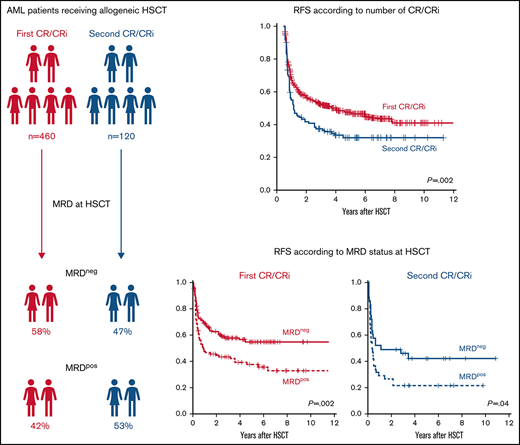
Key Points
HSCT during the second remission carries a higher relapse risk than in the first remission, regardless of the diagnostic ELN2017 risk.
The MRD status at HSCT was an independent risk factor, irrespective of the number of remissions.
Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) offers the best chance for relapse-free survival to most patients with acute myeloid leukemia (AML). It may be performed during complete remission or delayed until after the first relapse because of relevant treatment-related morbidity and mortality. The measurable residual disease (MRD) status at HSCT adds refined prognostic information to the assigned European LeukemiaNet (ELN) 2017 genetic risk at diagnosis. We analyzed 580 patients with AML who underwent allogeneic HSCT during either the first (79%) or second (21%) remission. Although, because of common treatment strategies, some adverse risk characteristics, such as monosomal or complex karyotypes, were less frequent in patients who underwent transplant in the second remission, those patients had worse outcomes compared with patients who had transplant in the first remission. The MRD status at HSCT was an independent prognostic factor, irrespective of the number of remissions at HSCT. Notably, patients who were MRD+ who underwent HSCT in the first remission and those who were MRD− and underwent transplant in the second remission had similar outcomes. In the clinically highly relevant group of individuals who had ELN2017 intermediate risk, the MRD status provided the highest prognostic value with very dismal outcomes for patients who were MRD+ and underwent second-remission transplants. The adverse outcomes of patients who are MRD+ and of those who undergo transplant in the second remission should be considered when planning consolidation treatment, to avert an allogeneic HSCT in MRD+ second remission when possible.
Introduction
Acute myeloid leukemia (AML) is a highly heterogeneous disease that demands individualized treatment approaches. Allogeneic hematopoietic stem cell transplantation (HSCT) offers a potentially curative consolidation option for most affected patients, but is accompanied by treatment-related toxicities and mortality. Subsequently, allogeneic HSCT in the first complete remission (CR) usually is offered to patients with increased relapse risks that outweigh the risk of nonrelapse mortality (NRM) associated with allogeneic HSCT.1,2 In most cases, this group comprises individuals with intermediate or adverse genetic risk according to European LeukemiaNet (ELN) 2017 risk stratification, which is commonly used to inform treatment decisions today.3 Besides the genetic risk at diagnosis, response to the applied therapy is an important disease-related prognostic factor. With the introduction of measurable residual disease (MRD) evaluation, rather than determining the mere presence of a morphologic remission, the assessments of treatment response have significantly increased in sensitivity.4-6 Hence, MRD evaluation increasingly contributes to the decision to treat with an allogeneic HSCT in the first CR in ELN2017 favorable- or intermediate-risk patients.3,4
However, a variety of additional factors may hamper allogeneic HSCT in the first remission, including availability of a matched donor, age, and comorbidities.2 Today, conditioning regimens of reduced intensity7-9 and alternative donor approaches10-12 increase our ability to offer an allogeneic HSCT to most eligible patients with AML. Finally, for individuals who do not undergo HSCT in the first CR and experience relapse, an allogeneic HSCT still provides the highest probability of achieving favorable long-term outcomes, especially if a second CR is achieved.13,14
Regarding comparative outcome analyses, retrospective data indicate that patients who have an HSCT during the second morphologic remission tend to have a higher cumulative incidence of relapse (CIR) and shorter overall survival (OS), but similar NRM, compared with patients who undergo HSCT during the first remission.13 With respect to MRD status at HSCT, an analysis of patients with AML who undergo myeloablative HSCT indicated a comparable prognostic value of flow cytometry–based pre-HSCT MRD status in the first and second remission.15 In contrast, a study conducted by the European Society for Blood and Marrow Transplantation, which analyzed the transplant centers reported MRD status, without specifying the applied heterogeneous techniques, suggested that the MRD status at HSCT has an impact on CIR and relapse-free survival (RFS), but not on OS in individuals who undergo HSCT in the second remission.16 However, comparative outcome analyses that take into account the currently used ELN2017 genetic risk as well as the MRD status before performing allogeneic HSCT in the first or second remission are missing, and considering those factors together was the main objective of the current study.
Patients and methods
Patients and treatment
We retrospectively analyzed 580 patients with AML who underwent their first allogeneic HSCT from January 1999 through November 2020 at our center and were either in the first (n = 460; 79%) or second (n = 120; 21%) morphologic CR or CR with incomplete peripheral cell count recovery (CRi). Median age at HSCT was 59.6 (range, 16.3-76.8) years. The majority of the patients (72%) received either nonmyeloablative (n = 383) or reduced-intensity (n = 36) conditioning,17-19 whereas 28% of the patients received myeloablative conditioning (n = 161). Details on the applied conditioning regimens and immunosuppression are given in the supplemental Information. Further information regarding characteristics at diagnosis and HSCT are shown in Table 1. Median follow-up after HSCT for living patients was 3.9 years. Data analyses were approved by the Institutional Review Board of the University Hospital Leipzig. Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Patients’ characteristics according to first vs second remission at diagnosis and before allogeneic HSCT
| . | All patients n = 580 . | HSCT in first CR/CRi n = 460 . | HSCT in second CR/CRi n = 120 . | P . |
|---|---|---|---|---|
| Characteristics at diagnosis | ||||
| Sex, n (%) | .68 | |||
| Male | 308 | 242 (53) | 66 (55) | |
| Female | 272 | 218 (47) | 54 (45) | |
| Disease origin, n (%) | .002 | |||
| Secondary/treatment related | 195 | 169 (37) | 26 (22) | |
| De novo | 385 | 291 (63) | 94 (78) | |
| Hemoglobin, g/dL, median (range) | 8.9 (3.2-15.7) | 8.7 (3.2-15.7) | 9.5 (4.3-14.9) | .01 |
| Platelet count, x 109/L, median (range) | 65 (1-950) | 65 (1-950) | 65 (3-289) | .98 |
| WBC, × 109/L, median (range) | 5.9 (0.1-385) | 5.4 (0.1-385) | 17.1 (0.6-366) | .006 |
| <10 × 109/L, n (%) | 118 | 199 (63) | 19 (40) | .002 |
| ≥10 × 109/L, n (%) | 144 | 115 (37) | 29 (60) | |
| Blood blasts, %, median (range) | 21 (0-98) | 20 (0-98) | 31 (0-92) | .16 |
| BM blasts, %, median (range) | 51 (0-95) | 50 (0-95) | 61 (22-95) | .03 |
| CD34+/CD38− cell burden, median (range) | 0.5 (0-89) | 0.5 (0-89) | 0.5 (0-21) | .77 |
| Normal karyotype, n (%) | <.001 | |||
| Absent | 288 | 253 (59) | 35 (34) | |
| Present | 247 | 178 (41) | 69 (66) | |
| ELN2017 group, n (%) | .15 | |||
| Favorable | 112 | 89 (25) | 23 (34) | |
| Intermediate | 147 | 123 (34) | 24 (36) | |
| Adverse | 168 | 148 (41) | 20 (30) | |
| Complex karyotype, n (%) | <.001 | |||
| Absent | 445 | 346 (84) | 99 (100) | |
| Present | 67 | 67 (16) | 0 (0) | |
| Monosomal karyotype, n (%) | <.001 | |||
| Absent | 459 | 359 (87) | 100 (98) | |
| Present | 58 | 56 (13) | 2 (2) | |
| NPM1, n (%) | .03 | |||
| Wild-type | 328 | 280 (77) | 48 (65) | |
| Mutated | 108 | 82 (23) | 26 (35) | |
| FLT3-ITD, n (%) | <.001 | |||
| Wild-type | 345 | 298 (81) | 47 (63) | |
| Mutated | 96 | 68 (19) | 28 (37) | |
| NPM1 and FLT3-ITD co-mutation, n (%) | .01 | |||
| NPM1 wild-type, FLT3-ITD negative | 281 | 246 (68) | 35 (47) | |
| NPM1 mutated, FLT3-ITD negative | 61 | 49 (14) | 12 (16) | |
| NPM1 wild-type, FLT3-ITD positive | 47 | 34 (9) | 13 (18) | |
| NPM1 mutated, FLT3-ITD positive | 45 | 31 (9) | 14 (19) | |
| CEBPAbiallelic, n (%) | .08 | |||
| Wild-type | 344 | 292 (99) | 52 (95) | |
| Mutated | 7 | 4 (1) | 3 (5) | |
| FLT3-ITD allelic ratio, n (%) | .23 | |||
| <0.5 | 46 | 36 (59) | 10 (43) | |
| ≥0.5 | 38 | 25 (41) | 13 (57) | |
| RUNX1, n (%) | .32 | |||
| Wild-type | 103 | 87 (86) | 16 (76) | |
| Mutated | 19 | 14 (14) | 5 (24) | |
| TP53, n (%) | >.99 | |||
| Wild-type | 109 | 90 (89) | 19 (90) | |
| Mutated | 13 | 11 (11) | 2 (10) | |
| ASXL1, n (%) | .73 | |||
| Wild-type | 114 | 94 (89) | 20 (87) | |
| Mutated | 15 | 12 (11) | 3 (13) | |
| Characteristics at HSCT | ||||
| Age at HSCT, y, median (range) | 59.6 (16.3-76.8) | 60.3 (16.3-76.0) | 59.6 (19.2-76.8) | .43 |
| Treatment cycles before HSCT, n (%) | <.001 | |||
| 1 | 157 | 72 (16) | 85 (71) | |
| 2 | 311 | 280 (61) | 31 (26) | |
| ≥3 | 111 | 107 (24) | 4 (3) | |
| Blood count regeneration at HSCT, n (%) | .41 | |||
| CR | 484 | 387 (84) | 97 (81) | |
| CRi | 96 | 73 (16) | 23 (19) | |
| MRD status at HSCT, n (%) | .10 | |||
| MRD− | 167 | 133 (58) | 34 (47) | |
| MRD+ | 133 | 95 (42) | 38 (53) | |
| Donor type, n (%) | .02 | |||
| Matched related | 124 | 109 (24) | 15 (13) | |
| Unrelated, HLA matched | 329 | 258 (56) | 71 (59) | |
| HLA mismatched | 112 | 80 (17) | 32 (27) | |
| Haploidentical | 14 | 12 (3) | 2 (2) | |
| Conditioning regimen, n (%) | .22 | |||
| NMA | 383 | 296 (64) | 87 (73) | |
| HSCT | 36 | 31 (7) | 5 (4) | |
| MAC | 161 | 133 (29) | 28 (23) | |
| HCT-CI score, n (%) | .59 | |||
| 0 | 244 | 195 (44) | 49 (42) | |
| 1 and 2 | 163 | 125 (26) | 38 (32) | |
| ≥3 | 159 | 129 (29) | 30 (26) | |
| CMV status, n (%) | .05 | |||
| Recipient+/donor− | 210 | 157 (35) | 53 (45) | |
| All others | 364 | 298 (65) | 66 (55) | |
| aGvHD ≥ grade 2, n (%) | .21 | |||
| Absent | 371 | 303 (74) | 68 (68) | |
| Present | 137 | 105 (26) | 32 (32) | |
| cGvHD, n (%) | .51 | |||
| Absent | 165 | 139 (41) | 26 (37) | |
| Limited | 58 | 45 (13) | 13 (19) | |
| Extended | 184 | 153 (45) | 31 (44) | |
| Donor sex, n (%) | .88 | |||
| All others | 489 | 388 (85) | 101 (86) | |
| Female into male | 82 | 66 (15) | 16 (14) | |
| . | All patients n = 580 . | HSCT in first CR/CRi n = 460 . | HSCT in second CR/CRi n = 120 . | P . |
|---|---|---|---|---|
| Characteristics at diagnosis | ||||
| Sex, n (%) | .68 | |||
| Male | 308 | 242 (53) | 66 (55) | |
| Female | 272 | 218 (47) | 54 (45) | |
| Disease origin, n (%) | .002 | |||
| Secondary/treatment related | 195 | 169 (37) | 26 (22) | |
| De novo | 385 | 291 (63) | 94 (78) | |
| Hemoglobin, g/dL, median (range) | 8.9 (3.2-15.7) | 8.7 (3.2-15.7) | 9.5 (4.3-14.9) | .01 |
| Platelet count, x 109/L, median (range) | 65 (1-950) | 65 (1-950) | 65 (3-289) | .98 |
| WBC, × 109/L, median (range) | 5.9 (0.1-385) | 5.4 (0.1-385) | 17.1 (0.6-366) | .006 |
| <10 × 109/L, n (%) | 118 | 199 (63) | 19 (40) | .002 |
| ≥10 × 109/L, n (%) | 144 | 115 (37) | 29 (60) | |
| Blood blasts, %, median (range) | 21 (0-98) | 20 (0-98) | 31 (0-92) | .16 |
| BM blasts, %, median (range) | 51 (0-95) | 50 (0-95) | 61 (22-95) | .03 |
| CD34+/CD38− cell burden, median (range) | 0.5 (0-89) | 0.5 (0-89) | 0.5 (0-21) | .77 |
| Normal karyotype, n (%) | <.001 | |||
| Absent | 288 | 253 (59) | 35 (34) | |
| Present | 247 | 178 (41) | 69 (66) | |
| ELN2017 group, n (%) | .15 | |||
| Favorable | 112 | 89 (25) | 23 (34) | |
| Intermediate | 147 | 123 (34) | 24 (36) | |
| Adverse | 168 | 148 (41) | 20 (30) | |
| Complex karyotype, n (%) | <.001 | |||
| Absent | 445 | 346 (84) | 99 (100) | |
| Present | 67 | 67 (16) | 0 (0) | |
| Monosomal karyotype, n (%) | <.001 | |||
| Absent | 459 | 359 (87) | 100 (98) | |
| Present | 58 | 56 (13) | 2 (2) | |
| NPM1, n (%) | .03 | |||
| Wild-type | 328 | 280 (77) | 48 (65) | |
| Mutated | 108 | 82 (23) | 26 (35) | |
| FLT3-ITD, n (%) | <.001 | |||
| Wild-type | 345 | 298 (81) | 47 (63) | |
| Mutated | 96 | 68 (19) | 28 (37) | |
| NPM1 and FLT3-ITD co-mutation, n (%) | .01 | |||
| NPM1 wild-type, FLT3-ITD negative | 281 | 246 (68) | 35 (47) | |
| NPM1 mutated, FLT3-ITD negative | 61 | 49 (14) | 12 (16) | |
| NPM1 wild-type, FLT3-ITD positive | 47 | 34 (9) | 13 (18) | |
| NPM1 mutated, FLT3-ITD positive | 45 | 31 (9) | 14 (19) | |
| CEBPAbiallelic, n (%) | .08 | |||
| Wild-type | 344 | 292 (99) | 52 (95) | |
| Mutated | 7 | 4 (1) | 3 (5) | |
| FLT3-ITD allelic ratio, n (%) | .23 | |||
| <0.5 | 46 | 36 (59) | 10 (43) | |
| ≥0.5 | 38 | 25 (41) | 13 (57) | |
| RUNX1, n (%) | .32 | |||
| Wild-type | 103 | 87 (86) | 16 (76) | |
| Mutated | 19 | 14 (14) | 5 (24) | |
| TP53, n (%) | >.99 | |||
| Wild-type | 109 | 90 (89) | 19 (90) | |
| Mutated | 13 | 11 (11) | 2 (10) | |
| ASXL1, n (%) | .73 | |||
| Wild-type | 114 | 94 (89) | 20 (87) | |
| Mutated | 15 | 12 (11) | 3 (13) | |
| Characteristics at HSCT | ||||
| Age at HSCT, y, median (range) | 59.6 (16.3-76.8) | 60.3 (16.3-76.0) | 59.6 (19.2-76.8) | .43 |
| Treatment cycles before HSCT, n (%) | <.001 | |||
| 1 | 157 | 72 (16) | 85 (71) | |
| 2 | 311 | 280 (61) | 31 (26) | |
| ≥3 | 111 | 107 (24) | 4 (3) | |
| Blood count regeneration at HSCT, n (%) | .41 | |||
| CR | 484 | 387 (84) | 97 (81) | |
| CRi | 96 | 73 (16) | 23 (19) | |
| MRD status at HSCT, n (%) | .10 | |||
| MRD− | 167 | 133 (58) | 34 (47) | |
| MRD+ | 133 | 95 (42) | 38 (53) | |
| Donor type, n (%) | .02 | |||
| Matched related | 124 | 109 (24) | 15 (13) | |
| Unrelated, HLA matched | 329 | 258 (56) | 71 (59) | |
| HLA mismatched | 112 | 80 (17) | 32 (27) | |
| Haploidentical | 14 | 12 (3) | 2 (2) | |
| Conditioning regimen, n (%) | .22 | |||
| NMA | 383 | 296 (64) | 87 (73) | |
| HSCT | 36 | 31 (7) | 5 (4) | |
| MAC | 161 | 133 (29) | 28 (23) | |
| HCT-CI score, n (%) | .59 | |||
| 0 | 244 | 195 (44) | 49 (42) | |
| 1 and 2 | 163 | 125 (26) | 38 (32) | |
| ≥3 | 159 | 129 (29) | 30 (26) | |
| CMV status, n (%) | .05 | |||
| Recipient+/donor− | 210 | 157 (35) | 53 (45) | |
| All others | 364 | 298 (65) | 66 (55) | |
| aGvHD ≥ grade 2, n (%) | .21 | |||
| Absent | 371 | 303 (74) | 68 (68) | |
| Present | 137 | 105 (26) | 32 (32) | |
| cGvHD, n (%) | .51 | |||
| Absent | 165 | 139 (41) | 26 (37) | |
| Limited | 58 | 45 (13) | 13 (19) | |
| Extended | 184 | 153 (45) | 31 (44) | |
| Donor sex, n (%) | .88 | |||
| All others | 489 | 388 (85) | 101 (86) | |
| Female into male | 82 | 66 (15) | 16 (14) | |
ASXL1, additional sex combs-like 1 gene; CEBPA, CCAAT/enhancer-binding protein α gene; CMV, cytomegalovirus; Hb, hemoglobin; HCT-CI, hematopoietic cell transplantation comorbidity index; NMA, nonmyeloablative conditioning; NPM1, nucleophosmin 1 gene; PB, peripheral blood; HSCT, reduced-intensity conditioning; RUNX1, runt-related transcription factor 1 gene; TP53, tumor protein 53 gene; WBC, white blood cell.
Cytogenetics and molecular markers
Cytogenetic analyses at diagnosis were performed using standard techniques of banding and in situ hybridization. The mutation status of the genes CEBPA and NPM1, as well as the presence or absence of internal tandem duplications in the FLT3 gene (FLT3-ITD), were evaluated as previously described.20 For patients with pretreatment samples available, at diagnosis, the mutation status of 54 genes included in the TruSight Myeloid Sequencing Panel (Illumina, San Diego, CA) was evaluated using next-generation sequencing, as previously described.21 ASXL1 mutations at codon 646 were validated in a proofreading polymerase–based Sanger sequencing approach.21 Patients were grouped according to ELN2017 recommendations.3
MRD assessment before allogeneic HSCT
For patients with remission samples up to 28 days before HSCT available (n = 300; n = 228 in first CR/CRi and n = 72 in second CR/CRi), the MRD status was assessed using digital droplet polymerase chain reaction (PCR) for at least 1 of the targets NPM1 mutations, BAALC/ABL1 copy numbers, and MN1/ABL1 copy numbers or using quantitative reverse transcriptase-PCR for WT1/ABL1 expression levels, adapting the previously published cutoffs.22-25 The pre-HSCT MRD status relied on 1 marker in 37 patients, 2 markers in 78 patients, 3 markers in 157 patients, and all 4 markers in 28 patients. For further analyses, patients with at least 1 positive test result were regarded as pre-HSCT MRD positive (MRD+). Only NPM1 droplet digital PCR results for patients who underwent HSCT after May 2015 (n = 24) were available to the transplanting physicians after introduction of the assay into the clinical routine. However, the outcome of patients with NPM1 mutations was not significantly different before or after May 2015 (supplemental Figure 1).
Statistical analyses
OS and RFS were calculated from HSCT until death from any cause and relapse or death from any cause, respectively. Survival estimates were calculated using the Kaplan-Meier method, and groups were compared using the log-rank test. The competing risks CIR and NRM were calculated from HSCT to relapse or death, respectively, using the Gray test.26 Associations with baseline clinical, demographic, and molecular features were compared by using the Wilcoxon rank-sum test and Fisher’s exact or χ2 (for categorical variables with more than 2 categories) tests for continuous and categorical variables, respectively. All P values are 2-sided, and the type I error rate is 5%. All statistical analyses were performed using the R statistical software platform (version 3.4.3).27 Multivariable analyses are described in the supplemental Information.
Results
Patients’ characteristics according to HSCT in the first vs the second CR/CRi
Compared with patients who underwent HSCT in the first CR/CRi, patients who underwent HSCT in the second CR/CRi harbored a variety of factors associated with beneficial outcomes at diagnosis, including a higher likelihood of having de novo AML (P = .002; Table 1), NPM1 mutation (P = .03), and a normal karyotype (P < .001), as well as a lower likelihood of having a monosomal (P < .001) or complex (P < .001) karyotype. Patients in the second CR/CRi were also more likely to have an FLT3-ITD (P < .001), whereas the ELN2017 genetic risk did not differ significantly between both groups. In addition, patients who underwent HSCT in the second CR/CRi had higher hemoglobin levels (P = .01), higher white blood cell counts (P = .006), and higher bone marrow blast counts at diagnosis (P = .03).
Before HSCT, patients who underwent transplantation in the second CR/CRi received significantly less chemotherapy (from relapse to HSCT; 71%, 26%, and 3% of patients received 1, 2, or 3 or more cycles, respectively) than transplant recipients in the first CR/CRi (diagnosis to HSCT; 16%, 61%, and 24% of patients received 1, 2, or 3 or more cycles, respectively; P < .001). They also more often received HSCT grafts from HLA mismatched donors and less often from HLA matched related donors (P = .01).
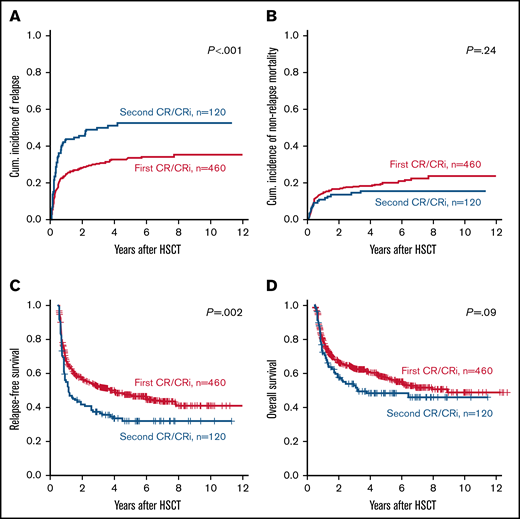
Distinct outcomes according to the number of remissions at HSCT
Despite a higher incidence of favorable-risk characteristics, patients who underwent HSCT in second CR/CRi had a significantly higher CIR (Figure 1A) and significantly shorter RFS (Figure 1C) than patients who underwent HSCT during the first CR/CRi, whereas OS did not differ significantly (Figure 1D), and NRM was similar in both groups (Figure 1B). Outcomes at 3 years after HSCT are given in supplemental Table 1. Also in multivariable analyses, the number of remissions (first vs second) remained a significant prognostic factor for CIR and RFS, but not for OS after adjustment for ELN2017 genetic risk and the MRD status at HSCT (Table 2). In separate multivariate analyses of patients undergoing HSCT in the first or second CR/CRi, the ELN2017 genetic risk and the MRD status before HSCT remained relevant prognostic factors. In addition, in patients who underwent HSCT in the first CR/CRi, more intensive conditioning regimens were associated with better OS, whereas in patients who underwent HSCT in the second CR/CRi, a remission duration of >1 year before first relapse was associated with lower CIR and longer RFS. Causes of deaths are given in the supplemental Information and supplemental Table 2.
Outcomes of patients with AML according to the number of CRs/CRis (first vs second) at allogeneic HSCT (n = 580). (A) CIR, (B) NRM, (C) RDS, and (D) OS.
Outcomes of patients with AML according to the number of CRs/CRis (first vs second) at allogeneic HSCT (n = 580). (A) CIR, (B) NRM, (C) RDS, and (D) OS.
Multivariable analyses
| . | Cumulative incidence of relapse/progression . | RFS . | OS . | |||
|---|---|---|---|---|---|---|
| sHR (95% CI)* . | P . | HR (95% CI)† . | P . | HR (95% CI)† . | P . | |
| All patients‡ | ||||||
| ELN2017 genetic risk (adverse vs intermediate vs favorable) | 1.89 (1.43-2.51) | <.001 | 1.99 (1.51-2.62) | <.001 | 1.56 (1.28-1.90) | <.001 |
| Number of remission at HSCT (second vs first) | 3.09 (1.85-5.17) | <.001 | 2.84 (1.79-4.51) | <.001 | — | — |
| Age at HSCT | — | — | — | — | 1.03 (1.02-1.05) | <.001 |
| Pre-HSCT remission status (MRD+ vs MRD−) | 2.77 (1.77-4.33) | <.001 | 2.73 (1-76-4.24) | <.001 | — | — |
| HSCT in first CR/CRi§ | ||||||
| ELN2017 genetic risk (adverse vs intermediate vs favorable) | 2.28 (1.36-3.82) | .002 | 2.22 (1.55-3.17) | <.001 | 1.58 (1.26-1.98) | <.001 |
| Pre-HSCT remission status (MRD+ vs MRD−) | 2.05 (1.43-2.93) | <.001 | 2.37 (1.40-4.00) | .001 | — | — |
| Conditioning regimen (MAC vs HSCT/NMA) | — | — | — | — | 0.66 (0.53-0.82) | <.001 |
| HSCT in second CR/CRiǁ | ||||||
| ELN2017 genetic risk (adverse vs intermediate vs favorable) | — | — | 1.57 (1.00-2.46) | .05 | 1.59 (1.04-2.45) | .03 |
| Duration of first remission (≥ 1 y vs <1 y) | 0.31 (0.16-0.59) | <.001 | 0.18 (0.07-0.51) | .001 | — | — |
| Pre-HSCT remission status (MRD+ vs MRD−) | 2.86 (1.44-5.69) | .003 | 4.04 (1.68-9.72) | .002 | — | — |
| . | Cumulative incidence of relapse/progression . | RFS . | OS . | |||
|---|---|---|---|---|---|---|
| sHR (95% CI)* . | P . | HR (95% CI)† . | P . | HR (95% CI)† . | P . | |
| All patients‡ | ||||||
| ELN2017 genetic risk (adverse vs intermediate vs favorable) | 1.89 (1.43-2.51) | <.001 | 1.99 (1.51-2.62) | <.001 | 1.56 (1.28-1.90) | <.001 |
| Number of remission at HSCT (second vs first) | 3.09 (1.85-5.17) | <.001 | 2.84 (1.79-4.51) | <.001 | — | — |
| Age at HSCT | — | — | — | — | 1.03 (1.02-1.05) | <.001 |
| Pre-HSCT remission status (MRD+ vs MRD−) | 2.77 (1.77-4.33) | <.001 | 2.73 (1-76-4.24) | <.001 | — | — |
| HSCT in first CR/CRi§ | ||||||
| ELN2017 genetic risk (adverse vs intermediate vs favorable) | 2.28 (1.36-3.82) | .002 | 2.22 (1.55-3.17) | <.001 | 1.58 (1.26-1.98) | <.001 |
| Pre-HSCT remission status (MRD+ vs MRD−) | 2.05 (1.43-2.93) | <.001 | 2.37 (1.40-4.00) | .001 | — | — |
| Conditioning regimen (MAC vs HSCT/NMA) | — | — | — | — | 0.66 (0.53-0.82) | <.001 |
| HSCT in second CR/CRiǁ | ||||||
| ELN2017 genetic risk (adverse vs intermediate vs favorable) | — | — | 1.57 (1.00-2.46) | .05 | 1.59 (1.04-2.45) | .03 |
| Duration of first remission (≥ 1 y vs <1 y) | 0.31 (0.16-0.59) | <.001 | 0.18 (0.07-0.51) | .001 | — | — |
| Pre-HSCT remission status (MRD+ vs MRD−) | 2.86 (1.44-5.69) | .003 | 4.04 (1.68-9.72) | .002 | — | — |
CI, confidence interval.
sHR, substitute hazard ratio, indicates lower (higher) risk of relapse for the first category listed for the dichotomous variables or for the lower (higher) values of the continuous variables.
HR, hazard ratio, <1 (>1) indicate lower (higher) risk of death or relapse for the first category listed for the dichotomous variables. Variables considered in the models were those significant at α = 0.10 in univariate analyses.
Multivariable model for the whole patient cohort: For CIR end point, variables considered were patient sex, ELN2017 genetic risk group, conditioning regimen (NMA/HSCT vs MAC), pre-HSCT remission status (MRD+ vs MRD−), number of remissions at HSCT (second vs first), and age at HSCT. For RFS end point, variables considered were patient sex, ELN2017 genetic risk group, conditioning regimen (NMA/HSCT vs MAC), pre-HSCT remission status (MRD+ vs MRD−), number of remission at HSCT (second vs first), and age at HSCT. For OS end point, variables considered were disease origin (secondary or therapy-related vs de novo), ELN2017 genetic risk group, donor type (matched related vs matched unrelated vs mismatched unrelated), number of chemotherapy cycles before HSCT, conditioning regimen (NMA/HSCT vs MAC), pre-HSCT remission status (MRD+ vs MRD−), number of remission at HSCT (second vs first), and age at HSCT.
Multivariable model for patients who underwent HSCT in first CR/CRi: for the CIR end point, variables considered were patient sex, ELN2017 genetic risk group, conditioning regimen (NMA/HSCT vs MAC), pre-HSCT remission status (MRD+ vs MRD−), and age at HSCT. For the RFS end point, variables considered were disease origin (secondary or therapy-related vs de novo), ELN2017 genetic risk group, conditioning regimen (NMA/HSCT vs MAC), pre-HSCT remission status (MRD+ vs MRD−), and age at HSCT. For OS end point, variables considered were disease origin (secondary or therapy-related vs de novo), ELN2017 genetic risk group, number of chemotherapy cycles before HSCT, conditioning regimen (NMA/HSCT vs MAC), pre-HSCT remission status (MRD+ vs MRD−), and age at HSCT.
Multivariable model for patients who underwent HSCT in the second CR/CRi: for the CIR end point, variables considered were patient sex, ELN2017 genetic risk group, duration of first remission (>1 y vs <1 y), conditioning regimen (NMA/HSCT vs MAC), and pre-HSCT remission status (MRD+ vs MRD−). For the RFS end point, the variables were disease origin (secondary or therapy-related vs de novo), ELN2017 genetic risk group, duration of first remission (>1 y vs <1 y), conditioning regimen (NMA/HSCT vs MAC), pre-HSCT remission status (MRD+ vs MRD−), and age at HSCT. For OS end point, the variables were disease origin (secondary or therapy-related vs de novo), ELN2017 genetic risk group, conditioning regimen (NMA/HSCT vs MAC), and age at HSCT.
Outcomes according to MRD at HSCT during the first or second CR/CRi
In patients who underwent HSCT in the first or second CR/CRi, the MRD status before allogeneic HSCT was a significant prognostic factor for a higher CIR (each P < .001; Figure 2A) and shorter RFS (P = .002 and P = .04, respectively; Figure 2B). In contrast, OS was not significantly different in patients who underwent HSCT in the first (P = .07) or second (P > .99) CR/CRi; Figure 2C), according to MRD status at HSCT. Comparable outcomes were noted when the 4 MRD markers were regarded separately (supplemental Figure 2).
Outcomes of patients with AML according to the number of CRs/CRis (first vs second) and the MRD status at allogeneic HSCT (positive vs negative; n = 300). (A) CIR. MRD+ vs MRD−: first CR/CRi (P < .001) and second CR/CRi (P < .001). (B) RFS. MRD+ vs MRD− first CR/CRi (P = .002) and second CR/CRi (P = .04). (C) OS. MRD+ vs MRD−: first CR/Cri (P = .07) and second CR/Cri (P > .99).
Outcomes of patients with AML according to the number of CRs/CRis (first vs second) and the MRD status at allogeneic HSCT (positive vs negative; n = 300). (A) CIR. MRD+ vs MRD−: first CR/CRi (P < .001) and second CR/CRi (P < .001). (B) RFS. MRD+ vs MRD− first CR/CRi (P = .002) and second CR/CRi (P = .04). (C) OS. MRD+ vs MRD−: first CR/Cri (P = .07) and second CR/Cri (P > .99).
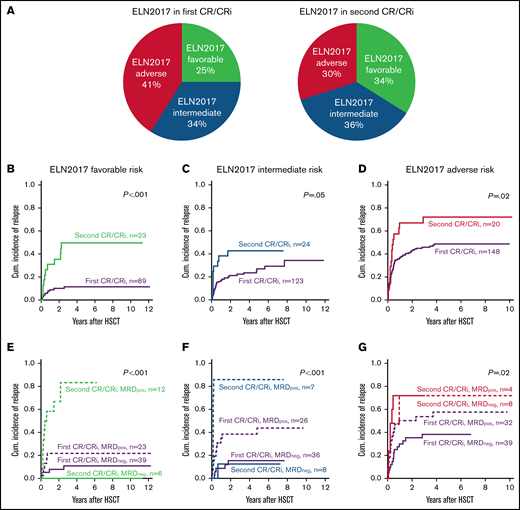
Composition of the 3 ELN2017 genetic risk groups in patients who underwent HSCT in the first or second CR/CRi
The distribution of the 3 ELN2017 risk groups did not differ significantly in those undergoing HSCT during the first or second CR/CRi (P = .15; Figure 3A).
Distribution and outcomes of patients that underwent allogeneic HSCT during first or second remission according to the ELN2017 genetic risk groups. (A) Distribution of the ELN2017 risk groups of patients who underwent allogeneic HSCT during the first or second CR/CRi. (B-D) CIR in patients with AML according to the number of CRs/CRis (first vs second) at allogeneic HSCT in the 3 ELN2017 genetic risk groups. ELN2017 favorable risk (B; n = 112), ELN2017 intermediate risk (C; n = 147), and ELN2017 adverse risk (D; n = 158). (E-G) Cumulative incidences of relapse of patients with AML according to the number of CRs/CRis (first vs second) and the MRD status at allogeneic HSCT (positive vs negative) in the 3 ELN2017 genetic risk groups. (E) ELN2017 favorable risk (n = 80), MRD+ vs MRD−: first CR/CRi (P = .21) and second CR/CRi (P = .003). (F) ELN2017 intermediate risk (n = 77), MRD+ vs MRD−: first CR/CRi (P = .02) and second CR/CRi (P = .002). (G) ELN2017 adverse risk (n = 83), MRD+ vs MRD-−: first CR/CRi (P = .06) and second CR/CRi (P = .81).
Distribution and outcomes of patients that underwent allogeneic HSCT during first or second remission according to the ELN2017 genetic risk groups. (A) Distribution of the ELN2017 risk groups of patients who underwent allogeneic HSCT during the first or second CR/CRi. (B-D) CIR in patients with AML according to the number of CRs/CRis (first vs second) at allogeneic HSCT in the 3 ELN2017 genetic risk groups. ELN2017 favorable risk (B; n = 112), ELN2017 intermediate risk (C; n = 147), and ELN2017 adverse risk (D; n = 158). (E-G) Cumulative incidences of relapse of patients with AML according to the number of CRs/CRis (first vs second) and the MRD status at allogeneic HSCT (positive vs negative) in the 3 ELN2017 genetic risk groups. (E) ELN2017 favorable risk (n = 80), MRD+ vs MRD−: first CR/CRi (P = .21) and second CR/CRi (P = .003). (F) ELN2017 intermediate risk (n = 77), MRD+ vs MRD−: first CR/CRi (P = .02) and second CR/CRi (P = .002). (G) ELN2017 adverse risk (n = 83), MRD+ vs MRD-−: first CR/CRi (P = .06) and second CR/CRi (P = .81).
In the favorable ELN2017 risk group, there were no significant differences in disease risk in patients who underwent HSCT in the first or second CR/CRi (supplemental Table 3). In the intermediate ELN2017 risk group, patients who underwent HSCT in the second CR/CRi more often had de novo AML (P = .04; supplemental Table 4). In the adverse ELN2017 risk group, patients who underwent HSCT in the second CR/CRi less often had a complex (P < .001) or monosomal (P < .001) karyotype, but more often had a normal (P < .001) one. In addition, they more often harbored a FLT3-ITD (P = .01), but had comparable incidences of ASXL1, RUNX1, and TP53 mutations (supplemental Table 5).
Outcomes in the ELN2017 risk groups
When the 3 ELN2017 risk groups were regarded separately, patients who underwent HSCT during the second CR/Cri consistently had a higher CIR compared with patients in the first CR/CRi (Figure 3B-D). This translated into an optical separation of RFS curve in patients with favorable ELN2017 risk (P = .07; supplemental Figure 3A-B) and shorter OS in patients with intermediate ELN2017 risk (P = .05; supplemental Figure 4B). In contrast, despite an optical separation of the outcome curves, OS and RFS were not significantly different between patients with adverse ELN2017 risk who underwent HSCT during the first or second CR/CRi (P = .50 and P = .30, respectively; supplemental Figure 5A-B).
In the favorable ELN2017 risk group, we observed a more pronounced impact of MRD status in patients who underwent HSCT during the second than during the first CR/CRi (CIR P = .003 vs P = .21, Figure 3E; RFS P = .02 vs P = .10, and OS P = .30 vs P = .07; supplemental Figure 3C-D). In patients with intermediate ELN2017 risk, the MRD status at HSCT affected CIR and RFS, irrespective of the number of remissions (second vs first CR/CRi, CIR: P = .002 vs P = .02, Figure 4F; RFS: P = .006 vs P = .05; supplemental Figure 4C), but did not translate into significantly different OS (P = .20 vs P = .30; supplemental Figure 4D). In patients with an adverse ELN2017 risk, only the MRD status in patients who underwent HSCT during the first CR/CRi affected CIR (P = .06; Figure 5G) and RFS (P = .05; supplemental Figure 5C), but not in patients who underwent HSCT in the second CR/CRi, as they had similarly high relapse rates.
An overview of the MRD results of each patient, their ELN2017 risk group, the number of remissions, and conditioning intensity are given in supplemental Table 6.
Discussion
When treating patients with AML, the decision for and the right timing of allogeneic HSCT remain a challenging task. Although it is often applied in patients who have achieved a first remission, select patient groups may benefit from a strategy that postpones allogeneic HSCT consolidation until after the first relapse. However, data on outcomes that compare the results of allogeneic HSCT performed after the first or second remission remain sparse.
Overall, in the analyzed cohort, undergoing a transplant during the second remission increased the risk of relapse ∼3 times (Table 2). Because of common treatment strategies, the patient cohort that had HSCT during the second remission was enriched for favorable diagnostic risk factors, including a higher incidence of NPM1 mutations and lower incidences of monosomal or complex karyotypes, as well as secondary AML, whereas the ELN2017 genetic risk did not differ significantly. Nevertheless, these patients had a higher CIR and shorter RFS than did patients who underwent HSCT during the first remission. This difference in outcome also remained significant in multivariable analyses for the whole patient cohort (Table 2) and confirms a previous analysis of patients who undergo myeloablative or reduced-intensity conditioning during the second remission.13
In terms of risk factors for patients with AML who undergo HSCT during the second remission, 2 retrospective studies found a low HCT-comorbidity index risk score and longer duration of first remission to be favorable prognostic factors.28,29 In both analyses the majority of individuals were younger (75% and 76% >55 years of age, respectively) and underwent myeloablative conditioning (69% and 72%, respectively).28,29 In addition, the diagnostic cytogenetic risk was observed as prognostically relevant,30,31 but that association was not consistently found in all studies.28 The data presented herein expand the knowledge of risk factors associated with HSCT performed during the second remission, including the current ELN2017 genetic risk stratification, the MRD status at HSCT, and a longer duration of first remission (>1 year; Table 2). Compared with patients in previous studies, most of our patients were older, reflecting the general age at AML diagnosis, and subsequently underwent less-intensive conditioning regimens.
The evaluation of MRD during and after AML treatment was identified as an important tool for estimating outcomes,32 including before allogeneic HSCT.6,15,33 In our analysis, the MRD status at HSCT in univariate and multivariable analyses was an important prognosticator in patients who underwent HSCT during the first or second remissions, increasing the risk of relapse ∼2 to 3 times (Table 2). Two previous retrospective analyses reported on the significance of MRD evaluation in patients who underwent HSCT in the second remission. Walter et al suggested equally poor outcomes of patients who were flow MRD+, irrespective of the number of remissions at HSCT,15 whereas we observed worse outcomes for patients in the second CR/CRi and also when the MRD status at HSCT was considered (Figure 2). Besides the different method of MRD assessment used by Walter et al, all patients had myeloablative conditioning. When we restricted our analysis to patients who underwent HSCT after myeloablative conditioning, with the caveat of a limited number of patients, patients who were MRD+ and underwent HSCT in the first or second remission had a similar high CIR and short RFS (supplemental Figure 6A-B). In contrast, patients who were MRD+ and underwent HSCT after nonmyeloablative or reduced-intensity conditioning had a high CIR and short RFS, especially when HSCT was performed during the second remission (supplemental Figure 6C-D). Although one could speculate that patients who are MRD+ and achieve a second CR/CRi may benefit from intensification of conditioning, as compared with reduced-intensity conditioning in younger individuals,31 the number of patients after myeloablative conditioning was limited in our analysis, and another study did not find such benefits.16 Finally, the benefit of a dose intensification before allogeneic HSCT in patients who were MRD+, also irrespective of the number of remissions, still remains unclear, as there is evidence of better OS after myeloablative compared with reduced-intensity conditioning in 1 study,34 but none for intensification of reduced-intensity conditioning in another study.35
Similar to our analysis a recent large European Society for Blood and Marrow Transplantation evaluation of the significance of transplant center–reported MRD status at HSCT showed distinct CIR and RFS, but no significant impact on OS in patients with AML treated with HSCT during the second remission.16 Despite no clear data on the method of MRD assessment and with the genetic risk assessed only by cytogenetics, the risk factors identified as significant for outcomes after allogeneic HSCT during the second remission confirm our findings.
Although, during the first remission, an allogeneic HSCT is usually offered to patients with AML with intermediate or adverse risk, some patients, especially those with intermediate risk, may benefit from delaying allogeneic HSCT until after the first relapse.13,14 It is important, however, to keep in mind that in approximately half of the patients with AML who relapse after chemotherapy, consolidation will not achieve a second remission that allows for HSCT13,14 and will have a significant survival disadvantage compared with patients who undergo allogeneic HSCT after achieving a second remission.13 However, it remains unclear whether an allogeneic HSCT during the first remission improves outcomes in all patients with AML of intermediate or adverse risk,36,37 or whether there are subgroups that may benefit from a transplant during the second remission. To our knowledge, our study is the first to incorporate the ELN2017 genetic risk stratification into outcome analyses of patients who undergo HSCT during the first or second remission. In the favorable risk group, OS was improved irrespective of the number of remissions at HSCT, and the MRD status affected outcomes similarly after HSCT during the first or second remission. In these patients, relapses are salvageable, indicating that in this group we are generally safe to delay an allograft until after the first relapse. For the clinically relevant ELN2017 intermediate risk group for which the optimal consolidation (chemotherapy vs allogeneic HSCT) remains a matter of debate, patients who were MRD− had favorable outcomes irrespective of the number of remissions (at 3 years, CIR 15% and 13%, respectively, and RFS, 64% and 63%, respectively). Outcomes were intermediate in patients who were MRD+ and underwent HSCT during the first remission (at 3 years, CIR 38% and RFS 46%) and poor in patients who were MRD+ and underwent HSCT in the second remission (at 3 years, CIR 86% and RFS 14%, Figure 3F; supplemental Figure 3D). Also bearing in mind that there may be a lower likelihood of achieving an MRD− second remission, our data support an allogeneic HSCT after achieving the first (ideally MRD−) remission. Patients with ELN2017 adverse risk had inferior outcomes, with the best long-term survival observed in patients who underwent HSCT during the first remission, which strengthens support for the early use of an allograft in this patient population. The additional clinical value of MRD analysis at HSCT in patients with ELN2017 adverse risk remain limited (Figure 3G), an observation we previously discussed as potentially related to the overall high relapse risk in the ELN2017 adverse risk group.38
Recently, some clinical trials demonstrated a benefit for maintenance strategies after chemotherapy39 or allogeneic HSCT40 consolidation. In the future, this development in conjunction with MRD-guided novel treatment approaches may have influence, not only on the timing, but also on the remission status at allogeneic HSCT, as well as on the risk of relapse during follow-up. These advancements hold promise for attenuating the risk factors and outcomes identified herein.
Our study was limited by its retrospective nature and the restricted size of the patient groups in the subanalyses, as well as the lack of an unbiased control patient set consolidated with chemotherapy alone. We also lacked systematic MRD assessments during chemotherapy cycles and after HSCT, which could have provided additional important outcome data and information on disease dynamics. However, in general, patients who underwent HSCT during the second remission had adverse outcomes compared with those who underwent HSCT during the first remission. The study indicates that the MRD status at HSCT is highly relevant in the first and second remissions. For patients with intermediate risk, those who were MRD+during the second remission and underwent HSCT fared worst. Subsequently, an allogeneic HSCT in MRD+ second remission should be avoided. This should be considered when discussing and planning individual consolidation treatments in AML. The benefit of additional therapies and therapeutics, including agents targeting specific molecular aberrations, such as IDH or FLT3 inhibitors, before or after HSCT may improve outcomes of eligible patients with MRD+AML, especially when they undergo HSCT in a second remission.
Acknowledgments
The authors thank Christel Müller, Daniela Bretschneider, Evelin Hennig, Sabine Leiblein, Martina Pleß, Ulrike Bergmann, Janet Bogardt, Annette Jilo, and Dagmar Cron for help in determining cytogenetic, morphologic, and immunological analyses; Marius Bill, Karoline Goldmann, and Juliane Grimm for help with the molecular analyses; and Scarlett Schwabe, Ines Kovacs, Claudia Diener, and Manuela Quandt for help with sample processing.
This study was supported by the Deutsche Gesellschaft für Innere Medizin (Clinician Scientist Program [M.J.]), Ein Herz für Kinder e.V., the Verein Zusammen gegen den Krebs e.V. (S.S.), the Deutsche Jose-Carreras-Stiftung (04R/2016) (S.S.), and an intramural scholarship of the University of Leipzig (L.B.).
Authorship
Contribution: M.J. and S.S. contributed to the design and analysis of this study and the writing of the manuscript, and all authors agreed on the final version; M.J., D. Brauer, D. Backhaus, L.B., and J.S. performed the laboratory-based research; M.J. and S.S. performed statistical analyses; and G.-N.F., V.V., D.N., U.P., and S.S. provided administrative support.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sebastian Schwind, Medical Clinic and Policlinic 1, Hematology Cellular Therapy and Hemostaseology, Leipzig University Hospital, Liebigstraße 22, Haus 7, 04103 Leipzig, Germany; e-mail: Sebastian.Schwind@medizin.uni-leipzig.de.
References
Author notes
Presented in abstract form (416) at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, December 2021.
For data sharing, contact the corresponding author (Sebastian.Schwind@medizin.uni-leipzig.de).
The full-text version of this article contains a data supplement.