Key Points
Enhanced efficacy outcomes can result from prolonged duration of copanlisib therapy in patients with relapsed/refractory MZL.
Long-term copanlisib treatment is tolerable and leads to durable responses, supporting its use in patients with relapsed/refractory MZL.
Abstract
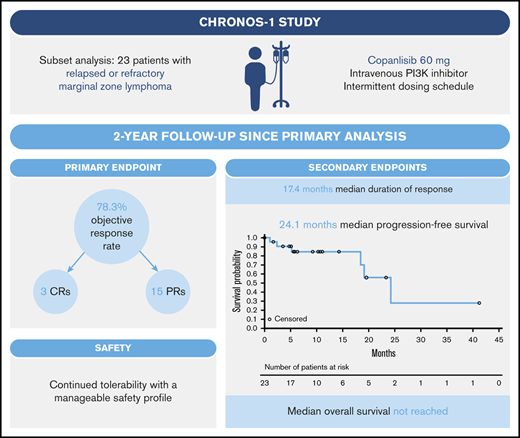
Marginal zone lymphoma (MZL) is challenging to treat, with many patients relapsing following initial treatment. We report the long-term efficacy and safety of copanlisib, a pan-class I phosphoinositide 3-kinase (PI3K) inhibitor, in the subset of 23 patients with relapsed/refractory MZL treated in the phase 2 CHRONOS-1 study (#NCT01660451, Part B; www.clinicaltrials.gov). Patients had a median of 3 prior lines of therapy, including rituximab and alkylating agents, and received IV copanlisib 60 mg on days 1, 8, and 15 of 28-day cycles for a median of 23 weeks. The objective response rate was 78.3% (18/23; 3 complete responses and 15 partial responses). The median duration of response was 17.4 months (median follow-up, 9.4 months), and median time to response was 2.1 months. Median progression-free survival was 24.1 months (median follow-up, 10.3 months), and median overall survival was not reached (median follow-up, 28.4 months). The most common all-grade treatment-emergent adverse events (TEAEs) included fatigue (52.2%, 12/23), diarrhea, and transient, infusion-related hyperglycemia (each 47.8%, 11/23). Nineteen patients (82.6%) had grade 3/4 TEAEs, most commonly transient, infusion-related hyperglycemia and hypertension (each 39.1%, 9/23). TEAEs led to dose reduction or dose interruptions /delays in 9 patients (39.1%) and 18 patients (78.3%), respectively. Patients with activated PI3K/B-cell antigen receptor signaling had improved response rates. Overall, copanlisib demonstrated strong efficacy, with a short time to objective response, improved objective response rate with longer treatment duration, durable responses, and manageable safety, in line with previous reports. These data provide rationale for long-term treatment with copanlisib in patients with relapsed/refractory MZL.
Introduction
Marginal zone lymphoma (MZL) comprises >10% of indolent B-cell lymphomas1 ; it is challenging to treat based on underlying disease heterogeneity,2 and many patients relapse. The Bruton tyrosine kinase inhibitor ibrutinib was the first targeted therapy approved in the United States3 for the treatment of patients with relapsed or refractory MZL, based on a phase 2 study reporting an objective response rate (ORR) of 48% and a median progression-free survival (PFS) of 14.2 months.4
Copanlisib is a pan-class I phosphoinositide 3-kinase (PI3K) inhibitor with potent activity against the PI3K-α and PI3K-δ isoforms, approved for the treatment of relapsed follicular lymphoma in patients who have received ≥2 lines of systemic therapy.5 Approval was based on results from the phase 2 CHRONOS-1 study in 142 patients with relapsed or refractory indolent B-cell lymphoma, including 23 patients with MZL; at the time of primary analysis, prespecified subgroup analysis of ORR in patients with MZL was 69.6% (overall ORR, 59.2%),6 resulting in breakthrough therapy designation for adults with MZL who have received ≥2 lines of systemic therapies.7 The long-term efficacy and safety of IV copanlisib in patients with relapsed or refractory indolent B-cell lymphoma from the CHRONOS-1 population (2-year follow-up) have recently been reported.8 Here, we report further details on the long-term efficacy and safety of copanlisib in the MZL subset from CHRONOS-1.
Methods
CHRONOS-1 was an open-label, single-arm, phase 2 study evaluating the efficacy and safety of single-agent copanlisib in patients with relapsed or refractory indolent B-cell lymphoma, as reported previously (#NCT01660451, Part B).6 Patients received a 1-hour IV infusion of copanlisib 60 mg on days 1, 8, and 15 of 28-day treatment cycles, until disease progression9 or unacceptable toxicity. The study protocol was compliant with the Declaration of Helsinki and Good Clinical Practice and approved by the appropriate ethics committees. All patients provided written, informed consent. All authors had a role in analyzing the data and had access to the primary clinical trial data.
In this analysis, eligible patients were aged ≥18 years with histologically confirmed MZL (splenic, nodal, or mucosa-associated lymphoid tissue lymphoma), relapsed or refractory after ≥2 lines of therapy, including rituximab and alkylating agents, with an Eastern Cooperative Oncology Group performance status of ≤2 and ≥1 bidimensionally measurable lesion.
The primary efficacy variable was centrally assessed ORR after ≥4 treatment cycles, defined as the proportion of patients with a best response of complete response (CR) or partial response (PR).9 Secondary efficacy variables included duration of response, PFS, and overall survival. Adverse events were graded using Common Terminology Criteria for Adverse Events (v4.03). Affymetrix GeneChip Human Gene 1.0 ST Arrays (AltheaDx, San Diego, CA) were used for messenger RNA extraction and gene expression profiling, using archival formalin-fixed paraffin-embedded tumor tissues.6
Response rate estimates and 95% confidence intervals (CIs) were calculated using exact Clopper-Pearson methodology. Time-to-event variables were described using Kaplan-Meier estimates. Gene expression analysis and weighted gene expression scores were conducted using logistic regression models.6
Results
Twenty-three patients with relapsed or refractory MZL who received copanlisib in the CHRONOS-1 study were included in this analysis. Fifteen patients (65%) were female; median age was 69 years (range, 39 to 81) (Table 1). Nodal MZL was the most common histological subtype, followed by mucosa-associated lymphoid tissue lymphoma and splenic MZL (Table 1). Patients had received a median of 3 lines of therapy; 11 (47.8%) were refractory to the last regimen and 19 (82.6%) were refractory to any regimen. Patients received a median of 5.8 copanlisib treatment cycles (range, 0.3 to 47.8), and the median duration of copanlisib treatment was 23 weeks (range, 1 to 191).
Patient demographics and baseline characteristics
| . | MZL subtype* . | . | ||
|---|---|---|---|---|
| . | Nodal MZL (n = 15) . | MALT lymphoma (n = 4) . | Splenic MZL (n = 4) . | Total (N = 23) . |
| Females, n (%) | 7 (46.7) | 4 (100) | 4 (100) | 15 (65.2) |
| Median age, y (range) | 71 (47-81) | 63 (39-75) | 62 (41-78) | 69 (39-81) |
| Baseline ECOG performance status, n (%) | ||||
| 0 | 9 (60.0) | 1 (25.0) | 2 (50.0) | 12 (52.2) |
| 1 | 6 (40.0) | 3 (75.0) | 2 (50.0) | 11 (47.8) |
| 2 | 0 | 0 | 0 | 0 |
| Median time from most recent progression, mo (range) | 3.2 (1.1-14.9) | 7.4 (2.3-7.8) | 2.6 (1.1-8.3) | 3.2 (1.1-14.9) |
| Median prior anticancer therapy lines, n (range) | 2 (2-5) | 3 (2-3) | 3.5 (2-9) | 3 (2-9) |
| Refractory to last regimen, n (%) | 6 (40.0) | 2 (50.0) | 3 (75.0) | 11 (47.8) |
| Rituximab | 7 (46.7) | 2 (50.0) | 1 (25.0) | 10 (43.5) |
| Alkylating agents | 7 (46.7) | 2 (50.0) | 2 (50.0) | 11 (47.8) |
| Rituximab and alkylating agents | 5 (33.3) | 2 (50.0) | 1 (25.0) | 8 (34.8) |
| . | MZL subtype* . | . | ||
|---|---|---|---|---|
| . | Nodal MZL (n = 15) . | MALT lymphoma (n = 4) . | Splenic MZL (n = 4) . | Total (N = 23) . |
| Females, n (%) | 7 (46.7) | 4 (100) | 4 (100) | 15 (65.2) |
| Median age, y (range) | 71 (47-81) | 63 (39-75) | 62 (41-78) | 69 (39-81) |
| Baseline ECOG performance status, n (%) | ||||
| 0 | 9 (60.0) | 1 (25.0) | 2 (50.0) | 12 (52.2) |
| 1 | 6 (40.0) | 3 (75.0) | 2 (50.0) | 11 (47.8) |
| 2 | 0 | 0 | 0 | 0 |
| Median time from most recent progression, mo (range) | 3.2 (1.1-14.9) | 7.4 (2.3-7.8) | 2.6 (1.1-8.3) | 3.2 (1.1-14.9) |
| Median prior anticancer therapy lines, n (range) | 2 (2-5) | 3 (2-3) | 3.5 (2-9) | 3 (2-9) |
| Refractory to last regimen, n (%) | 6 (40.0) | 2 (50.0) | 3 (75.0) | 11 (47.8) |
| Rituximab | 7 (46.7) | 2 (50.0) | 1 (25.0) | 10 (43.5) |
| Alkylating agents | 7 (46.7) | 2 (50.0) | 2 (50.0) | 11 (47.8) |
| Rituximab and alkylating agents | 5 (33.3) | 2 (50.0) | 1 (25.0) | 8 (34.8) |
ECOG, Eastern Cooperative Oncology Group; MALT, mucosa-associated lymphoid tissue.
Determined based on investigator assessment.
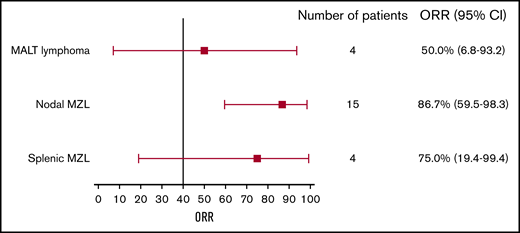
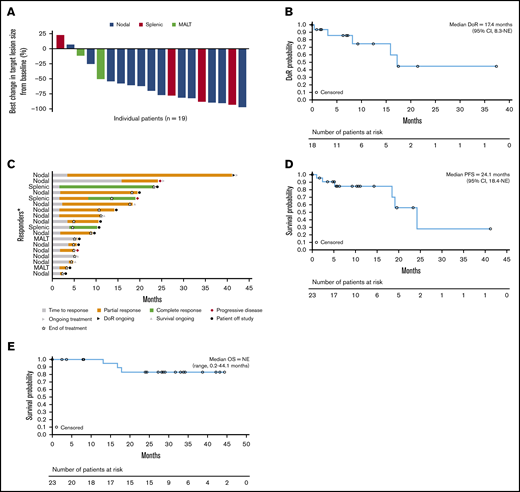
Eighteen patients had an objective tumor response, resulting in an ORR of 78.3%.8 ORRs by MZL subtype were 86.7% (13/15), 75% (3/4), and 50% (2/4) for nodal, splenic, and mucosa-associated lymphoid tissue MZL, respectively (Table 2; Figure 1). Three patients (13.0%, all with splenic MZL) had CRs; 1 of these transitioned from a PR with the longer treatment period. Fifteen evaluable patients (78.9%) showed ≥50% reduction in tumor size from baseline (Figure 2A).8
Objective tumor response by histological subtype
| . | MZL subtype . | . | ||
|---|---|---|---|---|
| n (%) . | Nodal MZL (n = 15) . | MALT lymphoma (n = 4) . | Splenic MZL (n = 4) . | Total (N = 23) . |
| CR | 0 | 0 | 3 (75.0) | 3 (13.0) |
| PR | 13 (86.7) | 2 (50.0) | 0 | 15 (65.2) |
| ORR | 13 (86.7) | 2 (50.0) | 3 (75.0) | 18 (78.3) |
| Disease control rate | 13 (86.7) | 4 (100) | 3 (75.0) | 20 (87.0) |
| Stable disease | 0 | 2 (50.0) | 0 | 2 (8.7) |
| Not available* | 2 (13.3) | 0 | 0 | 2 (8.7) |
| Not evaluable† | 0 | 0 | 1 (25.0) | 1 (4.3) |
| . | MZL subtype . | . | ||
|---|---|---|---|---|
| n (%) . | Nodal MZL (n = 15) . | MALT lymphoma (n = 4) . | Splenic MZL (n = 4) . | Total (N = 23) . |
| CR | 0 | 0 | 3 (75.0) | 3 (13.0) |
| PR | 13 (86.7) | 2 (50.0) | 0 | 15 (65.2) |
| ORR | 13 (86.7) | 2 (50.0) | 3 (75.0) | 18 (78.3) |
| Disease control rate | 13 (86.7) | 4 (100) | 3 (75.0) | 20 (87.0) |
| Stable disease | 0 | 2 (50.0) | 0 | 2 (8.7) |
| Not available* | 2 (13.3) | 0 | 0 | 2 (8.7) |
| Not evaluable† | 0 | 0 | 1 (25.0) | 1 (4.3) |
Includes patients with only post–baseline tumor assessment who could not be assessed.
Includes patients with no post–baseline tumor assessment, but who discontinued because of a drug-related toxicity, death, or progression by clinical judgment before disease was reevaluated.
ORR by histological subtype. Error bars represent 95% CI. MALT, mucosa-associated lymphoid tissue.
ORR by histological subtype. Error bars represent 95% CI. MALT, mucosa-associated lymphoid tissue.
Patient response and efficacy outcomes. (A) Best change in target lesion size by histological subtype. (B) Duration of response. (C) Response in patients achieving a CR or PR. (D) PFS. (E) Overall survival. Median DoR includes censored values for 13 patients, and median PFS includes censored values for 17 patients. The percentage best change in the sum of the diameters of all target lesions from baseline as assessed by the investigator is shown; the best change in target lesion size per the investigator onsite assessment could not be determined in 4 patients. DoR, duration of response; NE, not evaluable; OS, overall survival. *Each bar represents 1 patient.
Patient response and efficacy outcomes. (A) Best change in target lesion size by histological subtype. (B) Duration of response. (C) Response in patients achieving a CR or PR. (D) PFS. (E) Overall survival. Median DoR includes censored values for 13 patients, and median PFS includes censored values for 17 patients. The percentage best change in the sum of the diameters of all target lesions from baseline as assessed by the investigator is shown; the best change in target lesion size per the investigator onsite assessment could not be determined in 4 patients. DoR, duration of response; NE, not evaluable; OS, overall survival. *Each bar represents 1 patient.
The median duration of response was 17.4 months (range, 0 to 37.6; 95% CI, 8.3 to not evaluable), with a median follow-up of 9.4 months; 45.0% of patients were estimated to be in response after 2 years (95% CI, 12 to 74) (Figure 2B). In the subgroup of patients with nodal MZL, the median duration of response was 16.1 months (95% CI, 3.3 to not estimable). The overall median time to response was 2.1 months (range, 1.6 to 15.9; 95% CI, 1.7 to 3.7) (Figure 2C). Two of the 3 observed CRs were achieved after 2.1 months, and for 1 patient who had stable disease at the time of the primary analysis, a PR was achieved with prolonged treatment over 15 months (Figure 2C). Median PFS was 24.1 months (range, 0 to 41.1) (Figure 2D), with a median follow-up of 10.3 months; the estimated PFS rate at 2 years was ∼56% (95% CI, 20 to 82). Patients with nodal and nonnodal MZL had a median PFS of 24.1 months (range, 0 to 41.1) and 19.0 months (range, 1.0 to 23.2), respectively. Median overall survival was not reached (range, 0.2 to 44.1 months; median follow-up, 28.4 months), and the estimated survival rate at 2 years was 83% (Figure 2E).
Eight patients had available gene expression data for exploratory analysis, and 7 were evaluable for response; 6 had an objective response, including 1 CR (supplemental Figure 1). The patient with a CR demonstrated high expression levels of the PI3K and B-cell antigen receptor (BCR) pathway signatures; the 1 patient with stable disease had among the lowest expression levels.
At least 1 treatment-emergent adverse event (TEAE) was recorded in all 23 patients, with grade 3/4 TEAEs recorded in 19 patients (82.6%). No grade 5 TEAEs were reported. All-grade TEAEs most commonly reported (≥10%) included fatigue (52.2%, 12/23), diarrhea, and hyperglycemia (each 47.8%, 11/23) (Table 3). The most common grade 3/4 TEAEs (≥13%) included hyperglycemia, hypertension (each 39.1%, 9/23), fatigue, diarrhea, neutropenia, and pneumonia (each 13.0%, 3/23) (Table 3). Drug-related TEAEs of any grade were reported in 21 patients (91.3%), most commonly (≥25%) hyperglycemia (47.8%, 11/23), hypertension (43.5%, 10/23), diarrhea, and neutropenia (each 26.1%, 6/23). The most commonly recorded (≥5%) grade 3/4 drug-related TEAEs were hyperglycemia, hypertension (each 39.1%, 9/23), diarrhea (13.0%, 3/23), neutropenia, pneumonia, and fatigue (each 8.7%, 2/23). Serious TEAEs of any grade were recorded in 13 patients (56.5%), with grade 3/4 serious TEAEs recorded in 9 patients (39.1%). The most common (≥5%) all-grade serious TEAEs were pneumonia, pyrexia (13.0% each, 3/23), and hyperglycemia (8.7%, 2/23). The most common grade 3/4 serious TEAEs (≥5%) were hyperglycemia and pneumonia (each 8.7%, 2/23). Drug-related serious TEAEs of any grade were reported in 6 patients (26.1%), most commonly (≥5%) grade 3 pneumonia in 2 patients (8.7%) and hyperglycemia in 2 patients (8.7%, 1 grade 3 and 1 grade 4).
Frequency of most common TEAEs (occurring in ≥10% of the population)
| . | Any grade (n = 23) . | Grade 3/4 (n = 23) . |
|---|---|---|
| Any TEAE, n (%) | 23 (100) | 19 (82.3) |
| Fatigue | 12 (52.2) | 3 (13.0) |
| Diarrhea | 11 (47.8) | 3 (13.0) |
| Hyperglycemia | 11 (47.8) | 9 (39.1) |
| Hypertension | 10 (43.5) | 9 (39.1) |
| Nausea | 6 (26.1) | 0 |
| Neutropenia | 6 (26.1) | 3 (13.0) |
| Pruritus | 6 (26.1) | 0 |
| Anemia | 5 (21.7) | 1 (4.3) |
| Cough | 5 (21.7) | 0 |
| Pyrexia | 5 (21.7) | 1 (4.3) |
| Upper abdominal pain | 5 (21.7) | 0 |
| Constipation | 4 (17.4) | 0 |
| Pneumonia | 4 (17.4) | 3 (13.0) |
| Arthralgia | 3 (13.0) | 0 |
| Dizziness | 3 (13.0) | 0 |
| Insomnia | 3 (13.0) | 0 |
| Maculopapular rash | 3 (13.0) | 0 |
| Muscle spasms | 3 (13.0) | 1 (4.3) |
| Oropharyngeal pain | 3 (13.0) | 0 |
| Upper respiratory infection | 3 (13.0) | 1 (4.3) |
| Vomiting | 3 (13.0) | 0 |
| . | Any grade (n = 23) . | Grade 3/4 (n = 23) . |
|---|---|---|
| Any TEAE, n (%) | 23 (100) | 19 (82.3) |
| Fatigue | 12 (52.2) | 3 (13.0) |
| Diarrhea | 11 (47.8) | 3 (13.0) |
| Hyperglycemia | 11 (47.8) | 9 (39.1) |
| Hypertension | 10 (43.5) | 9 (39.1) |
| Nausea | 6 (26.1) | 0 |
| Neutropenia | 6 (26.1) | 3 (13.0) |
| Pruritus | 6 (26.1) | 0 |
| Anemia | 5 (21.7) | 1 (4.3) |
| Cough | 5 (21.7) | 0 |
| Pyrexia | 5 (21.7) | 1 (4.3) |
| Upper abdominal pain | 5 (21.7) | 0 |
| Constipation | 4 (17.4) | 0 |
| Pneumonia | 4 (17.4) | 3 (13.0) |
| Arthralgia | 3 (13.0) | 0 |
| Dizziness | 3 (13.0) | 0 |
| Insomnia | 3 (13.0) | 0 |
| Maculopapular rash | 3 (13.0) | 0 |
| Muscle spasms | 3 (13.0) | 1 (4.3) |
| Oropharyngeal pain | 3 (13.0) | 0 |
| Upper respiratory infection | 3 (13.0) | 1 (4.3) |
| Vomiting | 3 (13.0) | 0 |
TEAEs were coded according to the Medical Dictionary for Regulatory Activities.
Nine patients (39.1%) had TEAEs leading to dose reduction, with the most frequent (≥13%) being hypertension (17.4%, 4/23) and hyperglycemia (13.0%, 3/23). Eighteen patients (78.3%) had TEAEs leading to dose interruptions or delays, with the most frequent (≥13%) being decreased neutrophil count (17.4%, 4/23), diarrhea, fatigue, and hypertension (each 13.0%, 3/23). The median duration of dose interruption or delay was 1.0 week (range, 0.1 to 2.0). In total, 5 patients (21.7%) discontinued copanlisib because of drug-related TEAEs of lung infection (8.7%, 2/23), diarrhea, fatigue, and maculopapular rash (4.3%, 1 patient each).
Laboratory toxicities of interest included increased aspartate transaminase and increased alanine transaminase (all grade, 31.8% and 27.3%, respectively), both largely grade 1.
Discussion
The results of the 2-year follow-up of the CHRONOS-1 study demonstrate that copanlisib had highly effective antitumor activity in the 23 patients with MZL. At the 2-year follow-up, the ORR was marginally higher than previously reported at the time of the primary analysis (78.3% vs 69.6%).6 Responses were durable, with a median duration of response of 17.4 months. Likewise, although the overall median time to response was 2.1 months, 1 patient who had a PR at the time of the primary analysis achieved a CR and 1 patient who initially had stable disease achieved a PR following prolonged treatment with copanlisib. These results indicate that long-term treatment with copanlisib resulted in sustained, and possibly enhanced, responses.
Other measures of efficacy were also durable. The median PFS of 24.1 months exceeded previous findings (11.2 months)6 ; to our knowledge, this is considerably greater than in phase 1 and 2 studies of other PI3K inhibitors, such as umbralisib10 and idelalisib,11 and other targeted agents4 in MZL. Median overall survival was not reached, but the estimated survival rate at 2 years was 83%.
Patients with nodal histology comprised approximately two-thirds of the MZL subset. It is interesting to note that both the ORR (86.7%) and median PFS (24.1 months) for this subgroup were more than twice those previously reported for patients with nodal MZL treated with a Bruton tyrosine kinase inhibitor (41% and 8.3 months, respectively).4
In an exploratory gene expression analysis of 8 patients, higher expression levels of the PI3K and BCR pathway signatures were associated with improved response rates, and potentially enhanced antitumor activity of copanlisib in tumors with activation of PI3K/BCR pathway signaling, consistent with results reported in the CHRONOS-1 population.6 Although based on a very small number of patients, results may support the mechanism of action of copanlisib and a role for PI3K activation in MZL oncogenesis.
The most common TEAEs of any grade were consistent with the overall primary analysis and previous reports of copanlisib12-14 and included fatigue (52.2%), diarrhea, and hyperglycemia (each 47.8%). Hyperglycemia and hypertension were transient, infusion related, and manageable, in line with previous reports.13,15 Incidence of hyperglycemia, a known side effect of PI3K-α inhibition, was consistent with previous reports of copanlisib and of other pan-PI3K/PI3K-α inhibitors.13-17 Recently, appropriate consensus recommendations have been established to manage these temporary side effects.18
In conclusion, the long-term efficacy and tolerability data provide rationale for long-term treatment with copanlisib in patients with relapsed or refractory MZL, particularly in patients with activated PI3K/BCR signaling, providing an effective treatment option in this challenging-to-treat population. Phase 3 studies (CHRONOS-3 and CHRONOS-4) are ongoing in patients with indolent lymphoma, including MZL, to validate the efficacy and safety of copanlisib in combination with rituximab-based therapies; these studies could help to address the unmet need in relapsed or refractory MZL by providing additional therapeutic options.
Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents.
Acknowledgments
The authors thank the patients and their families, and the study teams at the participating centers. They thank Oliver Wirtz for providing statistical support, and Jess Loraine, of Complete HealthVizion, for medical writing support, based on detailed discussion and feedback from all the authors.
This study was funded by Bayer AG.
Authorship
Contribution: P.P. participated in the literature review and data collection, analysis, and interpretation; G.A.F., L.M., A.N., M.Ö., A.S., D.S., D.T., P.L.Z., and M.D. participated in data collection, analysis, and interpretation; F.H., J.G.-V., and B.H.C. participated in the literature review, study design, protocol development, and data analysis and interpretation; and all authors participated in writing and/or critically reviewing the manuscript and approved this version for submission.
Conflict-of-interest disclosure: P.P. has received honoraria from Genesis, Gilead, Roche, and Takeda and research support from Genesis, Roche, and Takeda. G.A.F. has received fees for consultancy and lecturing from AbbVie, AstraZeneca, Bayer, Janssen, and Roche. M.Ö. has received research funding from AbbVie, Archigen, Bayer, Celgene, Janssen, Merck Sharp & Dohme, Novartis, Roche, and Takeda; travel support from AbbVie, Abdi İbrahim, Amgen, Bristol Myers Squibb, Janssen, Jazz, Roche, Sanofi, and Takeda; and honoraria from Amgen and Takeda. A.S. has served on speaker bureaus for AbbVie, Amgen, ArQule, AstraZeneca, Bayer, Bristol Myers Squibb, Celgene, Eisai, Gilead, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sandoz, Servier, and Takeda; advisory boards for Bayer, Bristol Myers Squibb, Eisai, Gilead, Merck Sharp & Dohme, Pfizer, and Servier; and has received fees for consultancy from ArQule. F.H. is employed by Bayer AG. J.G.-V. is employed by Bayer HealthCare Pharmaceuticals, Inc. B.H.C. is employed by Bayer HealthCare Pharmaceuticals, Inc. P.L.Z. has received fees for consultancy from EUSA Pharma, Merck Sharp & Dohme, Sanofi, and Verastem; has served on speaker bureaus for Bristol Myers Squibb, Celgene, Celltrion, EUSA Pharma, Gilead, Immune Design, Janssen, Kyowa Kirin, Merck Sharp & Dohme, Portola, Roche, Servier, and Verastem; and advisory boards for Bristol Myers Squibb, Celgene, Celltrion, EUSA Pharma, Gilead, Immune Design, Janssen, Kyowa Kirin, Merck Sharp & Dohme, Portola, Roche, Sandoz, Servier, and Verastem. M.D. has received honoraria from Celgene, Janssen, and Roche; has served on scientific advisory boards for Acerta, Bayer, Celgene, Gilead, Janssen, Novartis, Roche, and Sandoz; has received speaker’s honoraria from Bayer, Celgene, Gilead, Janssen, and Roche; and has received institutional support from Celgene, Janssen, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Martin Dreyling, Medizinische Klinik III, Klinikum der Universität, LMU München, München, Germany; e-mail: martin.dreyling@med.uni-muenchen.de.
References
Author notes
The full-text version of this article contains a data supplement.