Key Points
Single-agent oral factor B inhibition led to rapid improvements of hemolytic markers, Hb levels, and transfusion requirements in PNH.
Abstract
Iptacopan (LNP023) is a novel, oral selective inhibitor of complement factor B under clinical development for paroxysmal nocturnal hemoglobinuria (PNH). In this ongoing open-label phase 2 study, PNH patients with active hemolysis were randomized to receive single-agent iptacopan twice daily at a dose of either 25 mg for 4 weeks followed by 100 mg for up to 2 years (cohort 1) or 50 mg for 4 weeks followed by 200 mg for up to 2 years (cohort 2). At the time of interim analysis, of 13 PNH patients enrolled, all 12 evaluable for efficacy achieved the primary endpoint of reduction in serum lactate dehydrogenase (LDH) levels by ≥60% by week 12 compared with baseline; mean LDH levels dropped rapidly and durably, namely by 77% and 85% at week 2 and by 86% and 86% at week 12 in cohorts 1 and 2, respectively. Most patients achieved a clinically meaningful improvement in hemoglobin (Hb) levels, and all but 1 patient remained transfusion-free up to week 12. Other markers of hemolysis, including bilirubin, reticulocytes, and haptoglobin, showed consistent improvements. No thromboembolic events were reported, and iptacopan was well tolerated, with no severe or serious adverse events reported until the data cutoff. In addition to the previously reported beneficial effect of iptacopan add-on therapy to eculizumab, this study showed that iptacopan monotherapy in treatment-naïve PNH patients resulted in normalization of hemolytic markers and rapid transfusion-free improvement of Hb levels in most patients. This trial was registered at www.clinicaltrials.gov as #NCT03896152.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare hematological disorder affecting ∼1 to 1.5 per million individuals worldwide, caused by somatic mutations in the PIGA (phosphatidylinositol glycan A) gene in hematopoietic stem cells (HSCs).1,2 The PIGA mutations lead to a deficiency of glycosylphosphatidylinositol-anchored proteins, resulting in ineffective inhibition of the complement alternative pathway with increased susceptibility of PNH-type erythrocytes to intravascular hemolysis and leading to hemolytic anemia, impaired bone marrow (BM) function, and potentially life-threatening thrombotic events.3,4
The current standard of care for PNH consists of monotherapy with either eculizumab or ravulizumab, both anticomplement component 5 (C5) monoclonal antibodies that significantly improve the clinical course of PNH patients, with a resolution of hemolysis-related symptoms, stabilization of Hb levels, reduction in both transfusion needs and thrombotic events, and, ultimately, improvement in both quality of life and long-term survival.5-12 Nevertheless, a significant proportion of patients remain anemic and continue to require transfusions, largely due to persistent, C3-mediated extravascular hemolysis.13-15 More proximal complement inhibitors, including anti-C3 and alternative pathway inhibitors, hold promise to alleviate both intra- and extravascular hemolysis.1,16 Indeed, the anti-C3 inhibitor pegcetacoplan was shown to be superior to eculizumab in terms of clinical and hematological outcomes at week 16 in PNH patients with residual anemia despite treatment with eculizumab.17 However, since the anti-C5 therapies and pegcetacoplan require IV and subcutaneous infusion, respectively, the unmet need for effective, orally-administered treatment options remains open.
Iptacopan (LNP023; Novartis Pharma AG, Switzerland) is a first-in-class, small-molecule, oral, and selective inhibitor of factor B, a key component of the complement alternative pathway. In a first phase 2 study in PNH patients with persistent hemolysis despite ongoing treatment with eculizumab, iptacopan add-on resulted in significant reductions in both intra- and extravascular hemolysis and transfusion-free improvements in Hb levels at week 13.18,19 These clinical benefits persisted even in patients who discontinued eculizumab (the protocol allowed this earliest after 6 months of combined therapy) and continued with iptacopan as monotherapy. This second phase 2 study evaluates the efficacy, safety, pharmacokinetics, and pharmacodynamics of upfront iptacopan monotherapy in adult PNH patients with active hemolysis and no complement inhibition in the prior 3 months.
Methods
Study design
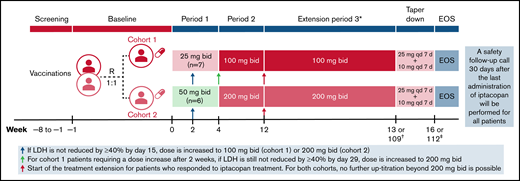
This is an ongoing, open-label, randomized, multicenter, phase 2 trial conducted in adult patients with active PNH with no complement inhibition within 3 months of study entry (NCT03896152). The total study duration from screening until the end of the study is approximately 28 months, including an 8-week screening phase, a 4-week treatment period (period 1), an 8-week treatment period (period 2), followed by an approximately 2-year extension period (period 3) (Figure 1).
Study design and iptacopan treatment duration. *Following period 3, patients will also have the possibility to transition directly to a long-term rollover extension program without a need for taper-down. †Week 13 for nonresponders or week 109 for responders. ‡Week 16 for nonresponders or week 112 for responders. bid, twice daily; EOS, end of study; qd, once daily; R, randomization.
Study design and iptacopan treatment duration. *Following period 3, patients will also have the possibility to transition directly to a long-term rollover extension program without a need for taper-down. †Week 13 for nonresponders or week 109 for responders. ‡Week 16 for nonresponders or week 112 for responders. bid, twice daily; EOS, end of study; qd, once daily; R, randomization.
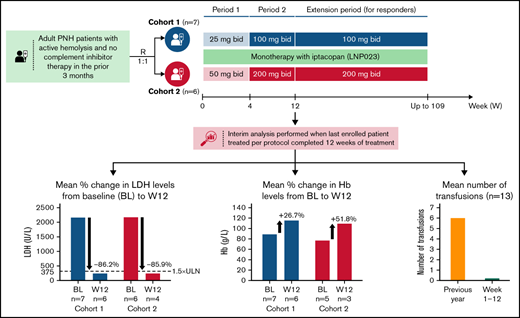
Patients were randomized 1:1 into 2 cohorts to receive either iptacopan 25 mg (twice daily) for 4 weeks followed by 100 mg twice daily for up to 2 years (cohort 1) or iptacopan 50 mg twice daily for 4 weeks followed by 200 mg twice daily for up to 2 years (cohort 2). If by day 15, lactate dehydrogenase (LDH) levels were not reduced by ≥40% from the mean of the pretreatment values (which was calculated separately for each patient based on all available LDH values in the 8 weeks before study day 1), patients were to be uptitrated to the higher dose (100 mg twice daily for cohort 1 and 200 mg twice daily for cohort 2) starting from study day 17. If by day 29 LDH was still not reduced by ≥40% from the mean of pretreatment values, cohort 1 patients were to be further uptitrated to 200 mg twice daily. No further uptitration was allowed beyond 200 mg twice daily. Eligible patients could enter period 3 based on the benefit of iptacopan treatment (reduced hemolytic parameters compared with screening and baseline). The data presented in this manuscript stem from an interim analysis performed when the last enrolled patient treated as per protocol completed 12 weeks of treatment (ie, periods 1 and 2) (data cutoff date: 8 April 2020).
The study was done according to the International Council for Harmonization E6 guidelines for Good Clinical Practice, with applicable local regulations, and in accordance with the ethical principles laid down in the declaration of Helsinki. Informed consent was obtained from each patient in writing.
Patients
Adult patients aged ≥18 years with a diagnosis of active PNH based on documented clone size of ≥10% by red blood cells (RBCs) and/or granulocytes, with LDH levels ≥1.5× upper limit normal (ULN) for ≥3 measurements over a maximum of 8 weeks before treatment initiation, and with a Hb level <105 g/L at baseline were eligible for enrollment. Exclusion criteria included treatment with eculizumab or any other complement inhibitor <3 months before treatment initiation, known or suspected hereditary or acquired complement deficiency, history of currently active primary or secondary immunodeficiency, history of splenectomy, history of BM/HSC or solid organ transplants, history of recurrent meningitis or meningococcal infections despite vaccination, any evidence of malignant disease within the previous 5 years, laboratory evidence of BM failure (reticulocytes <60 × 109/L, or platelets <50 × 109/L, or neutrophils <1 × 109/L), systemic corticosteroids (unless on a stable dose for ≥4 weeks before randomization), and severe concurrent comorbidities such as severe kidney disease, advanced cardiac disease, severe pulmonary arterial hypertension, or unstable thrombotic event.
Furthermore, all patients had to be vaccinated against Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae ≥4 weeks before initiating treatment with iptacopan, whereby the protocol recommended that the choice of vaccines should take into account the serotypes prevalent in the geographic areas in which study patients were enrolled. Prophylactic antibiotic treatment was mandatory for patients who started iptacopan treatment <4 weeks after vaccination. Prohibited medications besides those already mentioned included gemfibrozil, strong CYP2C8 inhibitors, P-glycoprotein substrates, and immunosuppressive agents such as cyclosporine, tacrolimus, mycophenolic acid, cyclophosphamide, and methotrexate, unless on a stable regimen for ≥3 months before study day 1.
Study objectives and assessments
The primary objective of this study was to evaluate the effect of iptacopan treatment on the reduction of PNH-associated hemolysis, as assessed by a 60% reduction in LDH levels or LDH below ULN by week 12 of treatment. As part of the secondary objectives, the effect of iptacopan treatment on other markers of intra- and extravascular hemolysis was assessed, including total and free Hb levels, RBC count, reticulocyte count, haptoglobin, bilirubin, PNH clone size, and C3 fragment deposition. Safety and tolerability were assessed throughout the study by monitoring adverse events (AEs), blood chemistry, hematology, urinalysis, electrocardiogram evaluation, physical examination, and vital signs. Pharmacokinetic samples were obtained predose on days 2, 15, 29, and 57, as well as 0.25, 1, 2, 4, and 6 hours after dose on days 29 and 57. The plasma concentrations of iptacopan were determined by a validated liquid chromatography with tandem mass spectrometry (LC-MS/MS) method with a lower limit of quantification of approximately 1 ng/mL, and the pharmacokinetics parameters, including Cmax, area under the curve, Ctrough, and Tmax, were calculated using noncompartmental methods. Pharmacodynamic assessments included the Wieslab assay, circulating fragment of factor B (Bb) levels, and quantification by flow cytometry of PNH-type RBCs and C3 fragment deposition on RBCs (see supplemental text for details).
Exploratory objectives of the study included the assessment of the relationship of iptacopan dose/ exposure levels with levels of blood Hb, LDH, plasma Bb, clone size, C3 deposition, and complement pathway activation (Wieslab). A model-based approach was applied to describe the relationship between iptacopan exposure (steady-state Ctrough) and biomarker variables. In addition, the effect of iptacopan on PNH-associated transfusion parameters was assessed as an exploratory objective.
Statistical analyses
The full analysis set and safety set included all patients who received any study drug. The pharmacodynamic analysis set included all patients with available pharmacodynamic data who received any study drug and had no protocol deviations with relevant impact on pharmacodynamic data. The pharmacokinetic analysis set included all patients with ≥1 available valid pharmacokinetic concentration measurement who received any study drug and had no protocol deviations with relevant impact on pharmacokinetic data. Approximately 10 patients (5 in each cohort) were planned to be enrolled in this study. With 10 patients, this study had an 80% probability of meeting the protocol-defined positive efficacy criteria of ≥50% responders (ie, 5 out of 10 patients or 3 out 5 patients in 1 of the 2 cohorts) if the true response rate is 58%.
The primary efficacy variable for assessing the effect of iptacopan was the response rate. A responder is defined as a patient with ≥60% reduction in LDH compared with baseline or LDH below ULN at any time up to and including week 12 for that patient, whether or not early escalation from the first dose level occurred. Baseline LDH was calculated as the mean of the last 3 screening values before randomization. For other laboratory-based variables, including total and free Hb, reticulocytes, C3 fragment deposition, haptoglobin, bilirubin, RBC count, platelet counts, ferritin, PNH type III RBC, and PNH clone size, the baseline was the average of all pretreatment values.
The relationship between plasma iptacopan concentration and response was modeled using a population approach (nonlinear mixed-effect modeling). Individual steady-state Ctrough concentrations were correlated to pharmacodynamic readouts of LDH, Hb, PNH clone size, C3d deposition, Bb, and Wieslab assay using sigmoid Emax models. Pharmacodynamic models were fitted to data at baseline and 4 weeks of treatment for each of the 4 tested dose levels (days 29 and 57) using the Stochastic Approximation Expectation-Maximization (SAEM) algorithm with a single random effect on the baseline. Individual time points at which either exposure or response measurements were missing or not collected were excluded from the exposure–response analysis. Baseline pharmacokinetic values were set to 0. Confidence intervals of the best-fit model for each pharmacodynamic parameter were calculated by resampling population parameters from the distribution of the estimates derived from the Fisher information matrix. SAS software version 9.4 was used for the statistical analysis, Phoenix WinNonlin version 6.2 was used for analyses of all pharmacokinetic parameters, and Monolix and Simulx 2019 (Lixoft) were used for nonlinear mixed-effect modeling and simulation.
Results
Patient characteristics
At the time of this interim analysis, 13 adult PNH patients (cohort 1, n = 7; cohort 2, n = 6) with a median age of 35 years (range, 20-62) were enrolled and treated across 5 study centers in Korea, Taiwan, Malaysia, and Singapore, with a median treatment duration of 212 days (range, 2-288) (Table 1; supplemental Figure 1). Eleven patients had completed 12 weeks of treatment per the protocol, though a total of 12 patients were evaluable for efficacy, including 1 patient in cohort 1 (patient 1) who was on a reduced iptacopan dose at the time of the interim analysis (a patient-specific dose reduction was started on day 36) and eventually tapered and discontinued treatment shortly after by patient preference and on physician decision; this patient is still evaluable for efficacy up until week 8. Of note, 1 patient in cohort 2 (patient 12) discontinued iptacopan after only 2 days of iptacopan dosing due to a nonsevere AE of headache and is thus not evaluable for efficacy.
Patient demographics and baseline characteristics (full analysis set)
| Characteristics . | Iptacopan 25 mg/100 mg, n = 7 . | Iptacopan 50 mg/200 mg, n = 6 . | Overall, n = 13 . |
|---|---|---|---|
| Age (y), median (range) | 30 (20-55) | 36 (34-62) | 35 (20-62) |
| Sex, male, n | 5 | 1 | 6 |
| Race, Asian, n | 7 | 6 | 13 |
| BMI, median (range) | 22 (16-27) | 23 (18-29) | 22 (16-29) |
| RBC transfusions in prior year, mean; median (range) | 7; 2.0 (0-19) | 5; 3.5 (0-13) | 6; 3.0 (0-19) |
| Anti-C5 naive, n (%) | 7 (100) | 5 (83) | 12 (92) |
| LDH level (U/L), mean; median (range) | 2228.0; 1686 (1008-3761) | 1946.0; 1737 (1212-2832) | 2097.8; 1686 (1008-3761) |
| Hb level (g/L), n*; mean; median (range) | 7; 90.3; 93 (70-107) | 5; 79.4; 73 (68-96) | 12; 85.8; 87 (68-107) |
| Free Hb level (IU/mL), n; mean; median (range) | 5; 43.9; 36.2 (31.6-82.5) | 4; 24.28; 25.2 (14.2-32.6) | 9; 35.19; 32.6 (14.2-82.5) |
| Red cell count (×1012/L), mean; median (range) | 3.14; 3.1 (2.4-3.8) | 2.63; 2.5 (1.9-3.7) | 2.91; 3.1 (1.9-3.8) |
| Reticulocyte level (×109/L), mean; median (range) | 205.4; 177 (136-284) | 200.8; 215 (30-352)† | 203.3; 209 (30-352)† |
| Reticulocyte/erythrocyte ratio (%), mean; median (range) | 6.7; 6.5 (4.2-10) | 8.35; 7.1 (1.2-18.9) | 7.46; 6.5 (1.2-18.9) |
| Platelet count (×109/L), mean; median (range) | 195.0; 194 (131-318) | 158.5; 180 (60-199) | 178.2; 188 (60-318) |
| Haptoglobin (g/L), mean; median (range)‡ | 0.05; 0.05 (0.05-0.05) | 0.05; 0.05 (0.05-0.05) | 0.05; 0.05 (0.05-0.05) |
| Total bilirubin (µmol/L), mean; median (range) | 32.4; 27 (24-48) | 32.3; 29 (20-51) | 32.4; 27 (20-51) |
| D-dimer (mg FEU/L), mean; median (range) | 0.380; 0.31 (0.24-0.88) | 0.735; 0.78 (0.40-1.14) | 0.544; 0.40 (0.24-1.14) |
| Characteristics . | Iptacopan 25 mg/100 mg, n = 7 . | Iptacopan 50 mg/200 mg, n = 6 . | Overall, n = 13 . |
|---|---|---|---|
| Age (y), median (range) | 30 (20-55) | 36 (34-62) | 35 (20-62) |
| Sex, male, n | 5 | 1 | 6 |
| Race, Asian, n | 7 | 6 | 13 |
| BMI, median (range) | 22 (16-27) | 23 (18-29) | 22 (16-29) |
| RBC transfusions in prior year, mean; median (range) | 7; 2.0 (0-19) | 5; 3.5 (0-13) | 6; 3.0 (0-19) |
| Anti-C5 naive, n (%) | 7 (100) | 5 (83) | 12 (92) |
| LDH level (U/L), mean; median (range) | 2228.0; 1686 (1008-3761) | 1946.0; 1737 (1212-2832) | 2097.8; 1686 (1008-3761) |
| Hb level (g/L), n*; mean; median (range) | 7; 90.3; 93 (70-107) | 5; 79.4; 73 (68-96) | 12; 85.8; 87 (68-107) |
| Free Hb level (IU/mL), n; mean; median (range) | 5; 43.9; 36.2 (31.6-82.5) | 4; 24.28; 25.2 (14.2-32.6) | 9; 35.19; 32.6 (14.2-82.5) |
| Red cell count (×1012/L), mean; median (range) | 3.14; 3.1 (2.4-3.8) | 2.63; 2.5 (1.9-3.7) | 2.91; 3.1 (1.9-3.8) |
| Reticulocyte level (×109/L), mean; median (range) | 205.4; 177 (136-284) | 200.8; 215 (30-352)† | 203.3; 209 (30-352)† |
| Reticulocyte/erythrocyte ratio (%), mean; median (range) | 6.7; 6.5 (4.2-10) | 8.35; 7.1 (1.2-18.9) | 7.46; 6.5 (1.2-18.9) |
| Platelet count (×109/L), mean; median (range) | 195.0; 194 (131-318) | 158.5; 180 (60-199) | 178.2; 188 (60-318) |
| Haptoglobin (g/L), mean; median (range)‡ | 0.05; 0.05 (0.05-0.05) | 0.05; 0.05 (0.05-0.05) | 0.05; 0.05 (0.05-0.05) |
| Total bilirubin (µmol/L), mean; median (range) | 32.4; 27 (24-48) | 32.3; 29 (20-51) | 32.4; 27 (20-51) |
| D-dimer (mg FEU/L), mean; median (range) | 0.380; 0.31 (0.24-0.88) | 0.735; 0.78 (0.40-1.14) | 0.544; 0.40 (0.24-1.14) |
The values at “baseline” visit are presented.
BMI, body mass index.
Patient 6 had Hb levels of 107 g/L at the baseline visit on study day −18 but was deemed eligible based on Hb levels of 99 g/L at a repeat unscheduled assessment on study day −8.
Patient 8, who had preexisting MDS with multilineage dysplasia, was enrolled despite significant reticulocytopenia (30 × 109/L) at baseline but was allowed to remain on study despite this protocol deviation.
All values are below the normal range.
All 13 enrolled patients had been vaccinated per the protocol before treatment initiation, with information about serotype coverage available for 10 patients: for N. meningitidis, all 10 patients received quadrivalent vaccines only (covering serotypes A, C, W-135, and Y, but not serotype B); for S. pneumoniae, 7 patients received 13-valent (PCV13), and 3 patients received 23-valent (PPSV23) vaccines. In addition, 2 patients received antibiotic prophylaxis for a short period of time (ceftibuten and ciprofloxacin, respectively).
At baseline, all patients had signs of active hemolysis (mean LDH: 2097.8 U/L [range, 1008-3761]) and clinically significant anemia (mean Hb: 85.8 g/L [range, 68-107]), with most patients being transfusion-dependent (mean RBC transfusions in the 12 months before to study entry: 6 [range, 0-19]) (Table 1). All patients were anti-C5 treatment-naive with the exception of the patient who discontinued iptacopan treatment on day 2 and is thus not considered for efficacy analyses (patient 12). Significant medical histories at study entry included 3 patients with aplastic anemia (patients 7, 9, and 11) and 2 patients with myelodysplastic syndrome (MDS); patient 8 had MDS with multilineage dysplasia with normal cytogenetics, reported as ongoing, whereas for patient 1, for whom MDS was reported as not ongoing, no additional diagnostic information was retrievable. With the exception of intermittent iron supplementation for patients 7 and 9, no concomitant therapy was given for the treatment of aplastic anemia or MDS.
Efficacy
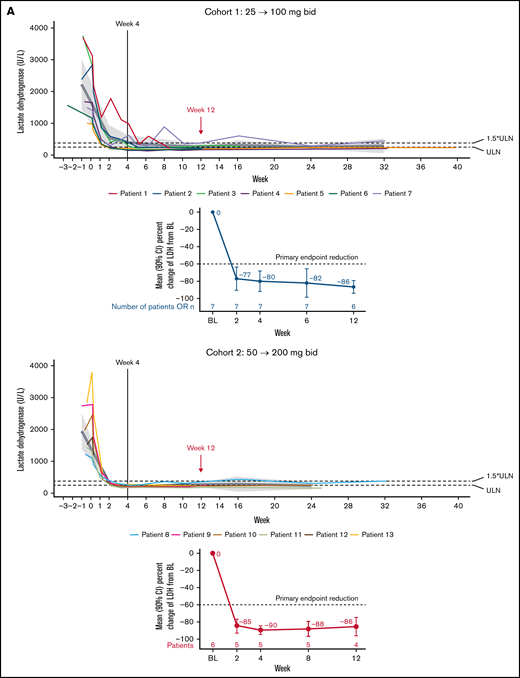
All 12 patients evaluable for efficacy achieved the primary endpoint with reductions in serum LDH concentrations by week 12 by ≥60% compared with baseline. A significant decline in LDH levels was observed already after only 2 weeks of treatment, with mean LDH levels declining by 76.5% (90% confidence interval [CI], −90.1-63.0; P < .0001) and 85.0% (90% CI, −92.8-77.2; P < .0001) in cohorts 1 and 2, respectively (Figure 2A), and the decline remained between 80% and 90% at weeks 4, 8, and 12; P < .001 for all (Figure 2A; supplemental Table 1) and persisted longer-term (into treatment extension period 3) for most patients with available data. Less variability was observed in the higher-dose cohort (cohort 2), where all patients maintained LDH levels <1.5× ULN until week 12. Of note, no patient in either cohort required an unscheduled uptitration of the iptacopan dose due to a lack in LDH reduction by ≥40% from baseline.
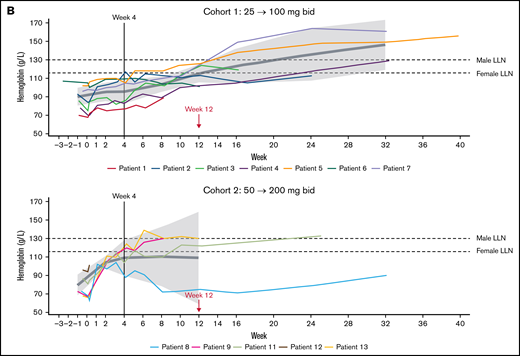
Effect of Iptacopan on LDH and Hb levels. (A) Change in LDH levels over time for iptacopan 25/100 mg and 50/200 mg cohorts (pharmacodynamic analysis set). (B) Change in Hb levels over time for iptacopan 25/100 mg and 50/200 mg cohorts (pharmacodynamic analysis set). Note: 1 patient in cohort 2 (patient 10) was excluded from the Hb analysis due to a protocol deviation, whereby an RBC transfusion was given between screening and baseline, raising the Hb level to above the protocol-defined upper limit of 105 g/L at baseline. bid, twice daily; BL, baseline; LLN, lower limit of normal.
Effect of Iptacopan on LDH and Hb levels. (A) Change in LDH levels over time for iptacopan 25/100 mg and 50/200 mg cohorts (pharmacodynamic analysis set). (B) Change in Hb levels over time for iptacopan 25/100 mg and 50/200 mg cohorts (pharmacodynamic analysis set). Note: 1 patient in cohort 2 (patient 10) was excluded from the Hb analysis due to a protocol deviation, whereby an RBC transfusion was given between screening and baseline, raising the Hb level to above the protocol-defined upper limit of 105 g/L at baseline. bid, twice daily; BL, baseline; LLN, lower limit of normal.
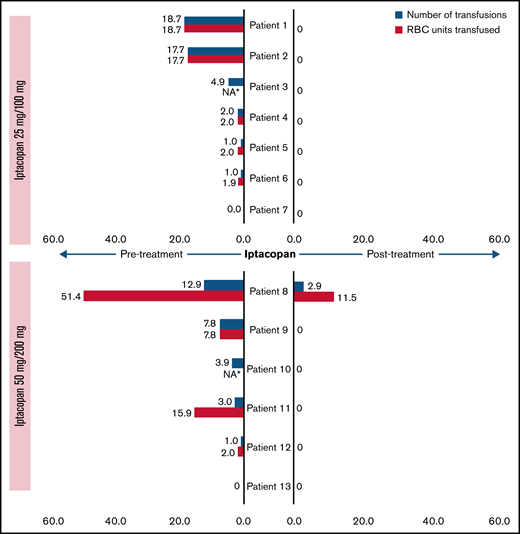
The dramatic effect on LDH levels was reflected in a significant hematological benefit (all treated and ongoing patients remained transfusion-free up until at least week 12), with the exception of 1 patient in cohort 2 (patient 8) who had preexisting MDS with multilineage dysplasia and was enrolled despite significant reticulocytopenia (30 × 109/L) at the baseline visit, requiring a total of 13 RBC transfusions (52 units) in the prior year; this patient received 1 transfusion (4 units) each on study days 3 and 156, which nevertheless is a meaningful improvement in terms of transfusion requirements (Figure 3).
Number of transfusions and packed RBC units transfused before entry and during study, normalized per year (pharmacodynamic analysis set). Blue bars represent the number of transfusions (normalized, per year) for each patient, and the associated red bars denote the number of packed RBC units transfused (normalized, per year). *Number of packed RBC units transfused was not available for 2 patients.
Number of transfusions and packed RBC units transfused before entry and during study, normalized per year (pharmacodynamic analysis set). Blue bars represent the number of transfusions (normalized, per year) for each patient, and the associated red bars denote the number of packed RBC units transfused (normalized, per year). *Number of packed RBC units transfused was not available for 2 patients.
Furthermore, treatment with iptacopan resulted in a clinically meaningful improvement in Hb levels, with a mean improvement from 88.5 (90% CI, 78.6-98.5) at baseline to 115.2 g/L (90% CI, 105.6-124.7) at week 12 and from 76.9 (90% CI, 64.9-88.9) at baseline to 109.0 g/L (90% CI, 58.9-159.1) at week 12 in cohorts 1 and 2, respectively (Figure 2B; supplemental Table 1). At week 12, 5 patients (3 patients in cohort 1 and 2 patients in cohort 2) had Hb levels above 120 g/L. A trend for a continued rise in Hb levels was apparent beyond week 12.
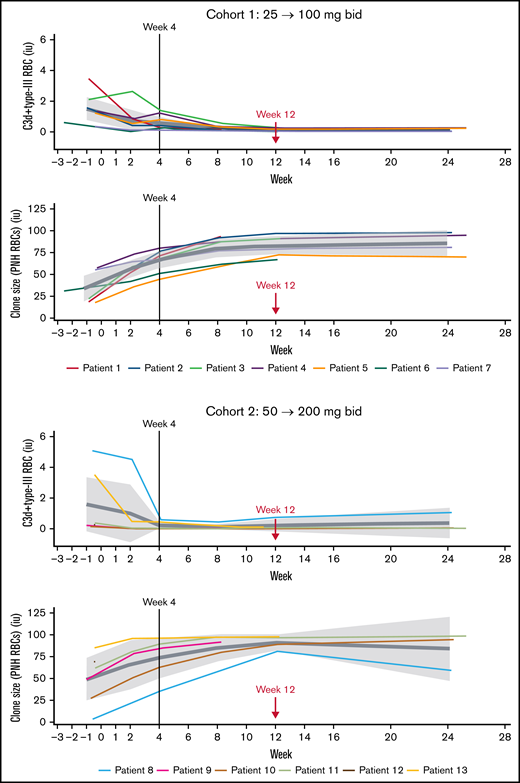
Treatment with iptacopan showed consistent improvements for other markers of hemolysis, including bilirubin, reticulocytes, free Hb, haptoglobin, and erythrocytes (supplemental Table 1; supplemental Figure 2). In particular, in cohorts 1 and 2, respectively, mean bilirubin levels decreased from 32.4 µmol/L and 35.3 µmol/L at baseline to 11.7 µmol/L and 14.8 µmol/L at week 12, and mean reticulocyte counts decreased from 217.6 × 109/L and 211.1 × 109/L to 130.8 × 109/L and 104.8 × 109/L, respectively. Overall, 4 patients (66.7%) in cohort 1 and 4 patients (100%) in cohort 2 (for 1 of the 5 cohort 2 patients, reticulocytes were not assessed at week 12) achieved a reticulocyte count ≤150 × 109/L at week 12. Finally, iptacopan treatment resulted in near-total abrogation of C3d deposition on RBC, already low at baseline in this mostly treatment-naive patient population, and a significant increase in PNH clone size (= type II+III RBC) (Figure 4; supplemental Table 2); the mean (90% CI) PNH clone size was 33.6% (18.7-48.6) at baseline and 82.9% (73.1-92.7) at week 12 in cohort 1 and 49.1% (24.7-73.6) at baseline and 91.1% (81.8-100.3) at week 12 in cohort 2.
Effect of iptacopan on C3d deposition and PNH clones (type II+III) (pharmacodynamic analysis set). bid, twice daily.
Effect of iptacopan on C3d deposition and PNH clones (type II+III) (pharmacodynamic analysis set). bid, twice daily.
Safety
Iptacopan was overall well-tolerated and safe. Of 13 patients, up until the data cutoff, 9 (69.2%) experienced ≥1 AE, with the majority of AEs being of mild intensity (Table 2). No deaths and no severe or serious AEs were reported. The most common AEs were headache, abdominal discomfort, an increase in blood alkaline phosphatase, cough, oropharyngeal pain, pyrexia, and upper respiratory tract infection (Table 2).
Incidence of AEs by preferred term and treatment sequence (full analysis set)
| . | nE, nS (%) . | ||
|---|---|---|---|
| . | Iptacopan 25 mg/100 mg, n = 7 . | Iptacopan 50 mg/200 mg, n = 6 . | Total, n = 13 . |
| Total AEs | 31, 4 (57.1) | 7, 5 (83.3) | 38, 9 (69.2) |
| AEs of mild intensity | 29, 4 (57.1) | 6, 4 (66.7) | 35, 8 (61.5) |
| AEs of moderate intensity | 2, 1 (14.3) | 1, 1 (16.7) | 3, 2 (15.4) |
| AEs of severe/serious intensity | 0 | 0 | 0 |
| Study drug-related AEs | 6, 1 (14.3) | 3, 3 (50.0) | 9, 4 (30.8) |
| AEs leading to discontinuation of study treatment | 0 | 1, 1 (16.7) | 1, 1 (7.7) |
| Study drug-related AEs leading to discontinuation of study treatment | 0 | 1, 1 (16.7) | 1, 1 (7.7) |
| Most common AEs by preferred terms (total incidence, n ≥2), n (%) | |||
| Headache | 1 (14.3) | 3 (50.0) | 4 (30.8) |
| Abdominal discomfort | 2 (28.6) | 0 | 2 (15.4) |
| Blood alkaline phosphatase increased | 1 (14.3) | 1 (16.7) | 2 (15.4) |
| Cough | 2 (28.6) | 0 | 2 (15.4) |
| Oropharyngeal pain | 2 (28.6) | 0 | 2 (15.4) |
| Pyrexia | 2 (28.6) | 0 | 2 (15.4) |
| Upper respiratory tract infection | 2 (28.6) | 0 | 2 (15.4) |
| . | nE, nS (%) . | ||
|---|---|---|---|
| . | Iptacopan 25 mg/100 mg, n = 7 . | Iptacopan 50 mg/200 mg, n = 6 . | Total, n = 13 . |
| Total AEs | 31, 4 (57.1) | 7, 5 (83.3) | 38, 9 (69.2) |
| AEs of mild intensity | 29, 4 (57.1) | 6, 4 (66.7) | 35, 8 (61.5) |
| AEs of moderate intensity | 2, 1 (14.3) | 1, 1 (16.7) | 3, 2 (15.4) |
| AEs of severe/serious intensity | 0 | 0 | 0 |
| Study drug-related AEs | 6, 1 (14.3) | 3, 3 (50.0) | 9, 4 (30.8) |
| AEs leading to discontinuation of study treatment | 0 | 1, 1 (16.7) | 1, 1 (7.7) |
| Study drug-related AEs leading to discontinuation of study treatment | 0 | 1, 1 (16.7) | 1, 1 (7.7) |
| Most common AEs by preferred terms (total incidence, n ≥2), n (%) | |||
| Headache | 1 (14.3) | 3 (50.0) | 4 (30.8) |
| Abdominal discomfort | 2 (28.6) | 0 | 2 (15.4) |
| Blood alkaline phosphatase increased | 1 (14.3) | 1 (16.7) | 2 (15.4) |
| Cough | 2 (28.6) | 0 | 2 (15.4) |
| Oropharyngeal pain | 2 (28.6) | 0 | 2 (15.4) |
| Pyrexia | 2 (28.6) | 0 | 2 (15.4) |
| Upper respiratory tract infection | 2 (28.6) | 0 | 2 (15.4) |
Only data from the on-treatment period are included.
nE, number of events; nS, number of patients.
Overall, 4 patients (30.8%) experienced AEs that were suspected to be drug-related by the investigator, most of which were transient and of mild intensity. Two patients experienced nonsevere headaches, 1 of whom (patient 12) discontinued the study after 2 days of treatment, the third patient experienced a transient increase in blood alkaline phosphatase, and the fourth patient experienced multiple AEs, including flatulence, blood alkaline phosphatase increase, blood luteinizing hormone increase, γ-glutamyl transferase increase, reverse tri-iodothyronine increase, and thyroxine free increase. Importantly, no breakthrough hemolysis was observed until the data cutoff, though none of the patients experienced any significant complement-amplifying conditions such as surgical interventions or severe infections after starting iptacopan. Moreover, no thromboembolic events were observed either, despite none of the patients being on prophylactic anticoagulation. Finally, no dose-dependent changes in the AE profile of the 2 cohorts were apparent (supplemental Table 3).
Pharmacokinetics and pharmacodynamics
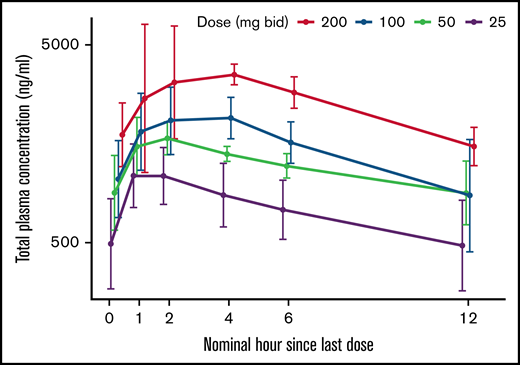
In line with earlier studies (data on file), iptacopan exposure (AUClast and Cmax) was underproportional in relationship to dose (Figure 5; supplemental Table 4). Mean AUClast values varied from 6290 h · ng/mL (day 29, 25 mg twice daily) to 19 900 h · ng/mL (day 57, 200 mg twice daily). Patients receiving iptacopan doses of ≥50 mg twice daily showed mean trough level exposures of ≥900 ng/mL, although it is only at the highest dose of 200 mg (mean Cmax 4520 ng/mL, mean AUC 19 900 h · ng/mL) that all patients maintained this exposure throughout the dosing interval (Figure 5). Target engagement was evaluated by quantifying the alternative pathway-dependent generation of fragment Bb and by the Wieslab assay. Bb levels decreased rapidly after the first iptacopan dose of 25 mg twice daily or higher, and Wieslab assay results confirmed a dose- and exposure-dependent suppression of alternative pathway activity, with near-maximal inhibition achieved at 200 mg twice daily (data not shown).
Pharmacokinetic profiles of iptacopan at steady state (days 29 and 57) (pharmacokinetic analysis). Data are represented as arithmetic means with standard deviation. bid, twice daily.
Pharmacokinetic profiles of iptacopan at steady state (days 29 and 57) (pharmacokinetic analysis). Data are represented as arithmetic means with standard deviation. bid, twice daily.
The exposure–response relationship for 6 pharmacodynamic variables was explored by population modeling using the sigmoid Emax function. Considering the predefined intrasubject dose escalation after 4 weeks of treatment, the modeling was performed on exposure–response data collected at baseline and after 4 weeks of treatment at each dose level (ie, at days 29 and 57). The analysis showed a decline in LDH levels, C3d deposition, Bb fragment, and the Wieslab assay and an increase in Hb levels and PNH clone size with increasing iptacopan exposures (supplemental Figure 3). Taking into account interindividual variability in exposure, it could be established that only the 200 mg twice daily dose has the potential to maximize the inhibition of the alternative pathway as it ensures an exposure that is sufficient to maintain a near-maximal Wieslab response in the majority of patients at the trough. Furthermore, 200 mg twice daily is the only dose that guarantees a near-maximal response in both LDH and Hb levels in the majority of patients.
Discussion
Iptacopan is a novel, oral, selective, and potent inhibitor of factor B, a key component of the complement alternative pathway. Preclinical studies have shown iptacopan to provide direct, reversible, high-affinity, and selective inhibition of factor B and alternative pathway-dependent complement activation, leading to reduced hemolysis of PNH-type erythrocytes.20,21 By targeting factor B, iptacopan inhibits the formation of the alternative pathway C3 convertase. It also blocks the amplification of classical and lectin pathway-dependent–C5 activation, although it does not impact amplification-independent complement activation via these pathways, which may potentially lead to lower infection risk in vaccinated patients as compared with other proximal complement inhibitors.1,16,22,23 Based on data from nonclinical and phase 1 studies, the pharmacokinetics of iptacopan is characterized by high absorption and moderate clearance (data on file). The main clearance pathway is hepatic metabolism (cytochrome P450 2C8-mediated oxidation, direct glucuronidation), followed by direct renal excretion (∼25%). The multitude of clearance pathways and the absence of significant inhibition of metabolizing enzymes both contribute to the low susceptibility of iptacopan to drug interactions. Furthermore, iptacopan has no food effect, no ethnic sensitivity, and has a terminal half-life in human plasma of approximately 20 hours.
Since the absence of a placebo response for LDH has previously been demonstrated in this patient population,7 this proof-of-concept study was designed as a nonplacebo-controlled trial. Iptacopan monotherapy in anti-C5 naive PNH patients with active hemolysis resulted in a rapid and durable reduction of hemolytic markers, including LDH, bilirubin, reticulocytes, and haptoglobin, as well as C3 fragment deposition on RBCs, resulting in stabilization and a dramatic increase in the proportion of PNH-type RBC. Consistently, a more gradual but clinically meaningful and durable increase in Hb levels was observed in most patients, with all but 1 remaining transfusion-free from the start of iptacopan treatment until the data cutoff. These results complement data from a previous phase 2 study in adult PNH patients with persistent hemolysis despite ongoing treatment with eculizumab, where iptacopan add-on resulted in similar hematological improvements that persisted even when eculizumab was discontinued.19 Together, both studies provide evidence that complement inhibition with iptacopan effectively controls both intra- and extravascular hemolysis and leads to significant, transfusion-free improvement in Hb levels in most patients with PNH. These findings are consistent with results from clinical trials of other proximal complement inhibitors, including pegcetacoplan and the factor D inhibitors, danicopan24,25 and BCX9930,26 which have demonstrated similar inhibition of both intra- and extravascular hemolysis.
Consistent with the previous phase 2 study findings,19 iptacopan treatment was safe and well-tolerated. Up until the data cutoff, no serious nor severe AEs, including none of infectious nature, were reported, and the majority of AEs suspected by the investigator to be drug-related, affecting roughly one-third of the patients, were transient and of mild intensity. Two patients discontinued iptacopan treatment, 1 due to nonsevere, though suspected-to-be-related headache after 2 days of dosing, the other by patient preference and upon physician decision. Importantly, up until the data cutoff for this interim analysis, no thromboembolic events or breakthrough hemolysis were reported. The absence of significant breakthrough hemolysis to date is particularly notable considering the significant increase in PNH clone size achieved with iptacopan treatment, although this observation remains to be confirmed with more patients, longer-term data, and upon the occurrence of complement-amplifying conditions such as surgical interventions or severe infections.
This study included a dose range of 25 mg to 200 mg twice daily (across 2 cohorts), reflecting the previous observation that iptacopan has an underproportional dose–exposure relationship, with the proposed dose range (eightfold) being larger than the expected exposure range (threefold) (data on file). Although the small patient numbers and significant exposure overlap across doses preclude any definite conclusions, the exposure and related alternative pathway inhibition assessed in this study are in line with unpublished data from previous clinical trials in healthy volunteers and across different indications.
A dose- and exposure-dependent effect of iptacopan was observed across all the efficacy parameters analyzed. The exposure–response analysis after 4 weeks of treatment at each dose level enabled the correlation of iptacopan exposure with each of 6 pharmacodynamic variables. Although some of the variables seemed to reach the maximal effect plateau at relatively low exposures (eg, Bb and C3d deposition), only exposures related to 200 mg twice daily achieved an effect maximization of all pharmacodynamic variables with potentially better disease control in the majority of patients. Longitudinal pharmacokinetic–pharmacodynamic modeling will be done when longer-term data on the higher doses become available.
Confirmatory phase 3 trials have recently been started. The first (APPLY-PNH; ClinicalTrials.gov identifier: NCT04558918) includes an active comparator arm with an anti-C5 antibody, eculizumab or ravulizumab, in an attempt to demonstrate the superiority of iptacopan (200 mg twice daily) compared with standard of care.27 The second (APPOINT-PNH; ClinicalTrials.gov identifier: NCT04820530) assesses the efficacy and safety of iptacopan monotherapy (200 mg twice daily) in patients with PNH who are naive to complement inhibitor therapy.28 With the phase 3 evaluation pending, iptacopan has the potential to become the first oral standard-of-care complement inhibitor monotherapy for adult patients with PNH.
Conclusions
Currently available clinical data with iptacopan support the strategy of complement alternative pathway inhibition for the treatment of PNH. In addition to the previously reported beneficial effect of iptacopan add-on to standard-of-care eculizumab, the interim results of this study demonstrate that iptacopan monotherapy in treatment-naïve PNH patients with active hemolysis results in normalization of hemolytic markers and in rapid and durable, transfusion-free improvement of Hb levels in the majority of patients, with a well-tolerated safety profile at all doses tested. The phase 3 evaluation of oral iptacopan monotherapy as a potential new standard of care for patients with PNH is ongoing.
Acknowledgments
The authors thank patients, investigators, and staff from all the participating sites for their collaboration and support. In addition, the authors would like to thank Lakshmi Kasthurirangan and Anupama Singh, MTech of Novartis Healthcare Pvt. Ltd. for providing medical editorial assistance with this study manuscript.
This study was sponsored by Novartis Pharmaceuticals Corporation.
Authorship
Contribution: All authors were involved in the conceptualization or execution of the study design of the NCT03896152 clinical trial; all authors contributed to manuscript draft content during its development; and all authors approved the final draft.
Conflict-of-interest disclosure: K.L., R.C., and G.J. are employees of the Novartis Institutes for Biomedical Research. I.B. is an employee of the Novartis Pharma AG. P.K.N. is an employee of the Novartis Healthcare Private Limited. The remaining authors declare no competing financial interests.
Correspondence: Jun Ho Jang, Division of Hematology-Oncology, Sungkyunkwan University School of Medicine, Samsung Medical Center, Seoul, South Korea; e-mail: jh21.jang@samsung.com.
References
Author notes
The data that support the findings of this study are available from the corresponding author upon reasonable request (e-mail: smcjunhojang@gmail.com). Clinical study documents (eg, clinical study report, clinical study protocol, and statistical analysis plan) and participant clinical study data are available to be shared after publication of the primary manuscript in a peer-reviewed journal and if regulatory activities are complete (ie, NCT03896152 study completion) and other criteria met per the Novartis Policy on Transparency and Publication of Clinical Study Data.
The full-text version of this article contains a data supplement.