessed by reverse-transcription quantitative real-time PCR for BCR::ABL1 transcripts with a sensitivity of at least 30 000 molecules of ABL. EFS was defined as the time from enrollment to failure of achieving CHR at week 6, lack of molecular response before HSCT, molecular or hematologic relapse, or death by any cause, whichever occurred first; the OS was calculated for the entire study population as the time from enrollment to death by any cause or last follow-up. Patients who withdrew from the trial were considered as censures at the time of withdrawal.
AEs and SAEs were recorded from the date informed consent was obtained up to 30 days after the last administration of ponatinib, and at any time if they were suspected to be related to study medication. AEs and SAEs were graded by using Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
Sample size estimation was based on demonstrating noninferiority in terms of CMR after consolidation with respect to the historical control (50%).19 For a unilateral α error of 0.05 and estimating the absence of losses, because all patients who started treatment are valid for induction, N = 30 was considered to be sufficient for detecting a difference ≥25.5%16 from the historical control with 90% power.
The main clinical and hematologic variables are expressed as frequency and as percentages for categorical variables. For continuous measurements, summary statistics included median, minimum, and maximum. Differences in subgroups by different covariates were evaluated with the χ2 test and Fisher’s exact tests, if necessary, for categorical variables and the median test for continuous variables. OS and EFS curves were plotted by using the Kaplan-Meier method and compared by using the log-rank test. Propensity score analysis with 1:1 matching was performed by using logistic regression to calculate the propensity score and the nearest neighbor matching method without calipers, as the matching method for comparison of outcomes of patients included in the PONALFIL trial vs the ALL P08 trial.20,21
Data collection and statistical analyses were performed at the PETHEMA Data Center for ALL using SPSS version 24 (IBM SPSS Statistics; IBM Corporation, Armonk, NY) and R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria) software. Two-sided P values <.05 were considered statistically significant.
Results
Patient characteristics
From April 2017 to February 2020, thirty patients were consecutively enrolled and treated in this trial. The data cutoff was November 2021, and the median duration of follow-up was 2.1 years (range, 0.2-4.0 years). Patient characteristics are presented in Table 2. The median age was 49 years (range, 19-59 years), and 13 of 30 patients were female. One patient exhibited CNS involvement at diagnosis. The ECOG score was <2 in 90% of patients. The median white blood cell count was 6.4 × 109/L (range, 0.6-359.3 × 109/L), hemoglobin was 90 g/L (range, 63-145 g/L), and platelet levels were 38 × 109/L (range, 11-206 × 109/L). The p190 isoform was shown in 20 (67%) patients, p210 in 9 (30%) patients, and p230 in 1 (3%) patient.
Baseline characteristics of the 30 patients included in the trial
| Characteristic . | Patients (N = 30) . |
|---|---|
| Male/female | 17 (57%)/13 (43%) |
| Median (range) age, y | 49 (19-59) |
| ECOG score <2 | 27 (90%) |
| CNS involvement | 1 (3%) |
| Median (range) WBC count, ×109/L | 6.4 (0.6-359.3) |
| Median (range) hemoglobin, g/L | 9.0 (6.3-14.5) |
| Median (range) platelets, ×109/L | 38.5 (11.0-206.0) |
| Median (range) LDH, U/L | 358.5 (160.0-3278.0) |
| Median (range) BCR-ABL1/ABL ratio, % | 39.7 (0.073-2763.3) |
| Phenotype | |
| Early pre-B | 2 (7%) |
| Common | 27 (90%) |
| Pre-B | 1 (3%) |
| Cytogenetics | |
| t(9;22) isolated | 18 (60%) |
| t(9;22) with additional chromosomal abnormalities | 12 (40%) |
| BCR rearrangement (bone marrow) | |
| p190 | 20 (67%) |
| p210 | 9 (30%) |
| p230 | 1 (3%) |
| Characteristic . | Patients (N = 30) . |
|---|---|
| Male/female | 17 (57%)/13 (43%) |
| Median (range) age, y | 49 (19-59) |
| ECOG score <2 | 27 (90%) |
| CNS involvement | 1 (3%) |
| Median (range) WBC count, ×109/L | 6.4 (0.6-359.3) |
| Median (range) hemoglobin, g/L | 9.0 (6.3-14.5) |
| Median (range) platelets, ×109/L | 38.5 (11.0-206.0) |
| Median (range) LDH, U/L | 358.5 (160.0-3278.0) |
| Median (range) BCR-ABL1/ABL ratio, % | 39.7 (0.073-2763.3) |
| Phenotype | |
| Early pre-B | 2 (7%) |
| Common | 27 (90%) |
| Pre-B | 1 (3%) |
| Cytogenetics | |
| t(9;22) isolated | 18 (60%) |
| t(9;22) with additional chromosomal abnormalities | 12 (40%) |
| BCR rearrangement (bone marrow) | |
| p190 | 20 (67%) |
| p210 | 9 (30%) |
| p230 | 1 (3%) |
Data are presented as no. (%) unless otherwise indicated.
LDH, lactate dehydrogenase; WBC, white blood cell; WHO, World Health Organization.
Response to treatment and outcome
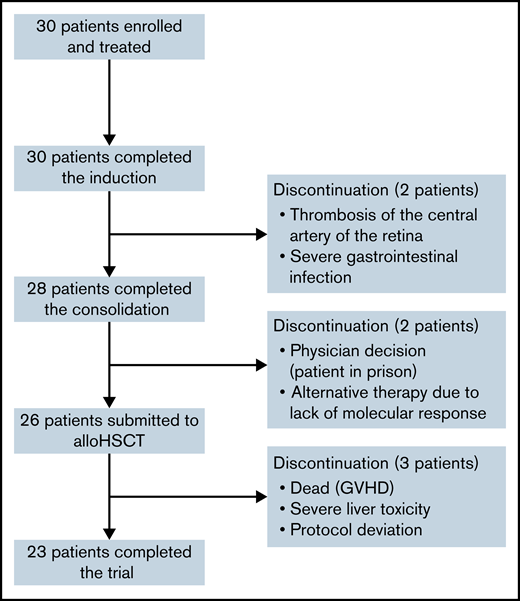
Figure 1 presents the flowchart of the study. All patients completed the induction phase and were evaluable for treatment response. CHR was achieved in all patients. At end induction, CMR was attained in 14 (47%) of 30 patients and MMR in 5 (17%) of 30 patients; no molecular response was observed in 11 (36%) of 30 patients. Two patients withdrew from the trial after induction due to thrombosis of the central artery of the retina and severe intestinal infection with bowel perforation and peritonitis (one case each). Both patients received dasatinib together with the consolidation chemotherapy prescribed by the trial.
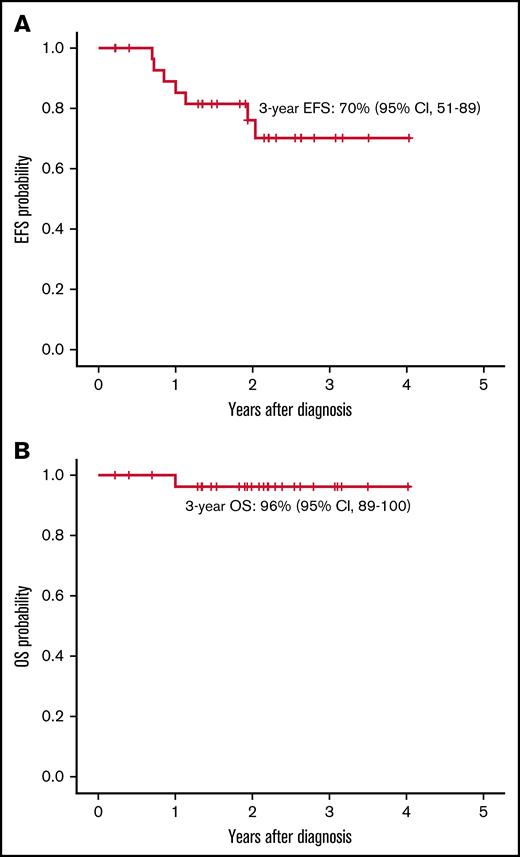
Consolidation was given to 28 patients. At the end of consolidation, 20 (71%) of 28 patients were in CMR, 7 (25%) of 28 were in MMR, and 1 patient (4%) did not achieve molecular response. Four of 7 patients in MMR had the p210 isoform, whereas the patient without molecular response exhibited the p190 isoform. Two patients withdrew from the trial at the end of consolidation by physician decision (1 patient was in prison, and the other received blinatumomab because of lack of molecular response). No relapses before alloHSCT were detected. alloHSCT was performed in 26 patients, and no patient underwent autologous HSCT. The median time from the start of treatment to transplant was 5.7 months (range, 4.2-9.6 months). One patient died of severe acute graft-versus-host disease, and another withdrew from the trial due to grade 4 hepatic toxicity. Both patients were in CMR before withdrawal. Five patients showed molecular relapse (median, 8 months; range, 3-21 months). One withdrew from the protocol and received alternative therapy (dasatinib) and presented hematologic relapse (being successfully rescued with fludarabine, high-dose cytarabine, idarubicin, and granulocyte-colony stimulating factor chemotherapy and ponatinib); the remaining 4 patients received ponatinib (30 mg/d until molecular remission, 15 mg/d afterward), with sustained CMR. One additional patient in CMR received dasatinib because of refusal of posttransplant IT CNS prophylaxis. In all, 20 of 26 patients (including the patient who died of graft-versus-host disease and the patient who withdrew from the trial because of liver toxicity) in continuous CMR did not receive any TKI after HSCT, with 18 remaining in the trial. The 3-year EFS probability was 70% (95% confidence interval [CI], 51-89) (Figure 2A), the 7 events being molecular refractoriness before HSCT (n = 1), TRM (n = 1) and molecular relapse after HSCT (n = 5). At the close of follow-up, 29 patients were alive, with a 3-year OS probability of 96% (95% CI, 89-100) (Figure 2B).
EFS and OS. (A) EFS for patients included in the PONALFIL trial from enrollment to failure of achieving CHR at week 6, lack of molecular response before HSCT, molecular or hematologic relapse or death by any cause. (B) OS for patients form diagnosis to death or last follow-up.
EFS and OS. (A) EFS for patients included in the PONALFIL trial from enrollment to failure of achieving CHR at week 6, lack of molecular response before HSCT, molecular or hematologic relapse or death by any cause. (B) OS for patients form diagnosis to death or last follow-up.
Results of biologic studies
According to Sanger sequencing, no ABL1 KD mutations at diagnosis were found in any patient. No T315I mutations were found by allele-specific PCR at diagnosis and in the 5 patients who experienced molecular relapse. The single nucleotide polymorphism array was performed in 19 patients with available DNA at diagnosis. All patients exhibiting MMR or no molecular response (n = 7), and those experiencing molecular relapse (n = 5), were also analyzed. Ikaros (IKZF1) deletion was detected in 11 (58%) of 19 patients, whereas CDKN2A/B, PAX5, or RB1 losses were observed in 8 (42%) of 19 patients, 7 (37%) of 19, and 5 (26%) of 19, respectively. The IKZF1plus profile (concomitant deletion of IKZF1 with CDKN2A, CDKN2B, PAX5, or P2RY8-CRLF2 in the absence of ERG deletion) was observed in 6 (32%) of 19 participants. Of the 7 of 16 patients with MMR (n = 6) or no molecular response (n = 1) before HSCT and with genetic material available at diagnosis, 4 showed IKZF1 deletion (IKZF1plus in 2), 1 showed CDKN2A/B and PAX5 deletion, 1 showed duplication from ABL1 to 9qter (derived from BCR::ABL1 rearrangement), and 1 only showed polymorphic CNAs. The patient not achieving molecular response showed a IKZF1 deletion together with 3p- (PTPRG), monosomy 9 (CDKN2A/B, PAX5), 13q- (RB1), and monosomy 15 (SPRED1). Among the 5 patients with molecular relapse, 3 showed IKZF1 deletion (1 of them being IKZF1plus), and 1 showed duplication from ABL1 to 9qter (derived from BCR::ABL1 rearrangement); 1 patient did not show any CNAs.
Propensity score analysis
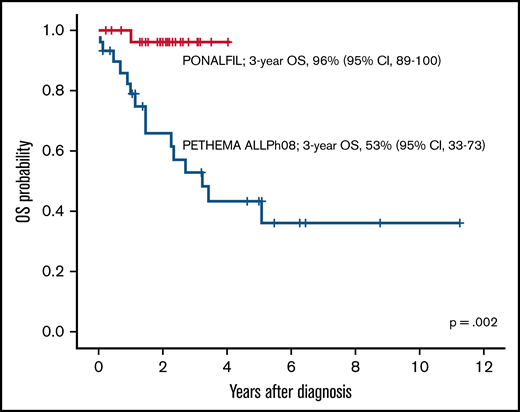
The following variables were selected for propensity score analysis in patients from the PONALFIL and the ALLPh08 trials: age, ECOG score, white blood cell count, CNS involvement at diagnosis, cytogenetics [isolated t(9;22) and t(9;22) with additional chromosomal abnormalities], and BCR::ABL1 isoform (p190, p210, and p230). Table 3 presents the characteristics of the 2 matched cohorts. The 3-year OS rates for the PONALFIL and ALLPh08 trials were 96% vs 53% (P = .002), respectively (Figure 3).
Characteristics of the 2 cohorts matched for propensity score analysis
| Characteristic . | ALLPh08 (N = 30) . | PONALFIL (N = 30) . | P . |
|---|---|---|---|
| Male | 16/30 (53%) | 17/30 (57%) | .795 |
| Age, median [minimum; maximum], y | 46 (19, 56) | 49 (19, 59) | .439 |
| ECOG score <2 | 27 (90%) | 27 (90%) | 1.0 |
| White blood cell count, ×109/L | 1.0 | ||
| <30 | 22 (73%) | 22 (73%) | |
| ≥30 | 8 (27%) | 8 (27%) | |
| CNS involvement | 2 (7%) | 1 (3%) | 1.0 |
| Cytogenetics | .602 | ||
| t (9;22) | 16 (53%) | 18 (60%) | |
| t (9;22) with additional chromosomal abnormalities | 14 (47%) | 12 (40%) | |
| Isoform | .278 | ||
| p190 | 14 (47%) | 20 (67%) | |
| p210 | 15 (50%) | 9 (30%) | |
| p230 | 1 (3%) | 1 (3%) |
| Characteristic . | ALLPh08 (N = 30) . | PONALFIL (N = 30) . | P . |
|---|---|---|---|
| Male | 16/30 (53%) | 17/30 (57%) | .795 |
| Age, median [minimum; maximum], y | 46 (19, 56) | 49 (19, 59) | .439 |
| ECOG score <2 | 27 (90%) | 27 (90%) | 1.0 |
| White blood cell count, ×109/L | 1.0 | ||
| <30 | 22 (73%) | 22 (73%) | |
| ≥30 | 8 (27%) | 8 (27%) | |
| CNS involvement | 2 (7%) | 1 (3%) | 1.0 |
| Cytogenetics | .602 | ||
| t (9;22) | 16 (53%) | 18 (60%) | |
| t (9;22) with additional chromosomal abnormalities | 14 (47%) | 12 (40%) | |
| Isoform | .278 | ||
| p190 | 14 (47%) | 20 (67%) | |
| p210 | 15 (50%) | 9 (30%) | |
| p230 | 1 (3%) | 1 (3%) |
Safety
A total of 106 AEs were registered in 20 patients (67%) (Table 4). The most frequent AEs of any grade were hematologic (28%), gastrointestinal (14%), hepatic (11%), and infectious (8%). Overall, 16 (53%) of 30 patients experienced a grade ≥3 AE. The most common were hematologic (42%), hepatic (22%), and infectious (17%). Cardiovascular events occurred in 1 patient (thrombosis of central artery of the retina). Three patients permanently discontinued the study due to SAEs (thrombosis of central retina artery, severe bowel infection and perforation with peritonitis, and grade IV hepatic toxicity after alloHSCT [1 case each]). Only 1 of 4 patients who received ponatinib after HSCT experienced transient grade 2 liver toxicity.
AEs of any grade and of grade ≥3 in the patients included in the series
| . | Any grade . | Grade ≥3 . |
|---|---|---|
| Total number | 106 | 36 |
| No. of patients | 20/30 (67%) | 16/30 (53%) |
| Hematologic | 30 (28%) | 15/36 (42%) |
| Infectious | 8 (8%) | 6/36 (17%) |
| Gastrointestinal | 15 (14%) | 1/36 (3%) |
| Skin | 5 (5%) | 1/36 (3%) |
| Respiratory | 6 (6%) | 2/36 (6%) |
| General | 4 (4%) | — |
| Neurologic | 4 (4%) | — |
| Ocular | 2 (2%) | 2/36 (6%) |
| Hepatic | 12 (11%) | 8/36 (22%) |
| Hypertension | 1 (1%) | — |
| Vascular occlusive event | 1 (1%) | 1/36 (3%) |
| Other | 17 (16%) | — |
| . | Any grade . | Grade ≥3 . |
|---|---|---|
| Total number | 106 | 36 |
| No. of patients | 20/30 (67%) | 16/30 (53%) |
| Hematologic | 30 (28%) | 15/36 (42%) |
| Infectious | 8 (8%) | 6/36 (17%) |
| Gastrointestinal | 15 (14%) | 1/36 (3%) |
| Skin | 5 (5%) | 1/36 (3%) |
| Respiratory | 6 (6%) | 2/36 (6%) |
| General | 4 (4%) | — |
| Neurologic | 4 (4%) | — |
| Ocular | 2 (2%) | 2/36 (6%) |
| Hepatic | 12 (11%) | 8/36 (22%) |
| Hypertension | 1 (1%) | — |
| Vascular occlusive event | 1 (1%) | 1/36 (3%) |
| Other | 17 (16%) | — |
Discussion
This study confirms the safety and high antileukemic efficacy (100% hematologic CR, and 71% CMR at the end of consolidation), the high rate of alloHSCT performance (26 of 30 patients [87%]), and the promising EFS (70% at 3 years) and OS (96% at 3 years) of the combination of ponatinib (30 mg/d) and standard chemotherapy followed by alloHSCT in adults aged <60 years with newly diagnosed Ph+ ALL. Moreover, these results compared favorably with those of the ALLPh08 trial with the same design and schedule, using imatinib as a TKI.
The efficacy of ponatinib as a single drug in relapsed or refractory Ph+ ALL15 prompted the inclusion of this drug in first-line therapy of adults with Ph+ ALL in combination with intensive chemotherapy,16 minimal chemotherapy,18 and recently with blinatumomab.12 A first report of a phase 2 trial conducted at the MD Anderson Cancer Center (MDACC) combined hyper-CVAD with ponatinib in 37 adult patients with de novo Ph+ ALL.22 An update of the trial found a 100% CHR for the 65 patients with active disease at enrollment, with CMR being achieved in 63 (83%) of 76 patients. The 3-year continuous complete remission (CR) was 83% (95% CI, 69-91), the 3-year EFS was 70% (95% CI, 56-80), and the 3-year OS was 76% (95% CI, 63-85).16 Fifteen patients (20%) underwent alloHSCT in first CR according to physician decision. A post hoc 6-month landmark analysis found no difference in OS in patients undergoing transplant vs nontransplanted patients. A phase 2 trial (LAL1811) from the Italian GIMEMA Group administered 45 mg of ponatinib per day plus steroids in 44 patients newly diagnosed with Ph+ ALL aged ≥60 years, or unfit for intensive chemotherapy and HSCT.18 The median age was 67 years. The CHR and CMR at 24 weeks were reached in 38 (86.4%) of 44 patients and in 18 (40.9%) of 44 patients, respectively. The median EFS was 14.31 months (95% CI, 9.30-22.31), whereas medians of OS and CR duration were not reached; the median duration of CMR was 11.6 months.
The current trial included only young and middle-aged adults (median age, 49 years) because it was aimed to perform alloHSCT in all patients at the best molecular response after consolidation. The dose of ponatinib was reduced to 30 mg to avoid or reduce vascular occlusive events. The chemotherapy schedule can be considered as standard, although asparaginase was omitted to avoid an increase in hepatic and vascular toxicity when combined with ponatinib. As expected, all patients displayed CHR, and the rate of CMR at the end of consolidation was 71%, a rate similar to that achieved with the trial of hyper-CVAD and ponatinib.16 Two patients withdrew from the trial after induction for an occlusive arterial event and a severe infection, respectively, whereas 2 additional patients abandoned the trial after consolidation due to a situation that precluded the performance of HSCT (patient in prison) and by physician decision to offer alternative therapy due to the lack of molecular response, respectively. The alloHSCT was performed in 87% of CR patients and was well tolerated, with only TRM occurring in 1 patient and severe liver toxicity in another. All patients were in CMR after HSCT. It is noteworthy that pre-emptive TKI administration after HSCT was scheduled in the trial; however, 20 of 26 patients never received ponatinib or another TKI as maintenance therapy. Molecular relapse was observed in 5 patients and was managed with ponatinib (n = 4) or dasatinib (n = 1, by physician decision), with hematologic relapse occurring in the patient who received dasatinib. The 3-year EFS and OS of 70% (95% CI, 51-89) and 96% (95% CI, 89-100) can be considered satisfactory.
It is also worth noting that a restrictive definition of EFS (including molecular failure or relapse as event) was used in this trial. A propensity score–matched analysis with comparable patients from the ALLPh08 study,19 with the same design and interventions except for the use of imatinib instead of ponatinib, showed a clear improvement in outcomes in favor of the PONALFIL trial. Identical results were observed in the propensity score–matched comparison of the trial with hyper-CVAD and ponatinib vs hyper-CVAD and dasatinib conducted at the MDACC.16
Overall safety and tolerability of the treatment were considered acceptable, and no unexpected AEs were observed. The rate of permanent discontinuation due to toxicity during the entire study was 13% and was directly attributable to ponatinib in only 1 case. The most frequent grade ≥3 AEs were hematologic, infectious, and hepatic, as observed in other similar trials.
Despite having a different postconsolidation approach, our study reported results similar to those of the trial with hyper-CVAD and ponatinib conducted at MDACC, and showed better outcomes compared with the ALLPh08 trial.19 However, several limitations should be highlighted. First, this is a single-arm phase 2 study. Second, the number of patients was limited, a fact that precludes analysis of subsets of patients according to genetic and molecular background. No ABL1 KD mutations were found at diagnosis by using Sanger sequencing, and the T315I mutation was not found in any patient by using allele-specific PCR. IKZF1 gene mutations were found at diagnosis in 4 of 7 patients who did not achieve CMR (IKZF1plus in 2 patients), but no conclusion can be drawn from this low number of cases.23 Third, the follow-up is still short, despite all patients having completed the trial.
Recently published clinical trials include immunotherapy with monoclonal antibodies (blinatumomab) combined with a TKI (dasatinib or ponatinib) without or with minimal chemotherapy as first-line therapy of Ph+ ALL in adults; they found a high rate of hematologic and molecular responses and promising short-term EFS and OS.11,12,24 In the D-ALBA (Frontline Sequential Dasatinib and Blinatumomab in Adult Philadelphia Positive Acute Lymphoblastic Leukemia) trial from the GIMEMA Group, CHR was achieved in all patients (N = 63) with the combination of ponatinib and a short course of steroids, and CMR achieved after the second blinatumomab course was 60%.11 Transplantation was performed in 29 (50%) of 58 patients who started blinatumomab. The 2-year OS probability was 95% in the first analysis and 87.8% with a median follow-up of 27 months (accessed 1 July 2021).25 In the last update of a phase 2 trial conducted at MDACC combining ponatinib and blinatumomab upfront during induction and consolidation, CHR was attained in all 24 patients with newly diagnosed Ph+ ALL, with CMR of 91% at the end of consolidation, and 2-year EFS and OS of 95%.12 No patients underwent alloHSCT in this trial. These impressive results have questioned the need for alloHSCT in all young adults with Ph+ ALL, at least in those treated with ponatinib as TKI. Further studies should establish the optimal role of alloHSCT in Ph+ ALL in trials combining TKI, attenuated or minimal chemotherapy, and immunotherapy.
In conclusion, our trial yielded good results in terms of the efficacy and tolerability of the combination of ponatinib and standard induction and consolidation chemotherapy followed by alloHSCT in adults up to age 60 years with newly diagnosed Ph+ ALL. It also showed that a pre-emptive strategy for ponatinib after transplantation is feasible and allows a reduction of ponatinib exposure in a significant number of patients.
Acknowledgments
The authors thank Incyte for providing ponatinib to all study patients free of cost, to CABYC for helping in the conduction of the trial, and to the following institutions for their contribution to the study: ICO-Hospital Germans Trias i Pujol, Josep Carreras Leukemia Research Institute (IJC) (J.-M.R., O.G.-C., J.R., A.T., and E.G.); Hospital Universitari i Politècnic La Fe (P. Montesinos, I.C.-F.); Hospital Doce De Octubre (P. Martínez, J.M.-L.); Hospital Clínic (J.E. and D.E.); Hospital Universitario Virgen De La Victoria (M.G.-F.); Complejo Hospitalario Universitario Santiago de Compostela (N.A.); Hospital Universitario Virgen del Rocío (J.G.-C.); Hospital Universitario Marqués de Valdecilla (A.B.); ICO-Hospital Duran i Reynals (S.M.); and Hospital Universitario de Salamanca (HUS/IBSAL), CIBERONC and Center for Cancer Research-IBMCC (USAL-CSIC) (R.G.-S.).
The PETHEMA Foundation, a nonprofit organization, was the sponsor of the trial, and the PETHEMA Data Center elaborated the database, and organized training meetings in which principal investigators and collaborative investigators participated. This project was supported in part by the ISCIII (PI14/01971 and PI19/01828), cofunded by ERDF/ESF, “A way to make Europe”/“Investing in your future,” CERCA/Generalitat de Catalunya SGR 2017 288 (GRC)/“La Caixa.” This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement no. 116026. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation and the European Federation of Pharmaceutical Industries and Associations.
Authorship
Contribution: J.-M.R. designed the study and coordinated the trial; P. Montesinos, I.C.-F., P. Martínez, J.E., D.E., M.G.-F., N.A., J.G.-C., A.B., A.T., E.G., S.M., and J.M.-L. were responsible for patient care; J.R. and R.G.-S. performed genetic analyses; O.G.-C. contributed to database set-up, reviewed and cleaned data, and contributed to data analysis; and all authors were involved in drafting of the manuscript and approved the final draft.
Conflict-of-interest disclosure: J.-M.R. has received honoraria and travel grants from Incyte, Novartis, Takeda, AMGEN, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Josep-Maria Ribera, ICO-Hospital Germans Trias i Pujol, Josep Carreras Leukemia Research Institute (IJC), C/Canyet s/n, o8916 Badalona, Spain; e-mail: jribera@iconcologia.net.
References
Author notes
Presented at the 64th American Society of Hematology Annual Meeting & Exposition, Atlanta, 11-14 December 2021 (Blood. 2021;138[supplement 1]:1230).
Requests for data sharing may be submitted to jribera@iconcologia.net or olga.garcia.calduch@fundacionpethema.es. The study protocol is also available at https://www.fundacionpethema.es.