Key Points
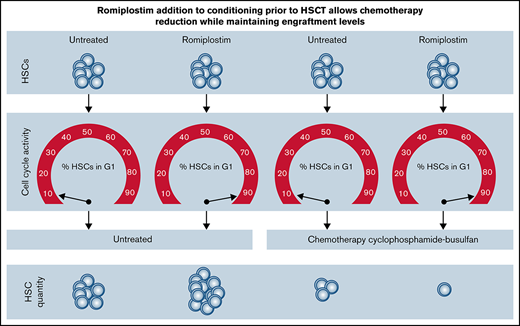
Romiplostim sensitizes HSCs to a clinically used chemotherapy-conditioning regimen.
Romiplostim allows reducing busulfan dose in conditioning while maintaining similar HSC engraftment levels in a mouse HSCT model.
Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) offers a curative treatment approach for certain benign and malignant hematologic diseases. The actual HSCT is preceded by a conditioning therapy that reduces host-vs-HSCT graft rejection and creates niche space for transplanted hematopoietic stem and progenitor cells (HSPCs). Conditioning consists of chemotherapy with or without irradiation and is a major cause of side effects in HSCT. However, reduction of the intensity of cytotoxic conditioning leads to higher rates of engraftment failure and increased rates of relapse. We here tested if the addition of an HSC cycling inducing agent during conditioning allows to diminish the dose of conditioning drugs without reducing subsequent transplanted HSC engraftment in a mouse HSCT model. The thrombopoietin receptor agonist romiplostim was shown to induce cell cycling activity in hematopoietic stem cells (HSCs). We thus tested if the addition of romiplostim to the clinically applied conditioning chemotherapy regimen cyclophosphamide and busulfan leads to increased efficacy of the chemotherapeutic regimen. We found that romiplostim not only sensitizes HSCs to chemotherapy but also enables a reduction of the main chemotherapeutic component busulfan by half while HSC engraftment levels are maintained in long-term, serial transplantation assays.
Introduction
Hematopoietic stem cells (HSCs) ensure life-long production of mature blood cells. However, most HSCs are quiescent and only rarely divide in steady state.1,2 Physiological stimuli such as inflammation and pharmacological stimuli like thrombopoietin receptor agonists can drive HSCs into active cell cycling, resulting in self-renewing divisions.3,4 Dividing cells are more susceptible to chemotherapy.5 One of the best studied and applied myeloablative conditioning regimen for patients undergoing hematopoietic stem cell transplantation (HSCT) is a combination of the alkylating drugs cyclophosphamide (Cy) and busulfan (Bu).6 Though highly effective, this conditioning regimen is associated with substantial toxicity and can contribute to nonrelapse mortality.7-9 Thus, reducing toxicity while maintaining conditioning efficacy is highly desirable. We here provide preclinical evidence for the benefit of adding the thrombopoietin receptor agonist romiplostim (RPL) to the conditioning regimen cyclophosphamide-busulfan (CyBu). Adding RPL to CyBu increases on-target toxicity on HSCs and thus allows the reduction of the busulfan dose while maintaining similar HSC engraftment levels in a mouse model for HSCT.
Methods
Mice
All procedures involving experimental animals were performed according to protocols approved by the Cantonal Veterinary Office Zurich (licenses #163/16 and #103/2020).
Chemotherapy conditioning
Cyclophosphamide was administered at a dose of 100 mg/kg followed by busulfan at doses of either twice 5 mg/kg (CyBu10), twice 15 mg/kg (CyBu30), or thrice 20 mg/kg (CyBu60). Doses were adapted from previous studies,10,11 including the order of cycophophamide before busulfan due to lower toxicity as compared with BuCy in mice and humans.9,10
Further methods are provided in supplemental Methods.
Results and discussion
We recently demonstrated that the thrombopoietin receptor agonist RPL induces self-renewing divisions in HSCs.4 Further, there is indirect evidence that thrompoietin receptor agonists expand human hematopoietic stem and progenitor cells (HSPCs) when applied in aplastic anemia.12,13 Given the assumption that cycling cells are more susceptible to cytotoxic treatment, we here tested the hypothesis that adding RPL to chemotherapy in mice increases the susceptibility of HSCs to cytotoxic treatment with cyclophosphamide and busulfan.
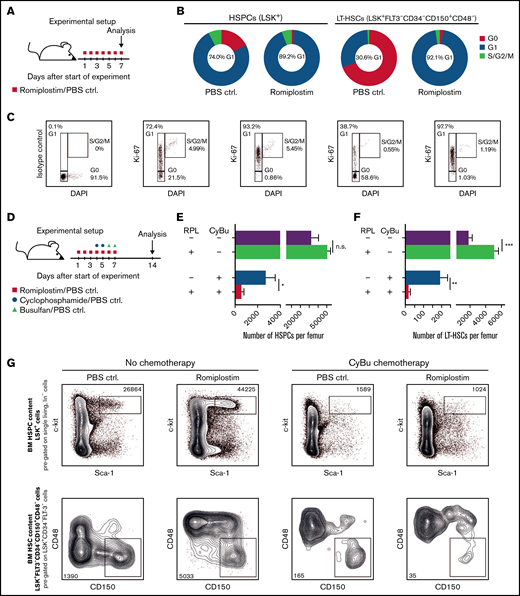
In a first step, we analyzed the impact of RPL on cell cycle activity of phenotypically defined HSPCs, (LSK+) as well as of LT-HSCs (LSK+CD34−FLT-3−CD48−CD150+, experimental setup depicted in Figure 1A). Confirming and extending our prior observations,4 we found that after 7 days of in vivo RPL treatment, HSPCs, and especially LT-HSCs, show a substantial shift from G0 to G1 cell cycle status (Figure 1B-C).
Romiplostim drives HS(P)Cs into active cell cycling and sensitizes them to CyBu chemotherapy. (A) The experimental setup to assess cell cycle activity upon RPL/PBS stimulation; BM is analyzed after 7 days of RPL/PBS application. (B) The cell cycle status of phenotypically defined HSPCs (LSK+) and LT-HSCs (LSK+FLT-3−CD34−CD150+CD48−) with or without RPL/PBS stimulation (n = 5 per group). (C) Representative flow cytometry plots from panel B. (D) The experimental setup to assess the impact of RPL on phenotypically defined HSPCs and LT-HSCs upon simultaneous treatment with CyBu30 chemotherapy; BM is analyzed 14 days after the start of RPL/PBS application equivalent to 7 days after the last dose of chemotherapy and RPL/PBS (n = 5 per group). The number of HSPCs (E) and the number of LT-HSCs (F) per 1 femur 1 week after treatment with RPL/PBS and CyBu30. (G) Representative flow cytometry plots from (E) and (F). BM, bone marrow; ctrl, control; DAPI, 4′,6-diamidino-2-phenylindole; PBS, phosphate-buffered saline.
Romiplostim drives HS(P)Cs into active cell cycling and sensitizes them to CyBu chemotherapy. (A) The experimental setup to assess cell cycle activity upon RPL/PBS stimulation; BM is analyzed after 7 days of RPL/PBS application. (B) The cell cycle status of phenotypically defined HSPCs (LSK+) and LT-HSCs (LSK+FLT-3−CD34−CD150+CD48−) with or without RPL/PBS stimulation (n = 5 per group). (C) Representative flow cytometry plots from panel B. (D) The experimental setup to assess the impact of RPL on phenotypically defined HSPCs and LT-HSCs upon simultaneous treatment with CyBu30 chemotherapy; BM is analyzed 14 days after the start of RPL/PBS application equivalent to 7 days after the last dose of chemotherapy and RPL/PBS (n = 5 per group). The number of HSPCs (E) and the number of LT-HSCs (F) per 1 femur 1 week after treatment with RPL/PBS and CyBu30. (G) Representative flow cytometry plots from (E) and (F). BM, bone marrow; ctrl, control; DAPI, 4′,6-diamidino-2-phenylindole; PBS, phosphate-buffered saline.
In order to test our hypothesis that RPL sensitizes HSCs to chemotherapy, we next treated mice with RPL or PBS, followed by cyclophosphamide/busulfan (CyBu30) (experimental setup outlined in Figure 1D). We then assessed the number of HSPCs and LT-HSCs in the BM of mice 7 days after the last application of chemotherapy. As previously shown, RPL treatment alone expands HSPCs and LT-HSCs.4 In strong contrast, RPL in combination with the chemotherapy regimen CyBu30 significantly reduced HSPCs and LT-HSCs counts, indicating an enhanced HSPC and HSC depletion effect of CyBu through RPL (Figure 1E-G). We also tested RPL application in combination with cyclophospamide or busulfan monotherapy and found that the effect was less pronounced compared with CyBu combination therapy (supplemental Figure 1A-F).
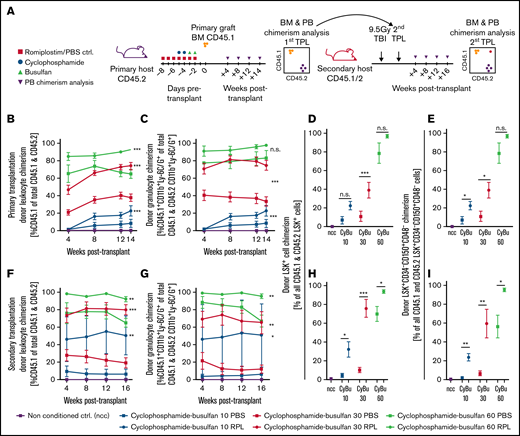
We next set out to test increased chemotherapy susceptibility of phenotypically defined HSPCs on a functional level. To this end, we implemented RPL into a CyBu conditioning regimen in an HSCT model. In this setting, we measured donor HSPC engraftment-chimerism as a surrogate parameter for on-target CyBu toxicity, assuming that a higher donor chimerism in recipient mice reflects an enhanced cytotoxic conditioning and clearing effect on recipient HSPCs (experimental setup shown in Figure 2A). In order to study if and in how far the dose of busulfan can potentially be decreased through the addition of RPL, we tested different dose levels of busulfan while leaving the dose of cyclophosphamide unchanged.10 We found that when RPL was added to CyBu10 and to CyBu30 conditioning regimen, a significantly higher donor leukocyte, granulocyte, and phenotypic LT-HSC chimerism was achieved at 14 weeks posttransplant in primary recipients (Figure 2B-E). Although numerically the chimerism was higher in the CyBu60-RPL conditioned animals, this difference was not significant. This is possibly due to the fact that conditioning is very efficient at the high doses of CyBu with the donor chimerism reaching almost 100% in the HSPC compartment in primary HSPC transplantation recipients. Importantly, we measured significantly higher engraftment levels in total leukocytes, granulocytes, LSK+ cells, and LT-HSCs in all busulfan dosing groups up to 16 weeks after secondary transplantation, the longest time-point analyzed (Figure 2F-I). Interestingly, the CyBu30-RPL regimen exhibited comparable chimerism values to CyBu60-PBS (Figure 2H-I), whereas CyBu10-RPL showed an enhanced effect as compared to CyBu30-PBS. RPL in combination with busulfan monotherapy did not substantially alter chimerism levels (supplemental Figure 2A-I,K).
Romiplostim sensitizes HS(P)Cs to CyBu chemotherapy in a preclinical HSCT model and allows a dose reduction of busulfan while maintaining a stable long-term chimerism. (A) The experimental setup to assess the effects of RPL on chemosensitivity to CyBu conditioning therapy in functionally defined long-term HSCs; animals were treated with RPL/PBS and cyclophosphamide and busulfan as also described in Figure 1D but in contrast received BM transplant after their course of chemotherapy. Experimental animals were followed for 14 weeks and bled regularly before their BM was analyzed and transplanted into secondary recipients and observed there for another 16 weeks. The peripheral blood donor leukocyte chimerism (CD45.1/CD45.2) (B) and the donor granulocyte chimerism (CD45.1/CD45.2 CD11b+Ly-6C/G+) (C) in primary transplant recipient mice. (D-E) The phenotypic HSPC and HSC chimerisms of primary transplant recipients 14 weeks after primary transplantation. (F-G) The peripheral blood donor leukocyte and donor granulocyte chimerisms in secondary transplant recipient mice. (H-I) The BM HSPC and HSC chimerisms of secondary transplant recipient mice. The number of mice used for this experimental setup is depicted in supplemental Figure 2J. ctrl, control; ncc, nonconditioned control; TBI, total body irradiation.
Romiplostim sensitizes HS(P)Cs to CyBu chemotherapy in a preclinical HSCT model and allows a dose reduction of busulfan while maintaining a stable long-term chimerism. (A) The experimental setup to assess the effects of RPL on chemosensitivity to CyBu conditioning therapy in functionally defined long-term HSCs; animals were treated with RPL/PBS and cyclophosphamide and busulfan as also described in Figure 1D but in contrast received BM transplant after their course of chemotherapy. Experimental animals were followed for 14 weeks and bled regularly before their BM was analyzed and transplanted into secondary recipients and observed there for another 16 weeks. The peripheral blood donor leukocyte chimerism (CD45.1/CD45.2) (B) and the donor granulocyte chimerism (CD45.1/CD45.2 CD11b+Ly-6C/G+) (C) in primary transplant recipient mice. (D-E) The phenotypic HSPC and HSC chimerisms of primary transplant recipients 14 weeks after primary transplantation. (F-G) The peripheral blood donor leukocyte and donor granulocyte chimerisms in secondary transplant recipient mice. (H-I) The BM HSPC and HSC chimerisms of secondary transplant recipient mice. The number of mice used for this experimental setup is depicted in supplemental Figure 2J. ctrl, control; ncc, nonconditioned control; TBI, total body irradiation.
In sum, we here show that adding RPL to CyBu conditioning prior to HSCT aids in achieving a comparable, stable donor chimerism with only half-dose busulfan than could be achieved without RPL. Mechanistically, this is likely due to RPL-induced HSPC cycling and therefore chemotherapy sensitization leading to a more efficient depletion of recipient HSPCs and thus more efficient donor HSCT engraftment.
We assume that reduction of busulfan will allow to indeed reduce short- and long-term toxicity in HSPC transplant recipients. However, the model used did not allow to indeed test this specific hypothesis. Also, we did not determine if combined RPL-chemotherapy conditioning might possibly lead to a survival advantage or alternatively to a desirable chemo-sensitization effect on malignant HSPCs such as acute myeloid leukemia or myelodysplastic syndrome cells.
Although these aspects will need evaluation in further studies, we believe that our data provide a stimulus to explore the addition of RPL in the context of chemotherapy and HSCT-conditioning approaches.
Acknowledgments
This work was supported by research grants of the Swiss National Science Foundation (310030B_166673/1) to M.G.M. and by a research grant of the Stiftung zur Förderung der Knochenmarktransplantation (SFK) to C.M.W.
Authorship
Contribution: C.M.W. and L.V.K. devised, performed, and analyzed experiments and wrote the manuscript; and M.G.M. directed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus G. Manz, Department of Medical Oncology and Hematology, Comprehensive Cancer Center Zurich (CCCZ), University Hospital Zurich and University of Zurich, Raemistrasse 100, CH-8091 Zurich, Switzerland; e-mail: markus.manz@usz.ch.
References
Author notes
All datasets and protocols will be made available upon reasonable request to the corresponding author: markus.manz@usz.ch.
The full-text version of this article contains a data supplement.