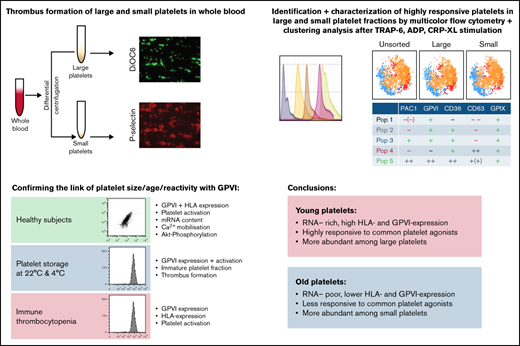
Key Points
Juvenile platelets show increased GPVI expression.
These platelets are highly responsive and more abundant among large platelets.
Abstract
Platelets within one individual display heterogeneity in reactivity, size, age, and expression of surface receptors. To investigate the combined intraindividual contribution of platelet size, platelet age, and receptor expression levels on the reactivity of platelets, we studied fractions of large and small platelets from healthy donors separated by using differential centrifugation. Size-separated platelet fractions were perfused over a collagen-coated surface to assess thrombus formation. Multicolor flow cytometry was used to characterize resting and stimulated platelet subpopulations, and platelet age was determined based on RNA and HLA-I labeling. Signal transduction was analyzed by measuring consecutive phosphorylation of serine/threonine-protein kinase Akt. Compared with small platelets, large platelets adhered faster to collagen under flow and formed larger thrombi. Among the large platelets, a highly reactive juvenile platelet subpopulation was identified with high glycoprotein VI (GPVI) expression. Elevated GPVI expression correlated with high HLA-I expression, RNA content, and increased platelet reactivity. There was a stronger difference in Akt phosphorylation and activation upon collagen stimulation between juvenile and older platelets than between large and small platelets. GPVI expression and platelet reactivity decreased throughout platelet storage at 22°C and was better maintained throughout cold storage at 4°C. We further detected higher GPVI expression in platelets of patients with immune thrombocytopenia. Our findings show that high GPVI expression is a feature of highly reactive juvenile platelets, which are predominantly found among the large platelet population, explaining the better performance of large platelets during thrombus formation. These data are important for studies of thrombus formation, platelet storage, and immune thrombocytopenia.
Introduction
Circulating platelets are heterogeneous in structure and age, as well as in their activation response upon stimulation during thrombus formation. This leads to a diversity of platelet populations with distinct or overlapping functions, including aggregating, secreting, or procoagulant platelet populations. This heterogeneity in function appears during the exposure of a platelet to different adhesive surfaces, agonists, blood flow patterns, and other cells.1 However, intrinsic differences in platelet size, levels of intracellular and membrane proteins, and platelet age are also important in defining these distinct platelet populations.2 How precisely these intrinsic factors arbitrate the reactivity of a platelet is not yet known.
More specifically, the exact relation between platelet age and platelet size is still not well established in humans. A larger platelet size (mean platelet volume) is associated with poorer outcomes in prothrombotic disorders such as sepsis and cardiovascular disease. In these conditions, platelet turnover is increased,3 and circulating platelets are younger.4 Currently, larger platelets are considered to be juvenile because they may not have yet shed their granules. Platelets can absorb many substances, however, which might increase platelet size during their life span.5 Others argue that the heterogeneity in platelet size originates from the thrombopoiesis from megakaryocytes and is not influenced by platelet age.6-8 Experiments in baboons revealed that size and age are independent determinants of platelet reactivity.7 It remains unclear how strong platelet age and size determine platelet reactivity and which additional features exist that characterize highly responsive platelets.
We and others have shown that larger platelets respond remarkably stronger to several platelet agonists, such as thrombin or collagen, than smaller platelets.7-10 Furthermore, large platelets contain and mobilize more Ca2+ from their intracellular stores upon activation and have a predisposition to expose phosphatidylserine on their membranes compared with small platelets.11 Conversely, small platelets exhibit enhanced integrin αIIbβ3 activation in response to adenosine 5′-diphosphate (ADP). Other groups found that reticulated juvenile platelets are more responsive compared with nonreticulated ones.12,13
In this study, we have combined a differential centrifugation protocol to separate size fractions and novel markers for juvenile platelet identification to further dissect the relation between platelet size, age, and reactivity. To determine the reactivity of the differently sized platelets in a more biologically relevant setting, we used a model of whole-blood thrombus formation under flow. In addition, we characterize the associations between platelet size and age, receptor expression, and their response to agonists using multicolor flow cytometry and clustering algorithms.
Methods
Materials and additional detailed methods can be found in the supplemental Methods.
Blood collection
The study was approved by the ethics committees of Universitätsmedizin Greifswald and Maastricht University. All participants provided written, informed consent before inclusion into the study and did not take antiplatelet drugs within 2 weeks before blood collection.
Blood was collected in acid citrate dextrose solution A (17 mM sodium citrate, 28 mM dextrose, 9 mM citric acid, 0.2 mM potassium sorbate, final in blood) from the antecubital vein with a Vacutainer 21-gauge needle (both, BD Biosciences, Franklin Lakes, NJ). Platelet indices, including platelet count, mean platelet volume, and platelet distribution width, were determined in whole blood with a Sysmex pocH-100i cell counter (Chuo-Ku, Kobe, Japan). Immature platelet fraction of the patients with immune thrombocytopenia (ITP) was measured by using a Sysmex XN-9000 analyzer (Chuo-Ku).
Platelet preparations
Preparation of platelet size fractions.
Whole blood was centrifuged (120g, 20 minutes) to obtain platelet-rich plasma (PRP). Per milliliter of PRP, 2.5 U apyrase and acid citrate dextrose solution A (final, 7.5 mM sodium citrate, 12.4 mM glucose, 3.8 mM citric acid) were added. Then, 1 mL PRP was spun down at 650g for 7 minutes to obtain a pellet with unseparated platelets. Large and small platelets were separated as described previously.10
Platelet storage.
Buffy coat–derived pooled platelet concentrates (PCs; n = 3) were collected from healthy donors according to the German guidelines for hemotherapy and prepared by using standard procedures at our institution. Buffy coats of 4 blood group identical donations were pooled, and 250 mL additive solution PAS-E (SSP+, Macopharma, Tourcoing, France) was added. Residual red blood cells and leukocytes were depleted (Leucoflex LXT Filter; Macopharma). PCs were split into 2 subunits and stored at room temperature or 4°C (cold storage). Stored platelets were investigated at day of preparation (day 0) and after 1, 2, 5, 7, and 12 days.
Whole-blood perfusion experiments
Whole blood perfusion experiments were performed as described previously.14 Citrate-anticoagulated blood samples (35%-40% hematocrit) were depleted from platelets and leukocytes by inline filtration, and fractions of unsorted, large, small, and stored platelets were added to a final concentration of 300 000 cells/µL (with the exception of plateletcrit-adjusted small platelets) as described elsewhere.15 Detailed descriptions of experimental procedures are given in the supplemental Methods.
Identification of platelet populations by multicolor flow cytometry and clustering analysis
Size-separated washed platelets (25 × 109/L) were stained with allophycocyanin-conjugated anti–HLA-A,B,C monoclonal antibody (mAb) (1:20), BV510-conjugated anti-CD42b mAb (1:50), fluorescein isothiocyanate–conjugated anti-CD61 mAb (1:20), phycoerythrin-conjugated anti– glycoprotein VI (GPVI) mAb (1:100), and PerCP-Cy5.5–conjugated anti-CD36 mAb (1:200) for the platelet surface marker orientation. For measuring both surface and activation markers on the platelets, size-separated washed platelets were maximally stimulated, in the presence of 2 mM CaCl2, by cross-linked collagen-related peptide (CRP-XL) (5 µg/mL), 2-MeSADP (5 µM), or thrombin receptor activator peptide 6 (TRAP-6) (25 µM) for 15 minutes and simultaneously stained for BV421-conjugated CD63 mAb (1:100), BV510-conjugated CD42 mAb (1:50), fluorescein isothiocyanate–conjugated PAC1 mAb (1:20), phycoerythrin-conjugated GPVI mAb (1:100), and PerCP-Cy5.5–conjugated CD36 mAb (1:200). After 15 minutes, 0.3% Cytofix was added (BD Biosciences), and the samples were fixated for at least 30 minutes at 4°C in the dark before measuring 10 000 events per sample on a BD FACSCanto II (BD Biosciences). Analysis of the multicolor flow cytometry data are described in the supplemental Methods.
Conventional flow cytometry
Platelet activation of GPVI-rich and poor platelets, determination of RNA content, HLA and GPVI quantification on reticulated platelets, signal transduction assays, and analyses of platelets from patients with ITP were performed by using conventional flow cytometric assays. Details are provided in the supplemental Methods.
Statistical analysis
The data are shown as mean ± standard error of mean. GraphPad Prism 7.0 software (GraphPad Software, La Jolla, CA) was used for statistical analysis. Flow cytometric comparisons of large and small, RNA-positive and RNA-negative platelets were performed by using Wilcoxon matched pairs tests. Thrombus formation comparisons were calculated by using two-way analysis of variance with Tukey’s multiple comparisons test. Significances of differences in frequencies of platelet populations found in the multicolor flow cytometry data between sample conditions were tested by χ2 with multiple comparisons test and false discovery rate correction. A P value <.05 was considered to be statistically significant: *P < .05, **P < .01, ***P < .001, and ****P < .0001.
Results
Large platelets adhere faster and form larger thrombi in whole blood under flow
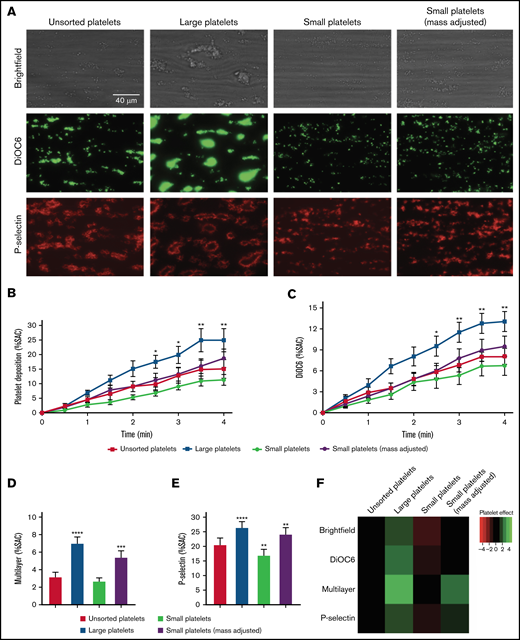
To assess the influence of platelet size on thrombus formation, the Maastricht flow chamber was used to perfuse (platelet- and leukocyte-depleted) whole blood reconstituted with unseparated, large, or small platelets over a coated collagen type I microspot at a wall shear rate of 1000 s−1. The large platelet fraction formed the largest sizes of thrombi compared with the unseparated and small platelet fractions (Figure 1A). In addition, when investigating thrombus formation over time, the large platelets adhered faster compared with the unsorted and small platelet fractions, judging by the percent surface area covered by platelets in brightfield imaging, and this was confirmed by fluorescent DiOC6 membrane staining (Figure 1B-C,F). Also, the large platelet fraction resulted in significantly increased thrombus multilayer formation compared with unsorted and small fractions, as well as higher P-selectin expression (Figure 1D-F). To equalize the platelet mass/crit in the small and large platelet fractions, mass adjustment was done by increasing the number of small platelets. Mass/crit adjustment resulted in enhanced thrombus formation, albeit still significantly lower than with the large platelet fraction (P = .01). P-selectin expression of mass/crit–adjusted small platelets was significantly higher compared with the unsorted condition but still lower than that observed for large platelets (Figure 1E-F).
Thrombus formation with different platelet fractions. Platelet- and leukocyte-depleted whole blood was reconstituted with unsorted, large, small, or small (mass adjusted) platelets and perfused for 4 minutes at a wall shear rate of 1000 s−1 over a coated microspot containing collagen type I. Thrombus build-up was evaluated by image capturing every 30 seconds during the 4 minutes of blood perfusion and end point analysis after postlabeling and perfusion. (A) Representative brightfield images (upper row) and fluorescent images of DiOC6 (middle row) or P-selectin expression (lower row) of the different platelet fractions after 4 minutes of perfusion. (B-C) Quantification of thrombus build-up (kinetics, n = 6) with brightfield images of platelet deposition (B) and fluorescence images of DiOC6 deposition (C). Correction for platelet surface differences (∼30% between large and small platelets10 ) would increase surface area coverage (SAC) of small platelets to similar values as the determined SAC for mass-adjusted small platelets. (D-E) Quantification of endpoint analysis (n = 6) of brightfield images of multilayer deposition (D) and fluorescence images of P-selectin expression (E). (F) Heatmap representing each parameter for the respective platelet size subpopulation at the end of the measurement. Unsorted platelets were set at 0 for reference. n = 4. *P < .05 and ****P < .0001 vs unsorted.
Thrombus formation with different platelet fractions. Platelet- and leukocyte-depleted whole blood was reconstituted with unsorted, large, small, or small (mass adjusted) platelets and perfused for 4 minutes at a wall shear rate of 1000 s−1 over a coated microspot containing collagen type I. Thrombus build-up was evaluated by image capturing every 30 seconds during the 4 minutes of blood perfusion and end point analysis after postlabeling and perfusion. (A) Representative brightfield images (upper row) and fluorescent images of DiOC6 (middle row) or P-selectin expression (lower row) of the different platelet fractions after 4 minutes of perfusion. (B-C) Quantification of thrombus build-up (kinetics, n = 6) with brightfield images of platelet deposition (B) and fluorescence images of DiOC6 deposition (C). Correction for platelet surface differences (∼30% between large and small platelets10 ) would increase surface area coverage (SAC) of small platelets to similar values as the determined SAC for mass-adjusted small platelets. (D-E) Quantification of endpoint analysis (n = 6) of brightfield images of multilayer deposition (D) and fluorescence images of P-selectin expression (E). (F) Heatmap representing each parameter for the respective platelet size subpopulation at the end of the measurement. Unsorted platelets were set at 0 for reference. n = 4. *P < .05 and ****P < .0001 vs unsorted.
Enhanced platelet reactivity of large platelets is linked to a highly responsive platelet subpopulation with high GPVI expression
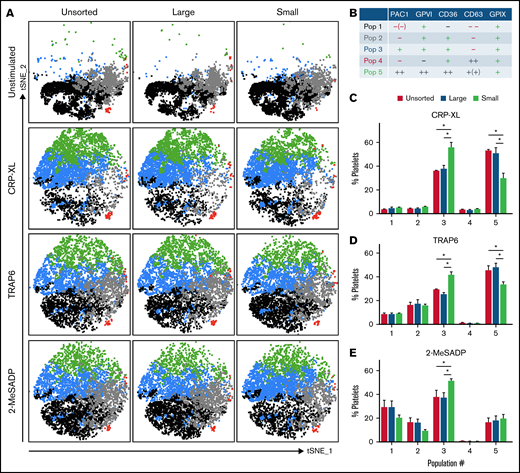
We next aimed to investigate the contribution of platelet size and receptor expression levels on the reactivity of platelets. For this purpose, we applied automated clustering algorithms as an exploration tool on multicolor flow cytometry data of the unsorted, large, and small platelet fractions that were maximally stimulated with CRP-XL, TRAP-6, or stable ADP. Platelets were costained for activated integrin αIIbβ3 (PAC1) and dense and lysosomal secretion (CD63), together with the surface markers GPVI (collagen receptor), CD36/GPIV (scavenger receptor) and CD42a/GPIX (part of the von Willebrand factor–binding GPIb-IX-V complex). Clustering analysis of these data identified 5 platelet populations (Figure 2A-B). Two of them were of high interest because they displayed differences in their abundancy among the various platelet size fractions. First, a strongly reactive platelet population with high GPVI and CD36 expression was identified after stimulation with CRP-XL and TRAP-6 (Pop 5, PAC1++, GPVI++, CD36++, CD63+[+], GPIX+). Pop 5 showed a significantly higher abundancy in the large (and unsorted) platelet fractions compared with the small platelet fraction (Figure 2C-D). The second population of interest (Pop 3) was a platelet population that exhibited integrin activation but did not expose CD63 (indicates granule release) after activation (Pop 3, PAC1+, GPVI+, CD36+, CD63–, GPIX+). Pop 3 was more abundant in the small platelet fraction compared with the other fractions for all agonists tested (Figure 2E).
Population analysis of multicolor flow cytometry data with platelet surface and activation markers on the size-separated platelet fractions. Isolated, size-separated platelets were stimulated with 5 µg/mL CRP-XL, 20 µM TRAP-6, or 5 µM 2-MeSADP and stained simultaneously for PAC1, GPVI, CD36, CD63, and GPIX for 15 minutes before fixation and measuring 10 000 events per sample. Clustering tools (FlowSOM) and a dimensionality reduction algorithm (tSNE) were used to analyze and visualize these multicolor flow cytometry data. (A) Representative tSNE plots with a FlowSOM population filter of the different platelet fractions (unsorted, large, and small) under different stimulations. A heatmap showing the individual profiles of platelet populations per sample is shown in supplemental Figure 2. (B) Characterization of the FlowSOM clusters (5 populations; 1-5). (C-E) Distribution profile of platelet populations upon stimulation with CRP-XL (C), TRAP-6 (D), or 2-MeSADP (E), respectively, resulting from FlowSOM analysis for the different platelet size fractions. Mean ± standard error of mean; n = 4. *P < .05.
Population analysis of multicolor flow cytometry data with platelet surface and activation markers on the size-separated platelet fractions. Isolated, size-separated platelets were stimulated with 5 µg/mL CRP-XL, 20 µM TRAP-6, or 5 µM 2-MeSADP and stained simultaneously for PAC1, GPVI, CD36, CD63, and GPIX for 15 minutes before fixation and measuring 10 000 events per sample. Clustering tools (FlowSOM) and a dimensionality reduction algorithm (tSNE) were used to analyze and visualize these multicolor flow cytometry data. (A) Representative tSNE plots with a FlowSOM population filter of the different platelet fractions (unsorted, large, and small) under different stimulations. A heatmap showing the individual profiles of platelet populations per sample is shown in supplemental Figure 2. (B) Characterization of the FlowSOM clusters (5 populations; 1-5). (C-E) Distribution profile of platelet populations upon stimulation with CRP-XL (C), TRAP-6 (D), or 2-MeSADP (E), respectively, resulting from FlowSOM analysis for the different platelet size fractions. Mean ± standard error of mean; n = 4. *P < .05.
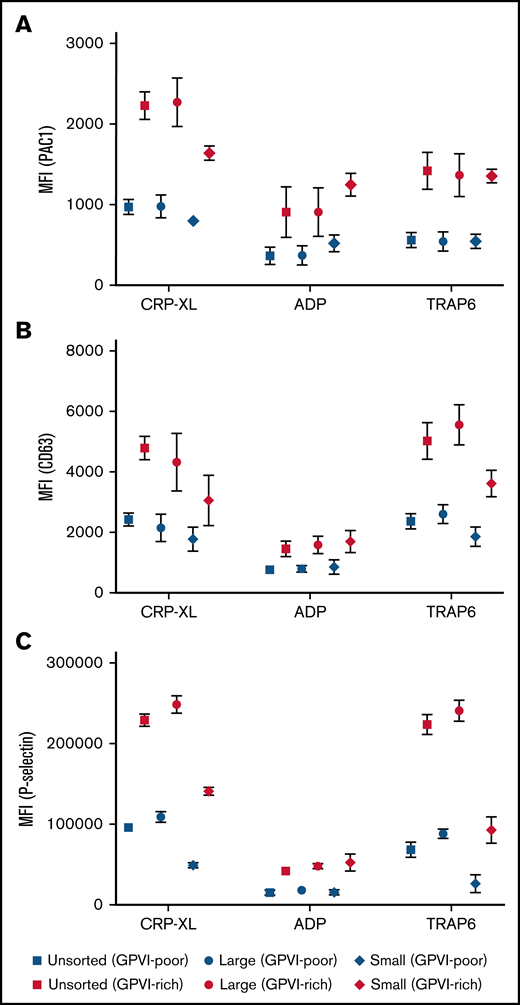
When comparing PAC1 binding (αIIbβ3 integrin activation), CD63 (dense granule secretion), or P-selectin expression (α-granule release) of the top 25% of GPVI-expressing platelets vs the rest of the platelets, GPVI-rich platelets always displayed higher reactivity among unsorted, large, and small platelets, regardless of the agonist used (Figure 3). Together, these results indicate that enhanced platelet reactivity of large platelets is related to a higher proportion of highly responsive platelets with high GPVI expression. In addition, small platelets predominantly exhibit aggregatory responses to ADP stimulation, being also pronounced when they express higher levels of GPVI.
GPVI-rich platelets are more reactive than GPVI-poor platelets, regardless of size. PAC1 (integrin activation) (A), CD63 (dense granule secretion) (B), and P-selectin (α-granule release) (C) expression levels were determined for GPVI-rich (top 25% expressing) platelets and for GPVI-poor (rest of platelets; 75%) platelets after 15 minutes of stimulation with CRP-XL (5 µg/ml), 2-MeSADP (5 µM), or TRAP-6 (20 µM) for the different size fractions. Mean ± standard error of mean; n ≥ 4.
GPVI-rich platelets are more reactive than GPVI-poor platelets, regardless of size. PAC1 (integrin activation) (A), CD63 (dense granule secretion) (B), and P-selectin (α-granule release) (C) expression levels were determined for GPVI-rich (top 25% expressing) platelets and for GPVI-poor (rest of platelets; 75%) platelets after 15 minutes of stimulation with CRP-XL (5 µg/ml), 2-MeSADP (5 µM), or TRAP-6 (20 µM) for the different size fractions. Mean ± standard error of mean; n ≥ 4.
We next analyzed Ca2+ mobilization and P-selectin expression of aspirin-treated large and small platelets, which did not affect the stronger response of large platelets (supplemental Figure 4). This finding excludes that a stronger thromboxane release by large platelets is the driver of these functional differences.
GPVI expression is increased in juvenile and large platelets
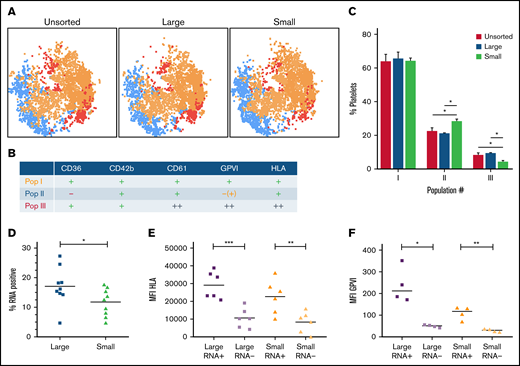
We determined how platelet age relates to the population of high-GPVI–expressing platelets. To do this, we again used multicolor flow cytometry and subsequent automated clustering algorithms, but this time we measured platelet surface markers on the platelet fractions. HLA-I expression was used as a marker for juvenile platelets.16 FlowSOM population analysis resulted in 3 populations (Figure 4A-B). Interestingly, high expression of GPVI is accompanied by high expression of HLA-I. This HLA++ and GPVI++ population (Pop III) was more abundant in the large platelet fraction compared with the small platelet fraction (±10% vs 5%, respectively) (Figure 4C). Hence, a higher proportion of juvenile platelets with increased HLA-I and GPVI expression was found among large platelets. In the small platelet fraction, a population with lower expression of CD36 and GPVI (Pop II) was more abundant compared with the unsorted and large platelet fractions (25% vs ±20%, respectively).
Population analysis of platelet surface markers on the size-separated platelet fractions. Isolated, size-separated platelets were stained for CD36, CD42b, CD61, GPVI, and HLA for 15 minutes before fixation, and 10 000 events were recorded per sample. Clustering tool (FlowSOM) and dimensionality reduction algorithm were used to analyze and visualize these multicolor flow cytometry data. (A) Representative dimensionality reduction algorithm plots with a FlowSOM populations filter for the different platelet fractions. (B) Characterization of the FlowSOM clusters (3 populations; I-III). (C) Distribution profile of platelet populations resulting from FlowSOM analysis for the different platelet size fractions. (D) The proportion of RNA-positive platelets was determined by T-oligonucleotides in separated large and small platelets. (E) Correlation of RNA-content and HLA-I expression was determined by costaining using T-oligonucleotides and HLA-A,B,C-antibody in separated large and small platelets. (F) Relation between RNA content and GPVI expression. Mean ± standard error of mean; n ≥ 4. *P < .05, **P < .01. MFI, mean fluorescence intensity.
Population analysis of platelet surface markers on the size-separated platelet fractions. Isolated, size-separated platelets were stained for CD36, CD42b, CD61, GPVI, and HLA for 15 minutes before fixation, and 10 000 events were recorded per sample. Clustering tool (FlowSOM) and dimensionality reduction algorithm were used to analyze and visualize these multicolor flow cytometry data. (A) Representative dimensionality reduction algorithm plots with a FlowSOM populations filter for the different platelet fractions. (B) Characterization of the FlowSOM clusters (3 populations; I-III). (C) Distribution profile of platelet populations resulting from FlowSOM analysis for the different platelet size fractions. (D) The proportion of RNA-positive platelets was determined by T-oligonucleotides in separated large and small platelets. (E) Correlation of RNA-content and HLA-I expression was determined by costaining using T-oligonucleotides and HLA-A,B,C-antibody in separated large and small platelets. (F) Relation between RNA content and GPVI expression. Mean ± standard error of mean; n ≥ 4. *P < .05, **P < .01. MFI, mean fluorescence intensity.
We then aimed to confirm these findings by measuring GPVI and HLA-I expression in RNA-positive (juvenile) and RNA-negative (older) platelets. To further establish a relation to platelet size, these parameters were determined in separated large and small platelet fractions. The large platelet fraction contained more RNA-positive platelets than the small fraction (Figure 4D). Also, HLA-I and RNA presence was corroborated as markers for platelet age (Figure 4E). The link between GPVI expression levels and platelet age was confirmed; juvenile RNA-positive platelets exhibited higher GPVI expression levels compared with older RNA-negative platelets, regardless of the platelet size fraction (Figure 4F). To rule out a potential bias due to the different size of RNA-positive and RNA-negative platelets, we compared both populations in 15 consecutive forward scatter gates separately. RNA-positive platelets always expressed higher levels of HLA and GPVI on their surface, excluding a size-related artifact (supplemental Methods).
Platelet age is a stronger determinant of platelet reactivity than platelet size
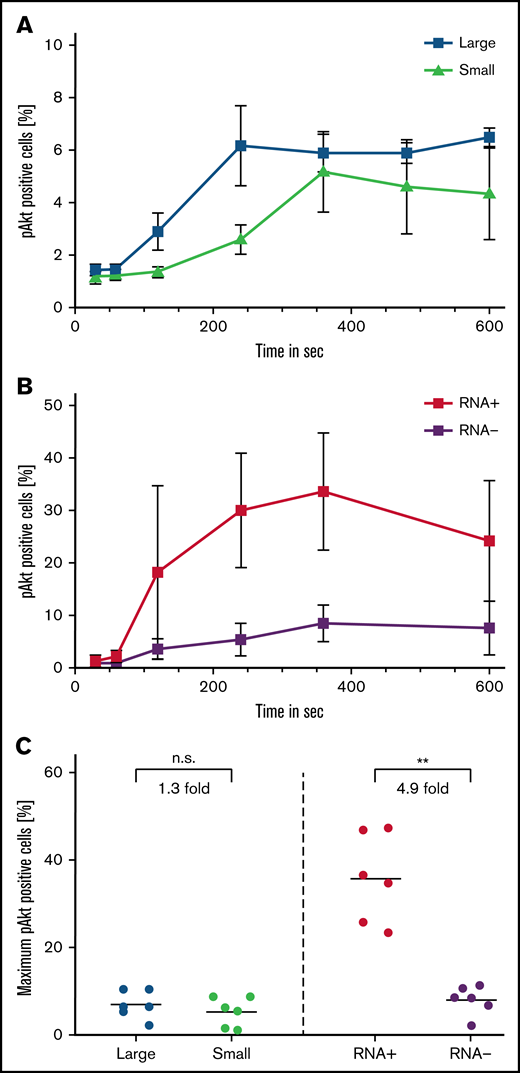
We determined the intensity and velocity of signal transduction for the large and small platelet fractions, as well as for RNA-positive and RNA-negative platelets, after stimulation with 5 µg/mL of collagen. This was done by measuring consecutive phosphorylation of serine/threonine-protein kinase Akt between 30 seconds and 10 minutes after stimulation.
Between the different size fractions, only a small difference in Akt phosphorylation was seen (1.3 fold higher maximum phosphorylation of large [7.0% ± 3.2%] to small platelets [5.4 ± 3.3]; P = .22) (Figure 5A,C). In contrast, juvenile, RNA-positive platelets exhibited a more rapid and higher amount of Akt phosphorylation compared with older, RNA-negative platelets (4.9-fold higher maximum phosphorylation of RNA-poitive [35.8% ± 10.1%] to RNA-negative platelets [8.1 ± 3.3]; P = .004) (Figure 5B-C).
Signal transduction is carried by RNA-positive platelets. Signal transduction was determined by the percentage of pAKT-AF488–positive cells after stimulation with 5 µg/mL (final) collagen in large and small platelets (A) and RNA-positive and RNA-negative platelets (B) as determined by costaining with T-oligonucleotides and pAKT-AF488 antibody. (C) Maximum pAKT-AF488 positive large and small as well as RNA-positive and RNA-negative platelets after stimulation with 5 µg/mL (final) collagen. Mean ± standard error of mean; n ≥ 4. **P < .01. pAKT, phosphorylated AKT; n.s., not significant.
Signal transduction is carried by RNA-positive platelets. Signal transduction was determined by the percentage of pAKT-AF488–positive cells after stimulation with 5 µg/mL (final) collagen in large and small platelets (A) and RNA-positive and RNA-negative platelets (B) as determined by costaining with T-oligonucleotides and pAKT-AF488 antibody. (C) Maximum pAKT-AF488 positive large and small as well as RNA-positive and RNA-negative platelets after stimulation with 5 µg/mL (final) collagen. Mean ± standard error of mean; n ≥ 4. **P < .01. pAKT, phosphorylated AKT; n.s., not significant.
GPVI expression and platelet function decrease during ex vivo platelet aging
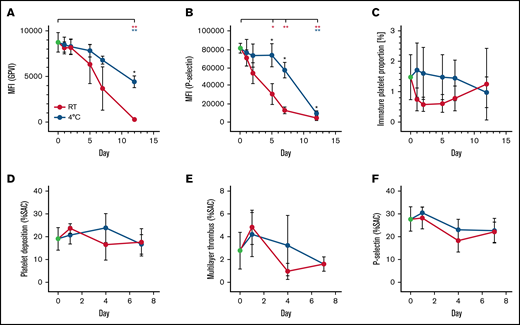
Platelets prepared and stored for transfusion develop progressive functional lesions associated with progressive ex vivo aging. GPVI expression (mean fluorescence intensity; day 0, 8736 ± 1085) was significantly reduced after 12 days on room temperature platelets (267.9 ± 129.9; P = .0056) as well as on cold-stored platelets (655.3 ± 378.4; P = .0049) . Nevertheless, cold-stored platelets better preserved GPVI expression compared with platelets stored at room temperature (Figure 6B). Likewise, CRP-XL–induced P-selectin expression decreased faster during storage at room temperature compared with cold-stored platelets (Figure 6A). The immature platelet fraction of stored platelets declined in both storing conditions (day 0, 1.467% ± 0.7506%; room temperature day 12, 1.233% ± 1.193%; 4°C day 12, 0.9667% ± 0.5033%) (Figure 6C).
Platelet function and GPVI expression decrease during platelet storage. Quantification of GPVI (A), P-selectin expression of platelets stimulated with CRP-XL 5 µg/mL (B). (C) Immature platelet fraction. Platelet deposition (D), Thrombus multilayer formation (E) and P-selectin expression (F), each given as a percent surface area coverage (SAC). Platelets were reconstituted in platelet- and leukocyte-depleted whole blood perfused over collagen-coated surfaces. Platelet concentrates were prepared from buffy coats, split and stored at room temperature (RT, red lines) or 4°C (blue lines). n ≥ 4. *P < .05, **P < .01. Blue and red asterisks represent significant changes over time; black asterisks represent significant differences between storage conditions.
Platelet function and GPVI expression decrease during platelet storage. Quantification of GPVI (A), P-selectin expression of platelets stimulated with CRP-XL 5 µg/mL (B). (C) Immature platelet fraction. Platelet deposition (D), Thrombus multilayer formation (E) and P-selectin expression (F), each given as a percent surface area coverage (SAC). Platelets were reconstituted in platelet- and leukocyte-depleted whole blood perfused over collagen-coated surfaces. Platelet concentrates were prepared from buffy coats, split and stored at room temperature (RT, red lines) or 4°C (blue lines). n ≥ 4. *P < .05, **P < .01. Blue and red asterisks represent significant changes over time; black asterisks represent significant differences between storage conditions.
Interestingly, thrombus formation capacity of stored platelets on collagen-coated surfaces only dropped slightly during storage. No significant differences between the storage conditions were observed for surface area coverage, multilayer formation, or P-selectin expression (Figure 6D-F).
Patients with ITP have younger platelets with increased GPVI expression
To examine our findings in a clinical situation with a higher proportion of immature platelets, we compared platelets of 2 patients with ITP vs platelets of 2 healthy control subjects. As expected, patients with ITP were thrombocytopenic; ITP patient 2 especially, who received a thrombopoietin agonist, exhibited an increased immature platelet fraction and a higher proportion of RNA-rich platelets compared with healthy donors (Figure 7A). Likewise, expression levels of HLA-I and GPVI were higher in patients with ITP compared with healthy control subjects (Figure 7B). Integrin activation response (PAC1 binding) was reduced upon GPVI stimulation in 1 patient and upon stimulation with TRAP and ADP in both patients. Dense granule secretion (CD63) was increased compared with healthy control subjects (especially in patient 2 receiving a thrombopoietin agonist) (Figure 7C-D). When platelet activation of GPVI-rich and GPVI-poor platelets was compared, the former platelet fraction always displayed the highest reactivity, independent of the agonist type and health condition (Figure 7E-F).
Findings confirmed in patients with higher proportion of immature platelets (ITP patients). (A) Platelet indices per donor, including count, mean platelet volume (MPV), flow cytometric forward scatter median fluorescence intensity (MFI), proportion of RNA-rich platelets obtained as in supplemental Figure 5, immature platelet fraction (IPF), and whether donor received thrombopoietin (TPO) receptor agonist. (B-D) Flow cytometric measurements of expression levels of GPVI and HLA-I in resting platelets (B), and PAC1 (C) and CD63 (D) upon stimulation with CRP-XL (5 µg/mL), 2-MeSADP (5 µM), or TRAP-6 (20 µM) per donor. Details on color coding are given in panel A. PAC1 (E) and CD63 (F) expression levels were determined for GPVI-rich (top 25% expressing) platelets and for GPVI-poor (rest of platelets; 75%) platelets for each agonist. Mean ± standard error of mean; n = 2 per group.
Findings confirmed in patients with higher proportion of immature platelets (ITP patients). (A) Platelet indices per donor, including count, mean platelet volume (MPV), flow cytometric forward scatter median fluorescence intensity (MFI), proportion of RNA-rich platelets obtained as in supplemental Figure 5, immature platelet fraction (IPF), and whether donor received thrombopoietin (TPO) receptor agonist. (B-D) Flow cytometric measurements of expression levels of GPVI and HLA-I in resting platelets (B), and PAC1 (C) and CD63 (D) upon stimulation with CRP-XL (5 µg/mL), 2-MeSADP (5 µM), or TRAP-6 (20 µM) per donor. Details on color coding are given in panel A. PAC1 (E) and CD63 (F) expression levels were determined for GPVI-rich (top 25% expressing) platelets and for GPVI-poor (rest of platelets; 75%) platelets for each agonist. Mean ± standard error of mean; n = 2 per group.
Discussion
In this study, we show that GPVI expression and platelet age are relevant factors to explain the heterogeneous interactions of platelets with different sizes upon collagen-induced thrombus formation. Juvenile GPVI-rich platelets are highly responsive to common platelet agonists and found predominantly among larger platelets. By linking GPVI expression to increased platelet size, juvenile age, and higher reactivity, we extend its relevance in thrombosis and thrombo-inflammation,17 platelet storage, and ITP.
A major problem for studying different platelet populations in thrombus formation is to establish an experimental setup that allows separate analysis of platelet subpopulations in whole blood, which is closer to the physiological situation, compared with studies with PRP or washed platelets. Here, we depleted whole blood from leukocytes and platelets by filtration, taking advantage of highly effective and standardized blood bank techniques. The remaining blood leaves erythrocytes and plasma factors unaltered and could be reconstituted with different platelet size fractions and stored platelets.
Using this setup, we found that platelet size clearly determines the extent and the velocity of thrombus growth. Specifically, large platelets were able to adhere faster to collagen matrices and to produce larger thrombi in whole blood under flow. This extends previous findings of a higher reactivity of large platelets compared with small platelets in PRP or buffer.4 We could exclude that the higher overall platelet mass of large platelets accounts for their better performance in thrombus formation by testing also mass adjusted small platelet fractions as described.10 Although a higher number of small platelets (= mass adjusted to the large platelet fraction) could form larger thrombi, they still formed smaller thrombi compared with the large platelet fraction (Figure 1). This makes an artifact produced by different platelet masses unlikely and emphasizes that large platelets perform stronger in collagen-induced thrombus formation.
We hypothesized that additional features exist as well as platelet size being associated with platelet reactivity. We therefore determined the abundance of the collagen receptor GPVI, CD36 (also known as “GPIV,”18 a highly abundant glycoprotein with a differential inter-donor expression pattern compared with other platelet receptors19 ), and GPIX. The latter is part of the GPIb-IX-V receptor complex binding von Willebrand factor and has similar receptor densities on large and small platelets.10 We thus included GPIX as a surface marker to control for size differences in activated and nonactivated platelets. Furthermore, we assessed their link to platelet reactivity in response to TRAP-6, CRP-XL, and ADP using multicolor flow cytometry and clustering algorithms. Platelets with increased GPVI expression displayed stronger responses to all 3 agonists, and these GPVI-rich platelets were higher distributed among large platelet fractions.
Importantly, GPVI-rich platelets had similar GPIX expression compared with the other platelet populations identified by using clustering algorithms, which excludes a size-related artifact in a way that larger platelets always exhibit stronger overall fluorescence signals. We also ruled out a bias of increased thromboxane release of large platelets upon stimulation by showing that aspirin could not diminish the differences between large and small platelets (supplemental Figure 4). Furthermore, the strongest response to ADP was seen in GPVI-rich small platelets, which confirms our previous studies that small platelets are prone to activate αIIbβ3 after ADP stimulation.10 We extent these findings by showing that high GPVI expression is also linked to this probably unique function of smaller platelets.
Next, we characterized highly responsive GPVI-rich platelets and found that platelets with high GPVI expression also express higher levels of HLA-I, an established marker for juvenile platelets.16 This was validated by showing that HLA-I and GPVI expression correlated with the RNA content independent of platelet size (Figure 4). Again, we found a higher proportion of highly reactive GPVI-rich juvenile platelets among large platelets, which can explain the differences detected between large and small platelets during thrombus formation. Notably, the population with high GPVI levels also exhibited high levels of CD36, which is known to support thrombus formation in response to collagen.20
To dissect the relevance of platelet size and age, we assessed signal transduction based on Akt phosphorylation in large vs small and RNA-rich vs RNA-poor platelets. Akt represents a key regulator position in the signal transduction of collagen-induced platelet activation. GPVI stimulation leads to phosphorylation of Akt via LYN and phosphatidylinositol 3-kinase and contributes to granule secretion and integrin activation.21-23 The absolute difference in AKT phosphorylation between large and small platelets was only 2.4%, whereas the differences between juvenile, RNA-rich, and older RNA-poor platelets was 28.6%. This corresponds to a 12-fold higher difference, which clearly indicates that platelet age is the dominant determinant of platelet reactivity. Apart from GPVI, autocrine stimulation with ADP also stimulates phosphatidylinositol 3-kinase,24 which may further benefit young platelets in this experimental setting.
We also showed that GPVI expression and platelet function decrease throughout routine platelet storage for transfusion, supporting the link between platelet age and GPVI expression. This finding is in line with earlier studies that have shown progressive shedding of GPVI in platelet storage.25 Interestingly, cold storage of platelets at 4°C better maintained platelet GPVI levels and platelet response to CRP-XL or collagen. Cold storage may protect GPVI from shedding and provide an easy strategy to maintain GPVI-related platelet function. However, this may selectively apply for buffy coat–derived PCs investigated in our study, as Miles et al26 recently described a pronounced GPVI decrease in apheresis-derived cold-stored platelets vs room temperature–stored platelets. Of note, shedding of GPVI may also occur during platelet aging in vivo because soluble GPVI is detectable in healthy subjects.27
Finally, we investigated GPVI expression and platelet function from 2 patients with ITP (Figure 7). In patients with ITP, platelets are younger because their platelet life span is decreased due to antibody-mediated platelet clearance resulting in an increased platelet generation. Our study confirms higher proportions of juvenile platelets in patients with ITP associated with higher expression levels of HLA-I and GPVI. This only in part correlated with platelet response after stimulation of GPVI: dense granule secretion was increased in the patients with ITP compared with that in healthy control subjects, whereas αIIbβ3 integrin activation was reduced upon GPVI stimulation (especially in 1 patient). The latter likely reflects the presence of autoantibodies in patients with ITP,28 which most commonly target αIIbβ3 (GPIIb/IIIa) and may inhibit binding of PAC1 and interfere with our applied readout system. Anti-GPVI autoantibody-induced GPVI shedding, however, has been observed in ITP,29 which could also explain the decreased response to GPVI activation. Nevertheless, GPVI-rich platelets were still the most reactive ones also in patients with ITP. This underscores the association of GPVI expression with platelet age and reactivity.
The increased abundance of GPVI on highly reactive juvenile human platelets is a new finding, although a relationship between GPVI expression and platelet function has been established by several previous studies. Genetic variants of GP6 determine GPVI-induced platelet activation and thrombus formation.19,30-32 Furthermore, GPVI is shed upon platelet activation17 by a metalloproteinase involving ADAM10.33,34 This process may be accelerated in sepsis and trauma patients, in whom GPVI expression decreases and results in an acquired platelet dysfunction.35,36 It may also occur in ITP due to autoantibody-induced GPVI shedding.29 Here we show that high GPVI receptor density is found on juvenile platelets, supporting the concept that progressive GPVI shedding occurs throughout their life span.33 Moreover, the findings indicate that elevated GPVI levels of juvenile platelets result from preferential packaging of GPVI protein into newly formed platelets by parent megakaryocytes rather than de novo synthesis of GPVI in mature platelets. Consequently, targeting GPVI and its downstream signaling pathways is reasonable as a therapeutic strategy aiming to interfere with platelet reactivity.17
Our study is somewhat in contrast to a recent study by Gupta et al,37 who found a GPVI signaling defect in juvenile mouse platelets after GPIbα antibody–mediated platelet depletion. The different observations may result from various antibodies inducing thrombocytopenia. Moreover, a complete depletion of platelets resembling similar conditions in humans compared with the mouse study is, for many reasons, not possible and therefore makes comparisons difficult.
In accordance with the pivotal studies performed by Thompson et al6 -8,38 in the 1980s, our results underpin that platelet size and age independently affect platelet reactivity for 2 reasons: (1) RNA-poor large platelets exist with apparently lower reactivity; and (2) RNA-rich small platelets exist with a higher reactivity compared with their RNA- counterparts. We found that reactivity was closely linked to GPVI expression. We suggest that ADP activation promotes the formation of a pro-aggregatory platelet population, which may predominantly involve smaller platelets. This concept needs further evaluation in upcoming studies.
In summary, we could establish a link between GPVI expression, platelet size, age, and reactivity. Our findings are important to better target highly reactive juvenile platelets in future studies addressing platelet subpopulations, to improve platelet storage, and to investigate diseases with increased proportions of juvenile platelets such as ITP.
Acknowledgments
Support is acknowledged from the Cardiovascular Center (HVC) Maastricht University Medical Centre+ and the Interreg Euregio Meuise-Rhin program Polyvalve, Liege-Maastricht-Hasselt-Aachen, and the Netherlands Landsteiner Foundation for Transfusion Research (grant no. 1711), and the DFG (project no. 374031971–TRR 240).
Authorship
Contribution: A.V. performed the multicolor flow cytometry and ITP patient experiments, evaluated the data, prepared the figures, and wrote the manuscript; S.H. performed the conventional flow cytometry experiments, contributed to the whole-blood perfusion experiments and the multicolor flow cytometry experiments, evaluated the data, and wrote the manuscript; K.A. contributed to the whole-blood perfusion experiments and storage experiments; B.M.E.T. and S.L.N.B. contributed to the whole-blood perfusion experiments and the multicolor flow cytometry experiments; S.L.S. performed the storage experiments; F.C.J.I.H.-M. enrolled the ITP patients; A.G. and J.W.M.H. supervised the project, evaluated the data, and edited the manuscript; and P.E.J.v.d.M. and T.T. designed the project, supervised the project, evaluated the data, and wrote and edited the manuscript; and all authors reviewed the final version of the manuscript.
Conflict-of-interest disclosure: A.G. reports grants from Deutsche Forschungsgemeinschaft, during the conduct of the study; grants and nonfinancial support from Aspen, Boehringer Ingelheim, MSD, Bristol Myers Squibb, ParinGenix, Bayer Healthcare, Gore Inc., Rovi, Sagent, and BioMarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, MSD, Macopharma, Bristol Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, and Ergomed, outside the submitted work. T.T. reports personal fees and other from Bristol Myers Squibb, Pfizer, and Chugai Pharma; personal fees from Bayer and Novartis; and other from Novo Nordisk and Daichii Sankyo, outside the submitted work. J.W.M.H. is founder and co-owner at FlowChamber, and is a consultant at Synapse. The remaining authors declare no competing financial interests.
Correspondence: Thomas Thiele, Transfusionsmedizin, Sauerbruchstr, 17487 Greifswald, Germany; e-mail: thomas.thiele@med.uni-greifswald.de.
References
Author notes
A.V. and S.H. are joint first authors.
P.E.J.v.d.M. and T.T. are joint senior authors.
Requests for data sharing may be submitted to the corresponding author (thomas.thiele@med.uni-greifswald.de).
The full-text version of this article contains a data supplement.