Key Points
PFS favoring denosumab vs zoledronic acid was prominent in patients intended for ASCT and who received PI-based triplet therapy.
Based on this PFS advantage, these patient subsets warrant further investigation.
Abstract
An exploratory end point from a recent trial in patients with newly diagnosed multiple myeloma showed that median progression-free survival (PFS) was increased by 10.7 months with denosumab vs zoledronic acid. We performed additional analyses to identify factors that may have contributed to the favorable PFS with denosumab. Ad hoc analyses were performed for patients intending to undergo autologous stem cell transplantation (ASCT; ASCT intent), not intending to undergo ASCT (ASCT no intent), and intent-to-treat according to age (<70 or ≥70 years) and baseline renal function (≤60 mL/min or >60 mL/min creatinine clearance [CrCl]). Of 1718 patients, 930 (54.1%) were in the ASCT-intent subgroup, and 788 (45.9%) were in the ASCT-no-intent subgroup. In the ASCT-intent subgroup, frontline triplet (median PFS, not estimable vs 35.7 months; hazard ratio [HR] [95% confidence interval (CI)], 0.65 [0.47-0.90]; descriptive P = .009) or bortezomib-only (median PFS, not estimable vs not estimable; HR [95% CI], 0.61 [0.39–0.95]; descriptive P = .029) induction regimens demonstrated the strongest PFS benefit favoring denosumab vs zoledronic acid. In the ASCT-no-intent subgroup, no benefit with denosumab vs zoledronic acid was observed. PFS favored denosumab vs zoledronic acid in patients with CrCl >60 mL/min and in patients <70 years old, but no difference was observed in patients with CrCl ≤60 mL/min or patients ≥70 years old. The PFS difference observed with denosumab is one of the notable benefits reported in newly diagnosed multiple myeloma and was most pronounced in patients intending to undergo ASCT and those who received proteasome inhibitor (PI)−based triplet regimens. This study was registered at www.clinicaltrials.gov as #NCT01345019.
Introduction
Multiple myeloma is a plasma cell malignancy that accounts for an estimated 150 000 new cases annually worldwide.1 Myeloma is characterized by development of osteolytic lesions, renal dysfunction, hypercalcemia, anemia, and elevated monoclonal paraprotein.2 Osteolytic lesions commonly lead to skeletal-related events (SREs; ie, spinal cord compression, pathological fracture, or surgery or radiotherapy to affected bone).3 Up to 80% of patients with newly diagnosed multiple myeloma develop detectable bone lesions, which result from deregulation of normal bone remodeling, thus resulting in cancer-induced bone loss and destruction4 and increased risk for fracture.5,6
In the skeleton, interactions among myeloma cells and cells of the bone marrow microenvironment, including osteoblasts, osteocytes, and stromal cells, secrete factors (eg, receptor activator of nuclear factor κ-B ligand [RANKL], C-C motif ligand-3, interleukin-3, interleukin-6, and others) that increase osteoclast formation, function, and activity, as well as additional factors (eg, Dickkopf-1 [DKK-1], soluble Frizzle-related protein 2, and sclerostin) that inhibit osteoblast function.4 This leads to an imbalance in bone homeostasis due to increased bone resorption rates and decreased bone formation activity, resulting in the development of clinically important osteoporosis and lytic bone lesions.7
RANKL is an essential mediator of osteoclast formation, activation, and survival.4,7 In myeloma, RANKL is secreted by bone marrow stromal cells,8,9 osteocytes,10,11 and myeloma cells,12,13 resulting in increased osteoclast activity. In turn, osteoclasts can directly recruit myeloma cells and promote proliferation and survival and induce resistance to apoptosis.14-16 Thus, excessive RANKL is correlated with increased bone disease and decreased survival in multiple myeloma.17 Furthermore, RANKL levels are significantly increased in patients with active multiple myeloma compared with patients with monoclonal gammopathy of undetermined significance or smoldering multiple myeloma. Interestingly, serum RANKL decreases upon response to chemotherapy.18 Additionally, in experimental models, soluble RANKL, by inducing osteoclast formation, is associated with the reactivation of “dormant” myeloma cells.19
Both bisphosphonates and denosumab are approved for the prevention of SREs in multiple myeloma. International guidelines recommend initiating bone-targeted therapy concurrently with antimyeloma therapy, even in the absence of overt osteolytic lesions.20,21 Clinical trials have suggested that IV bisphosphonates also have possible survival benefits beyond their effects on bone. In the Myeloma IX trial, zoledronic acid improved progression-free survival (PFS; median improvement of 2 months; hazard ratio [HR], 0.88) and reduced mortality (median improvement of 5.5 months; HR, 0.84) when compared with oral clodronate.22
Denosumab, a fully human monoclonal antibody that binds to and neutralizes RANKL, inhibits osteoclasts and has been shown to reduce the rates of SREs not only in solid tumors but also in multiple myeloma.23-26 A recent double-blind, placebo-controlled trial conducted in 1718 patients with newly diagnosed, active multiple myeloma and ≥1 osteolytic lesion or focal lesion showed similar activity of zoledronic acid and denosumab in delaying SREs23 and provided the basis for the label extension of denosumab in multiple myeloma patients in March 2018. Although comparable efficacy of denosumab and zoledronic acid was observed in terms of time to first on-study SRE and overall survival (HR, 0.90 [95% confidence interval (CI), 0.70-1.16]; P = .41), an exploratory analysis revealed an increase in median PFS in the denosumab arm by 10.7 months with an HR rate of 0.82 (95% CI, 0.68-0.99; descriptive P = .036). Even though the PFS in this study was an exploratory end point, the observed large and clinically meaningful difference in favor of denosumab was unexpected and suggested that RANKL inhibition with denosumab may provide an antimyeloma effect.23 Therefore, in the present study, further analysis of the PFS observation, according to induction therapy, intent to undergo autologous stem cell transplantation (ASCT), renal function, and age, was performed to identify subgroups of patients who may benefit most. The analysis of induction therapies for patients intending to undergo ASCT in both arms should largely account for any bias that may have contributed to the favorable PFS in the denosumab arm.
Methods
Patients and study design
The current study is a subgroup analysis of patients from a double-blind, randomized, active-controlled phase 3 study for which the study design, patient information, and results have been previously published.23 This study is registered at www.clinicaltrials.gov as #NCT01345019. In brief, patients ≥18 years with newly diagnosed multiple myeloma were included in the study. The key inclusion criteria were radiographic evidence of ≥1 lytic bone lesion or ≥1 focal lesion; ≤1 previous dose of IV bisphosphonate therapy; and Eastern Cooperative Oncology Group (ECOG) performance status ≤2, with adequate organ function and a creatinine clearance (CrCl) ≥30 mL/min. Patients with plasma cell leukemia were excluded, as were those who received >1 previous dose of IV bisphosphonate and those with prior history or current evidence of osteonecrosis/osteomyelitis of the jaw. Written informed consent was provided by patients before any study-specific procedure, and the study was approved by each study site’s institutional review board and local ethics committee.
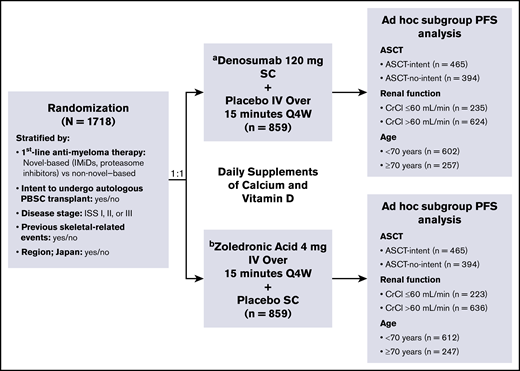
Patients were randomized (1:1) to receive either denosumab or zoledronic acid (859 patients in each treatment arm). Patients were stratified according to intent to undergo autologous transplantation (yes or no), first-line antimyeloma therapy (novel therapy based [includes bortezomib, lenalidomide, or thalidomide] or nonnovel therapy), International Staging System (ISS) stage at diagnosis (I, II, or III), previous SREs (yes or no), and region: Japan (yes or no) (Figure 1). Blinded treatment continued until 676 patients had ≥1 on-study SRE and the primary efficacy and safety analysis was completed.
Study design, treatment schema, and subgroups.aSC dose adjustments were not permitted. bPer protocol and zoledronic acid label, IV product was dose adjusted for baseline CrCl, and subsequent dose intervals were determined by serum creatinine levels. PBSC, peripheral blood stem cell; Q4W, every 4 weeks; SC, subcutaneous.
Study design, treatment schema, and subgroups.aSC dose adjustments were not permitted. bPer protocol and zoledronic acid label, IV product was dose adjusted for baseline CrCl, and subsequent dose intervals were determined by serum creatinine levels. PBSC, peripheral blood stem cell; Q4W, every 4 weeks; SC, subcutaneous.
Study treatment
Patients received the investigators’ choice of first-line antimyeloma therapy and either subcutaneous denosumab 120 mg plus IV placebo or IV zoledronic acid 4 mg plus subcutaneous placebo every 4 weeks in the double-blind treatment period. As a positive benefit-risk profile of denosumab was demonstrated in the primary analysis of the study, patients who were still undergoing the every-4-week scheduled assessments were offered open-label subcutaneous denosumab 120 mg every 4 weeks for up to 2 years. Dose adjustments were not permitted for denosumab on study. Dose adjustments for zoledronic acid were permitted according to the guidelines approved in the prescribing information.23 Daily supplementation with calcium (>500 mg) and vitamin D (400 IU) was recommended.
Assessments
An ad hoc analysis of the following subgroups was performed: patients intending to undergo ASCT (ASCT intent, prespecified subgroup), not intending to undergo ASCT (ASCT no intent), intent-to-treat (ITT) according to age (<70 or ≥70 years of age), and ITT according to renal function (CrCl >60 mL/min or ≤60 mL/min at baseline).
For the ASCT-intent subgroup, patient demographics, baseline characteristics, and frontline therapy were analyzed in detail. PFS was defined as the time in days from the randomization date to the date of first overall disease progression recorded by an investigator or death during the treatment phase from any cause, whichever occurred earlier. PFS events were collected during the study. The median number of months on study was 17.3 months for patients treated with denosumab and 17.6 months for zoledronic acid.
PFS subgroup analyses were conducted according to the first-line therapy (proteasome inhibitor [PI], immunomodulatory drug [IMiD], PI plus IMiD, and triplet vs doublet) and intent to receive ASCT (yes or no).
Analysis
PFS was estimated using the Kaplan-Meier method and analyzed using a Cox proportional-hazards model. The model was adjusted for baseline covariates (ie, SRE history, baseline age, and time from diagnosis of bone metastasis to study enrollment; for patients with multiple myeloma, time from diagnosis to study enrollment was used) and stratified by randomization stratification factors; HR was presented as denosumab vs zoledronic acid (ie, HR <1 favored denosumab).
Results
Of the 1718 patients included in the study, 930 out of 1718 (54.1%) were in the ASCT-intent subgroup and 788 out of 1718 (45.9%) were in the ASCT-no-intent subgroup. Of those in the ASCT-intent subgroup, 571 out of 930 (61.4%) underwent ASCT, while 74 out of 788 (9.4%) of those in the ASCT-no-intent subgroup underwent ASCT.
ASCT-intent subgroup
Of the 930 patients in this subgroup, 465 (50.0%) were randomized to the denosumab arm and 465 (50.0%) to the zoledronic acid arm. Baseline characteristics were well balanced between these treatment arms, including age, performance status, ISS stage, bone marrow plasma cell percentage, and previous SRE (Table 1). Triplet therapy use was balanced, with 354 out of 465 patients (76.1%) in the denosumab arm and 347 out of 465 patients (74.6%) in the zoledronic acid arm receiving cyclophosphamide-bortezomib-dexamethasone (CVd), bortezomib-thalidomide-dexamethasone (VTd), cyclophosphamide-thalidomide-dexamethasone (CTd), or bortezomib-lenalidomide-dexamethasone (VRd) triplet therapy as first-line multiple myeloma therapy. Overall, 67 out of 465 patients (14.4%) in the denosumab arm and 87 out of 465 patients (18.7%) in the zoledronic acid arm did not receive any triplet therapies at any time during their induction treatment but received doublet therapies, defined as thalidomide-dexamethasone (Td), lenalidomide-dexamethasone (Rd), or bortezomib-dexamethasone (Vd). Among “novel” myeloma therapies, bortezomib-based regimens, such as CVd and Vd, were the most commonly used frontline treatments (see Table 2 for frontline therapies). Patients who received CVd or Vd but did not receive any other triplet or doublet therapies were rather balanced between the 2 arms (denosumab arm, 186/465 [40.0%]; zoledronic acid arm, 214/465 [46.0%]). A small number of patients, who were not treated with a triplet therapy or Vd received IMiD-only therapy (Rd or Td) (20 [4.3%] patients in the denosumab arm and 33 [7.1%] patients in the zoledronic acid arm).
Baseline demographics and disease characteristics in the ASCT-intent subgroup
| . | Denosumab (n = 465) . | Zoledronic acid (n = 465) . | All patients (N = 930) . |
|---|---|---|---|
| Male | 269 (57.8) | 278 (59.8) | 547 (58.8) |
| Age, median (IQR), y | 59.0 (54.0-64.0) | 59.0 (54.0-64.0) | 59.0 (54.0-64.0) |
| Race | |||
| White | 380 (81.7) | 378 (81.3) | 758 (81.5) |
| Asian | 66 (14.2) | 59 (12.7) | 125 (13.4) |
| Black | 14 (3.0) | 17 (3.7) | 31 (3.3) |
| Other | 5 (1.1) | 11 (2.4) | 16 (1.7) |
| Geographic region | |||
| Europe | 251 (54.0) | 267 (57.4) | 518 (55.7) |
| North America | 133 (28.6) | 134 (28.8) | 267 (28.7) |
| Rest of the world | 81 (17.4) | 64 (13.8) | 145 (15.6) |
| Bone marrow plasma cell percentage at initial diagnosis, median (IQR) | 40.0 (20.0-70.0) | 39.0 (20.0-65.0) | 40.0 (20.0-68.0) |
| ECOG performance status at study entry | |||
| 0 | 181 (38.9) | 171 (36.8) | 352 (37.8) |
| 1 | 194 (41.7) | 207 (44.5) | 401 (43.1) |
| 2 | 90 (19.4) | 87 (18.7) | 177 (19.0) |
| Previous SRE | |||
| Any | 314 (67.5) | 314 (67.5) | 628 (67.5) |
| Pathological fracture | 264 (56.8) | 245 (52.7) | 509 (54.7) |
| Spinal cord compression | 52 (11.2) | 68 (14.6) | 120 (12.9) |
| Radiation therapy to bone | 67 (14.4) | 79 (17.0) | 146 (15.7) |
| Surgery to bone | 69 (14.8) | 88 (18.9) | 157 (16.9) |
| ISS stage at diagnosis | |||
| I | 167 (35.9) | 164 (35.3) | 331 (35.6) |
| II | 166 (35.7) | 174 (37.4) | 340 (36.6) |
| III | 122 (26.2) | 115 (24.7) | 237 (25.5) |
| Not available | 10 (2.2) | 12 (2.6) | 22 (2.4) |
| Prior oral bisphosphonate exposure, median (IQR), months | 5.4 (0.5-6.5) | 2.5 (0.6-4.2) | 3.8 (0.6-5.4) |
| . | Denosumab (n = 465) . | Zoledronic acid (n = 465) . | All patients (N = 930) . |
|---|---|---|---|
| Male | 269 (57.8) | 278 (59.8) | 547 (58.8) |
| Age, median (IQR), y | 59.0 (54.0-64.0) | 59.0 (54.0-64.0) | 59.0 (54.0-64.0) |
| Race | |||
| White | 380 (81.7) | 378 (81.3) | 758 (81.5) |
| Asian | 66 (14.2) | 59 (12.7) | 125 (13.4) |
| Black | 14 (3.0) | 17 (3.7) | 31 (3.3) |
| Other | 5 (1.1) | 11 (2.4) | 16 (1.7) |
| Geographic region | |||
| Europe | 251 (54.0) | 267 (57.4) | 518 (55.7) |
| North America | 133 (28.6) | 134 (28.8) | 267 (28.7) |
| Rest of the world | 81 (17.4) | 64 (13.8) | 145 (15.6) |
| Bone marrow plasma cell percentage at initial diagnosis, median (IQR) | 40.0 (20.0-70.0) | 39.0 (20.0-65.0) | 40.0 (20.0-68.0) |
| ECOG performance status at study entry | |||
| 0 | 181 (38.9) | 171 (36.8) | 352 (37.8) |
| 1 | 194 (41.7) | 207 (44.5) | 401 (43.1) |
| 2 | 90 (19.4) | 87 (18.7) | 177 (19.0) |
| Previous SRE | |||
| Any | 314 (67.5) | 314 (67.5) | 628 (67.5) |
| Pathological fracture | 264 (56.8) | 245 (52.7) | 509 (54.7) |
| Spinal cord compression | 52 (11.2) | 68 (14.6) | 120 (12.9) |
| Radiation therapy to bone | 67 (14.4) | 79 (17.0) | 146 (15.7) |
| Surgery to bone | 69 (14.8) | 88 (18.9) | 157 (16.9) |
| ISS stage at diagnosis | |||
| I | 167 (35.9) | 164 (35.3) | 331 (35.6) |
| II | 166 (35.7) | 174 (37.4) | 340 (36.6) |
| III | 122 (26.2) | 115 (24.7) | 237 (25.5) |
| Not available | 10 (2.2) | 12 (2.6) | 22 (2.4) |
| Prior oral bisphosphonate exposure, median (IQR), months | 5.4 (0.5-6.5) | 2.5 (0.6-4.2) | 3.8 (0.6-5.4) |
Data are n (%) unless indicated otherwise.
ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range.
Frontline treatment regimens for multiple myeloma
| . | ASCT-intent . | ||
|---|---|---|---|
| . | Denosumab (n = 465) . | Zoledronic acid (n = 465) . | All patients (N = 930) . |
| Triplet therapy | |||
| CVd | 159 (34.2) | 177 (38.1) | 336 (36.1) |
| VTd | 80 (17.2) | 70 (15.1) | 150 (16.1) |
| VRd | 80 (17.2) | 61 (13.1) | 141 (15.2) |
| CTd | 52 (11.2) | 53 (11.4) | 105 (11.3) |
| MPV | 12 (2.6) | 12 (2.6) | 24 (2.6) |
| Vincristine, Adriamycin (doxorubicin), dexamethasone (VAD) | 11 (2.4) | 10 (2.2) | 21 (2.3) |
| KRd | 6 (1.3) | 5 (1.1) | 11 (1.2) |
| dVd | 3 (0.6) | 5 (1.1) | 8 (0.9) |
| MPT | 1 (0.2) | 0 | 1 (0.1) |
| CRd | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| Liposomal doxorubicin, vincristine, dexamethasone (VAD) | 0 | 1 (0.2) | 1 (0.1) |
| Doublet therapy | |||
| Vd | 55 (11.8) | 59 (12.7) | 114 (12.3) |
| Rd | 12 (2.6) | 17 (3.7) | 29 (3.1) |
| Td | 14 (3.0) | 24 (5.2) | 38 (4.1) |
| VT | 2 (0.4) | 2 (0.4) | 4 (0.4) |
| VP | 2 (0.4) | 2 (0.4) | 4 (0.4) |
| Vd2 | 1 (0.2) | 0 | 1 (0.1) |
| Pd | 0 | 1 (0.2) | 1 (0.1) |
| Single agent | 40 (8.6) | 46 (9.9) | 86 (9.2) |
| Other | 28 (6.0) | 22 (4.7) | 50 (5.4) |
| DCEP | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| VTD-PACE | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| C-VAd | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Not applicable | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| . | ASCT-intent . | ||
|---|---|---|---|
| . | Denosumab (n = 465) . | Zoledronic acid (n = 465) . | All patients (N = 930) . |
| Triplet therapy | |||
| CVd | 159 (34.2) | 177 (38.1) | 336 (36.1) |
| VTd | 80 (17.2) | 70 (15.1) | 150 (16.1) |
| VRd | 80 (17.2) | 61 (13.1) | 141 (15.2) |
| CTd | 52 (11.2) | 53 (11.4) | 105 (11.3) |
| MPV | 12 (2.6) | 12 (2.6) | 24 (2.6) |
| Vincristine, Adriamycin (doxorubicin), dexamethasone (VAD) | 11 (2.4) | 10 (2.2) | 21 (2.3) |
| KRd | 6 (1.3) | 5 (1.1) | 11 (1.2) |
| dVd | 3 (0.6) | 5 (1.1) | 8 (0.9) |
| MPT | 1 (0.2) | 0 | 1 (0.1) |
| CRd | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| Liposomal doxorubicin, vincristine, dexamethasone (VAD) | 0 | 1 (0.2) | 1 (0.1) |
| Doublet therapy | |||
| Vd | 55 (11.8) | 59 (12.7) | 114 (12.3) |
| Rd | 12 (2.6) | 17 (3.7) | 29 (3.1) |
| Td | 14 (3.0) | 24 (5.2) | 38 (4.1) |
| VT | 2 (0.4) | 2 (0.4) | 4 (0.4) |
| VP | 2 (0.4) | 2 (0.4) | 4 (0.4) |
| Vd2 | 1 (0.2) | 0 | 1 (0.1) |
| Pd | 0 | 1 (0.2) | 1 (0.1) |
| Single agent | 40 (8.6) | 46 (9.9) | 86 (9.2) |
| Other | 28 (6.0) | 22 (4.7) | 50 (5.4) |
| DCEP | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| VTD-PACE | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| C-VAd | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Not applicable | 1 (0.2) | 2 (0.4) | 3 (0.3) |
Data are n (%).
C-CVd, cyclophosphamide, vincristine, Adriamycin (doxorubicin), dexamethasone; CRd, cyclophosphamide, lenalidomide, dexamethasone; DCEP, dexamethasone, cyclophosphamide, etoposide, cisplatin; dVd, liposomal doxorubicin, bortezomib, dexamethasone; KRd, carfilzomib, lenalidomide, dexamethasone; MPT, melphalan, prednisone, thalidomide; MPV, melphalan, prednisone, bortezomib; Pd, pomalidomide, dexamethasone; VAD, vincristine, Adriamycin (doxorubicin), dexamethasone; Vd2, bortezomib, liposomal doxorubicin; VP, bortezomib, prednisone; VT, bortezomib, thalidomide; VTD-PACE, dexamethasone, thalidomide, cisplatin, Adriamycin (doxorubicin), cyclophosphamide, etoposide, bortezomib.
PFS favored denosumab vs zoledronic acid for patients in the ASCT-intent subgroup (Table 3; Figure 2A). The median PFS was 46.1 months for the denosumab arm and 35.7 months for zoledronic acid arm (HR [95% CI], 0.65 [0.49–0.85]; descriptive P = .002). The probability of patients remaining progression-free was higher over time in patients treated with denosumab in the ASCT-intent subgroup compared with the ITT analysis (Table 3).
PFS and probability of patients with PFS
| . | ITT . | ASCT-intent . | ASCT-no-intent . | |||
|---|---|---|---|---|---|---|
| . | Denosumab (n = 859) . | Zoledronic acid (n = 859) . | Denosumab (n = 465) . | Zoledronic acid (n = 465) . | Denosumab (n = 394) . | Zoledronic acid (n = 394) . |
| Patients with a PFS event, n (%) | 219 (25.5) | 260 (30.3) | 91 (19.6) | 130 (28.0) | 128 (32.5) | 130 (33.0) |
| Median PFS (95% CI), mo | 46.1 (34.3-NE) | 35.4 (30.2-NE) | 46.1 (46.1-NE) | 35.7 (31.3-NE) | 30.4 (25.8-40.0) | 34.7 (25.1-NE) |
| HR* (95% CI) | 0.82 (0.68-0.99) | 0.65 (0.49-0.85) | 1.01 (0.79-1.30) | |||
| Descriptive P value | .036 | .002 | .92 | |||
| Probability of patients with PFS, %† | ||||||
| At month 6 | 92.3 | 90.3 | 95.8 | 91.9 | 88.1 | 88.3 |
| At month 12 | 83.6 | 81.5 | 89.6 | 84.9 | 76.5 | 77.5 |
| At month 18 | 76.5 | 72.1 | 82.7 | 75.4 | 69.1 | 68.3 |
| At month 24 | 69.2 | 63.7 | 77.7 | 68.0 | 58.9 | 58.5 |
| At month 30 | 60.6 | 55.1 | 68.9 | 58.5 | 50.5 | 51.4 |
| At month 36 | 54.7 | 48.7 | 63.6 | 49.9 | 42.8 | 48.1 |
| At month 42 | 51.4 | 44.2 | 61.2 | 44.6 | 38.0 | 44.4 |
| . | ITT . | ASCT-intent . | ASCT-no-intent . | |||
|---|---|---|---|---|---|---|
| . | Denosumab (n = 859) . | Zoledronic acid (n = 859) . | Denosumab (n = 465) . | Zoledronic acid (n = 465) . | Denosumab (n = 394) . | Zoledronic acid (n = 394) . |
| Patients with a PFS event, n (%) | 219 (25.5) | 260 (30.3) | 91 (19.6) | 130 (28.0) | 128 (32.5) | 130 (33.0) |
| Median PFS (95% CI), mo | 46.1 (34.3-NE) | 35.4 (30.2-NE) | 46.1 (46.1-NE) | 35.7 (31.3-NE) | 30.4 (25.8-40.0) | 34.7 (25.1-NE) |
| HR* (95% CI) | 0.82 (0.68-0.99) | 0.65 (0.49-0.85) | 1.01 (0.79-1.30) | |||
| Descriptive P value | .036 | .002 | .92 | |||
| Probability of patients with PFS, %† | ||||||
| At month 6 | 92.3 | 90.3 | 95.8 | 91.9 | 88.1 | 88.3 |
| At month 12 | 83.6 | 81.5 | 89.6 | 84.9 | 76.5 | 77.5 |
| At month 18 | 76.5 | 72.1 | 82.7 | 75.4 | 69.1 | 68.3 |
| At month 24 | 69.2 | 63.7 | 77.7 | 68.0 | 58.9 | 58.5 |
| At month 30 | 60.6 | 55.1 | 68.9 | 58.5 | 50.5 | 51.4 |
| At month 36 | 54.7 | 48.7 | 63.6 | 49.9 | 42.8 | 48.1 |
| At month 42 | 51.4 | 44.2 | 61.2 | 44.6 | 38.0 | 44.4 |
NE, not estimable.
HR <1 favors denosumab.
Kaplan-Meier estimates.
Progression-free survival by intent to undergo transplant. PFS in the ASCT-intent subgroup (A) and ASCT-no-intent subgroup (B).
Progression-free survival by intent to undergo transplant. PFS in the ASCT-intent subgroup (A) and ASCT-no-intent subgroup (B).
In the ASCT-intent subgroup, 2 subsets of patients demonstrated the strongest PFS benefit with denosumab treatment relative to zoledronic acid treatment (Table 4; supplemental Figure 1): patients who received frontline triplet induction therapy or those who received a bortezomib-only (no IMiD) induction regimen, such as CVd or Vd. Only a limited number of patients in the ASCT-intent subgroup received an IMiD-based doublet as frontline therapy (denosumab arm, n = 20; zoledronic acid arm, n = 33), thus making it difficult to interpret the PFS results in this subset (Table 4; supplemental Figure 1).
PFS in treatment subgroups by first-line therapy in the ASCT-intent subgroup
| . | Denosumab . | Zoledronic acid . |
|---|---|---|
| Patients who received triplet therapy (VRd, CVd, CTd, or VTd) | ||
| No. of patients* | 354 | 347 |
| Patients with a PFS event, n (%) | 65 (18.4) | 92 (26.5) |
| Median PFS (95% CI), mo | NE (NE-NE) | 35.7 (30.2-NE) |
| HR† (95% CI) | 0.65 (0.47-0.90) | |
| Descriptive P value | .009 | |
| Patients who received bortezomib-only (no IMiD) therapy (CVd or Vd) | ||
| No. of patients‡ | 186 | 214 |
| Patients with a PFS event, n (%) | 34 (18.3) | 59 (27.6) |
| Median PFS (95% CI), mo | NE (38.7-NE) | NE (30.2-NE) |
| HR† (95% CI) | 0.61 (0.39-0.95) | |
| Descriptive P value | .029 | |
| Patients who received doublet therapy (Rd, Td, or Vd) | ||
| No. of patients* | 67 | 87 |
| Patients with PFS event, n (%) | 16 (23.9) | 30 (34.5) |
| Median PFS (95% CI), mo | NE (25.1-NE) | 36.7 (22.2-NE) |
| HR† (95% CI) | 0.83 (0.40-1.74) | |
| Descriptive P value | .62 | |
| Patients who received VRd | ||
| No. of patients* | 80 | 61 |
| Patients with a PFS event, n (%) | 18 (22.5) | 12 (19.7) |
| Median PFS (95% CI), mo | NE (30.2-NE) | 35.7 (19.4-NE) |
| HR† (95% CI) | 1.20 (0.53-2.75) | |
| Descriptive P value | .66 | |
| Patients who received IMiD-only therapy (Rd or Td) | ||
| No. of patients* | 20 | 33 |
| Patients with a PFS event, n (%) | 5 (25.0) | 11 (33.3) |
| Median PFS (95% CI), mo | NE (18.4-NE) | 34.3 (22.3-NE) |
| HR† (95% CI) | 1.82 (0.18–18.25) | |
| Descriptive P value | .61 | |
| . | Denosumab . | Zoledronic acid . |
|---|---|---|
| Patients who received triplet therapy (VRd, CVd, CTd, or VTd) | ||
| No. of patients* | 354 | 347 |
| Patients with a PFS event, n (%) | 65 (18.4) | 92 (26.5) |
| Median PFS (95% CI), mo | NE (NE-NE) | 35.7 (30.2-NE) |
| HR† (95% CI) | 0.65 (0.47-0.90) | |
| Descriptive P value | .009 | |
| Patients who received bortezomib-only (no IMiD) therapy (CVd or Vd) | ||
| No. of patients‡ | 186 | 214 |
| Patients with a PFS event, n (%) | 34 (18.3) | 59 (27.6) |
| Median PFS (95% CI), mo | NE (38.7-NE) | NE (30.2-NE) |
| HR† (95% CI) | 0.61 (0.39-0.95) | |
| Descriptive P value | .029 | |
| Patients who received doublet therapy (Rd, Td, or Vd) | ||
| No. of patients* | 67 | 87 |
| Patients with PFS event, n (%) | 16 (23.9) | 30 (34.5) |
| Median PFS (95% CI), mo | NE (25.1-NE) | 36.7 (22.2-NE) |
| HR† (95% CI) | 0.83 (0.40-1.74) | |
| Descriptive P value | .62 | |
| Patients who received VRd | ||
| No. of patients* | 80 | 61 |
| Patients with a PFS event, n (%) | 18 (22.5) | 12 (19.7) |
| Median PFS (95% CI), mo | NE (30.2-NE) | 35.7 (19.4-NE) |
| HR† (95% CI) | 1.20 (0.53-2.75) | |
| Descriptive P value | .66 | |
| Patients who received IMiD-only therapy (Rd or Td) | ||
| No. of patients* | 20 | 33 |
| Patients with a PFS event, n (%) | 5 (25.0) | 11 (33.3) |
| Median PFS (95% CI), mo | NE (18.4-NE) | 34.3 (22.3-NE) |
| HR† (95% CI) | 1.82 (0.18–18.25) | |
| Descriptive P value | .61 | |
Numbers were based on the ranked therapies in a descending order of efficacy: VRd>VTd>CVd>CTd>Vd>Rd>Td; a patient was counted only 1 time in the highest ranking therapy group received.
HR <1 favors denosumab.
Number of patients who received CVd or Vd but did not receive any other triplet or doublet therapies.
Approximately two-thirds of the ASCT-intent subgroup (denosumab arm, 295/465 [63.4%]; zoledronic acid arm, 276/465 [59.4%]) actually received ASCT as part of the frontline treatment. Additionally, a small subset of patients in the ASCT-no-intent subgroup also received ASCT (36 patients in the denosumab arm and 38 in the zoledronic acid arm).
ASCT-no-intent subgroup
Overall, 394 patients in each arm were stratified into the ASCT-no-intent subgroup, and baseline characteristics were well balanced between these treatment arms (data not shown). This prespecified subgroup did not demonstrate a PFS benefit in the denosumab arm (HR [95% CI], 1.01 [0.79–1.30]; descriptive P = .92; Table 3; Figure 2B). The median PFS for patients in the ASCT-no-intent subgroup was 30.4 months for the denosumab arm and 34.7 months for the zoledronic acid arm. Of note, those patients were less intensively treated, with only 158 (40.1%) patients in the denosumab arm and 148 (37.6%) in the zoledronic acid arm receiving a triplet therapy consisting of CVd, VTd, VRd, or CTd.
PFS according to renal function and age
In the ITT population, patients were stratified by impaired renal function with CrCl ≤60 mL/min (denosumab, n = 235/1718 [13.7%]; zoledronic acid, n = 223/1718 [13.0%]) and moderate or good baseline renal function with CrCl >60 mL/min (denosumab, n = 624/1718 [36.3%]; zoledronic acid, n = 636/1718 [37.0%]). When grouped based on renal function, no difference in PFS was observed for denosumab compared with zoledronic acid in patients with baseline renal function with CrCl ≤60 mL/min (Figure 3A). In contrast, a PFS benefit was observed in patients in the denosumab arm compared with the zoledronic acid arm in patients with mild renal impairment or good baseline renal function with CrCl >60 mL/min (Figure 3B). Impaired renal function was associated with a worse outcome in terms of PFS compared with those patients with baseline CrCl >60 mL/min for the 2 treatment arms pooled (Figure 3C).
Progression-free survival according to renal function. PFS in patients with baseline CrCl ≤60 mL/min (A), patients with baseline CrCl >60 mL/min (B), and by baseline CrCl (C).
Progression-free survival according to renal function. PFS in patients with baseline CrCl ≤60 mL/min (A), patients with baseline CrCl >60 mL/min (B), and by baseline CrCl (C).
Patients <70 years old (denosumab, n = 602/859 [70.1%]; zoledronic acid, n = 612/859 [71.2%]) demonstrated a better outcome in terms of PFS in the denosumab arm (HR [95% CI], 0.74 [0.59–0.94]; descriptive P = .012). In the subgroup of patients ≥70 years of age (denosumab, n = 257/859 [29.9%]; zoledronic acid, n = 247/859 [28.8%]), a PFS benefit with denosumab was not observed (HR [95% CI], 0.97 [0.71–1.33]; descriptive P = .85).
Discussion
Treatment with denosumab was previously shown to be associated with a significant and clinically meaningful PFS benefit compared with zoledronic acid. Here, we demonstrate that this benefit is most pronounced within the subgroups of patients who have planned ASCT and in those who received a PI-based triplet regimen. Baseline patient demographics and characteristics were well balanced in the ASCT-intent and ASCT-no-intent subgroups. The median age of patients in the ASCT-intent subgroup was lower compared with the ASCT-no-intent subgroup (59 years and 71 years), but this was expected, as patients with multiple myeloma for whom ASCT is planned are usually younger.27
The ASCT-intent subgroup demonstrated the largest PFS benefit for denosumab compared with zoledronic acid. This PFS effect was most pronounced in patients who received triplet therapy with novel agents (mainly CVd, but also VRd, VTd, or CTd) or a bortezomib-based induction regimen (eg, CVd or Vd). Importantly, in the ASCT-intent subgroup, the PFS benefit was not limited to patients who actually received an ASCT, with no heterogeneity of effect observed between those who did and did not.
The results observed with bortezomib-based regimens suggest a potentially synergistic effect between bortezomib and denosumab that may lead to improved PFS. However, this effect may be abrogated in the presence of an IMiD, as patients receiving VRd, one of the most effective induction regimens currently available, did not demonstrate any PFS improvement with denosumab. However, these results should be interpreted with caution, as this subgroup of patients receiving VRd was small and imbalances cannot be excluded.
Synergy between bortezomib and denosumab is suggested by the fact that both affect RANKL; bortezomib reduced circulating RANKL and DKK-1 levels in relapsed/refractory multiple myeloma.28,29 PIs, including bortezomib, reduce osteoclast differentiation,30,31 stimulate osteoblastogenesis and bone formation in vivo and in vitro,32,33 and show bone-anabolic activity in multiple myeloma.28,29 In another study, patients who achieved complete response or very good partial response after 4 cycles of bortezomib had greater elevations of bone-specific alkaline phosphatase (ALP) levels than those not achieving a complete response or very good partial response.28
IMiD alone or in combination with a PI is unlikely to have a synergistic effect when used with denosumab. Treatment with thalidomide and lenalidomide significantly inhibited osteoblast development in vitro, as reflected by a reduction of ALP activity and matrix mineralization. Similar effects were observed with the addition of bortezomib. IMiDs upregulated DKK-1 and inhibin β A, but blocking these molecules did not restore osteoblast development.34 In patients with relapsed/refractory myeloma, bortezomib increased serum levels of bone-specific ALP with or without dexamethasone.35 However, when combined with melphalan, dexamethasone, and intermittent thalidomide (VMDT regimen), no increases in bone-specific ALP and osteocalcin were observed, 35 suggesting that bortezomib in combination with other antimyeloma agents may lose its beneficial effect on osteoblasts. Even in post-ASCT patients with low myeloma burden, bortezomib in combination with thalidomide and dexamethasone as consolidation therapy failed to produce a significant bone-anabolic effect.36 Both dexamethasone and thalidomide are known to reduce bone formation markers, and thus, bortezomib’s effect on bone formation seems not to override the negative impact of these drugs on osteoblast function.37,38 In another study in patients with relapsed/refractory myeloma, Rd treatment reduced C-terminal telopeptide of type 1 collagen (CTX), a bone resorption marker, in responders but did not affect bone formation. However, VRd reduced DKK-1, soluble RANKL, and CTX and increased bone-specific ALP and osteocalcin levels.39
Stem cell transplantation is an important treatment option that has prognostic value. Patients who receive an ASCT usually gain deeper remission, which is associated with a better outcome in terms of PFS, than those who do not.40 However, patients who undergo transplant are also usually younger and fitter than those who do not, as we observed in this trial, and more often receive triplet induction therapy. In the ASCT-no-intent subgroup, only ∼40% received triplet therapy with novel agents compared with 75% in the ASCT-intent subgroup. Both ASCT and a highly effective induction regimen may have led to deeper remissions and may have led to the hypothesis that denosumab supports the maintenance or extends the state of deep remission by blocking osteoclastic resorption, which supports myeloma growth and disease progression.4,41,42 This theory is supported by preclinical work on the cell dormancy hypothesis in multiple myeloma. Experimental studies using animal models show that inhibition of osteoclast activity not only prevents myeloma-induced osteolysis but also reduces growth of medullary, but not extramedullary, myeloma.19,43,44 The mechanism is poorly understood, but data suggest that osteoclasts reactivate dormant myeloma cells.19
The improved PFS observed in our analysis therefore could be due to the effective denosumab-induced inhibition of osteoclast activity and subsequent decrease in osteoclast-mediated stimulation of myeloma growth. In other words, denosumab may help to extend remission in multiple myeloma patients who have received an effective antimyeloma treatment, such as ASCT or a bortezomib-based regimen. Differences in the mechanism of action may explain the observed benefit of denosumab over zoledronic acid in the current study. Denosumab binds to RANKL, blocking it from interacting with RANK, thus inhibiting osteoclast formation and decreasing bone resorption.45 In contrast, bisphosphonates inhibit protein prenylation, likely via inhibition of farnesyl pyrophosphate synthase, downregulate stromal cell adhesion molecules, and increase apoptosis.46-48 It is possible that the relative osteoclast inhibition is more complete with denosumab compared with zoledronic acid.
This hypothesis is also supported by the work of Vij et al,49 who described a small phase 2 study with 96 patients with relapsed or plateau-phase multiple myeloma pretreated with subcutaneous denosumab. Denosumab effectively inhibited the RANKL pathway regardless of previous exposure to bisphosphonates, as evidenced by suppressed levels of the bone turnover marker, serum CTX. Furthermore, 11 subjects (21%) who relapsed within 3 months before study entry maintained stable disease for up to 16.5 months, whereas 19 patients (46%) with plateau-phase myeloma maintained stable disease for up to 18.3 months.49
Because lenalidomide maintenance is another factor that could affect patient outcomes, we looked specifically at patients receiving VRd or Rd as part of their frontline treatment, as these patients may have continued lenalidomide treatment until disease progression. Overall, the use of lenalidomide maintenance and VRd induction was low in this trial, as maintenance was not an approved or a widely accepted standard of care in Europe at the time the study was enrolling. VRd induction was only used in a small number of patients and was not fully balanced between the treatment arms, with more patients in the denosumab arm receiving VRd than in the zoledronic acid arm. As VRd is currently one of the most effective available induction regimens,40,50 and an imbalance could introduce bias favoring denosumab, PFS was assessed in this subgroup. In line with our hypothesis that PIs combined with IMiD may abrogate the beneficial effect on PFS, the VRd subgroup did not demonstrate a PFS benefit in the denosumab arm. Furthermore, the mean treatment duration of a single agent or Rd (n = 75 for denosumab; n = 79 for zoledronic acid) was 8.2 months for patients in the denosumab arm and 5.9 months in the zoledronic acid arm, suggesting no evidence of maintenance therapy in these subgroups. Importantly, the same holds true for VTd and thalidomide maintenance. No evidence of any maintenance with thalidomide was found.
Denosumab is not excreted via the kidneys and therefore can be used in patients with impaired renal function without any dose reductions. This is in contrast to zoledronic acid, which must be dose adjusted for patients with CrCl 30 to 60 mL/min and is not recommended in patients with CrCl <30 mL/min. We therefore hypothesized that the improved PFS in patients receiving denosumab vs zoledronic acid could be driven by patient renal function. To test this, we first looked at the ITT population and specifically at the subgroups of patients with baseline CrCl ≤60 mL/min and CrCl >60 mL/min. The majority of patients had a moderate or good renal function at baseline, with a CrCl of >60 mL/min, and interestingly, a PFS benefit with denosumab was only observed in these patients and not in those with an impaired renal function and a CrCl ≤ 60 mL/min.
Because younger and healthier patients are usually deemed eligible for stem cell transplantation27 in multiple myeloma, and because we saw a PFS benefit in patients in the ASCT-intent subgroup, we also analyzed PFS according to age. Patients <70 years of age demonstrated better PFS, with larger improvement in the denosumab arm when compared with the zoledronic acid arm. It is difficult to determine if this is due to the more intensive treatment delivered to younger patients planned for ASCT or their age, as these factors are so entwined.
This study has several limitations. The ad hoc analyses were not prespecified in the statistical analysis plan, are not statistically powered, and are only descriptive in nature. Disease progression was investigator identified, and the statistics for PFS are descriptive because the analysis was exploratory and not included in the formal hypothesis-testing scheme that controls overall type I error rates. Due to the nature of primary and secondary end points, response rates were not collected within this trial, and, therefore, a direct correlation between response, PFS, and denosumab cannot be established. The conclusions based on the subgroup analyses of first-line therapy may be biased, because patients were grouped based on postrandomization information. However, we have verified that the majority of the patients were treated with the first-line therapies at baseline prior to randomization, which minimizes potential bias. While the large and clinically meaningful PFS benefit observed in the denosumab arm was impressive and unexpected, especially as it was observed on top of novel antimyeloma therapy, these data must still be interpreted with caution.
It is difficult to interpret these data in the context of recent treatment developments and approvals, as well as shifts in the multiple myeloma treatment landscape. Data regarding the use of lenalidomide maintenance was not collected as part of the trial, as it was not approved at the time this study was enrolling. Additionally, only approximately one-third of patients received an induction regimen consisting of a PI and an IMiD before undergoing ASCT, which is now the standard of care in many countries in patients with newly diagnosed multiple myeloma who are eligible for transplantation; therefore, any potential effects of denosumab in the context of these new standards of care are not known.
Based on these data, denosumab is currently being investigated in an academic study (Eudra-CT 2018-000924-32) in high-risk smoldering myeloma and SLiM CRAB (calcium, renal failure, anemia, bone lesions)–positive myeloma to determine if denosumab can elongate the time to transition to active (CRAB positive) multiple myeloma, thus delaying the onset of active myeloma requiring antimyeloma therapy. The goal is to stabilize the microenvironment through RANKL inhibition to impair myeloma growth.
In summary, the PFS difference observed with denosumab in this analysis is one of the largest PFS benefits seen in newly diagnosed multiple myeloma. The PFS advantage in the denosumab arm was found to be most pronounced in patients in the ASCT-intent subgroup and patients who received a PI-based triplet regimen. Based on this PFS advantage, our results suggest that these patient subsets warrant further investigation.
Presented in abstract form at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018, and the 61st annual meeting of the American Society of Hematology, Orlando, FL, 7-10 December 2019.
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.
Acknowledgments
The authors thank Rick Davis and Erin O’Keefe (Complete Healthcare Communications, a CHC Group company, North Wales), whose work was funded by Amgen, and Yin C. Lin (Amgen) for medical writing assistance in the preparation of this manuscript.
This study was sponsored and funded by Amgen.
Authorship
Contribution: E.T., N.R., and W.P. wrote the manuscript; E.T., N.R., P.C., R.G.-S., X.L., W.P., Y.W., A.G., J.C., and C.P. analyzed and interpreted the data and were involved in the development, review, and approval of the manuscript; and Y.W. performed statistical analysis.
Conflict-of-interest disclosure: E.T. has received research funding from Amgen, Genesis, Janssen, and Takeda; honoraria from Amgen, Celgene, Genesis, Janssen, Medison, and Takeda; and travel expenses from Genesis, Janssen, and Takeda. N.R. has served as a consultant for Amgen, Bristol Myers Squibb, Celgene, Janssen, and Karyopharm. P.C. has served as a consultant and on the speakers’ bureau for Amgen and has received research funding from Amgen. R.G.-S. has received honoraria from Gilead, Amgen, Janssen, and Takeda; has served as a consultant for Gilead and Janssen; has received research funding from Gilead, Novartis, Amgen, Janssen, and Takeda (SEHH); has patents, royalties, or other intellectual property from Biomed-2 primers for clonality assessment; has provided expert testimony for IVS Technologies (patent recognition); and has received travel expenses from Gilead, Amgen, Janssen, and Takeda. X.L. has received honoraria from and has served as a consultant for Janssen, Celgene, Sanofi, Amgen, Takeda, Roche, Oncopeptides, Karyopharm, Harpoon Therapeutics, Novartis, and CARsgen Therapeutics. W.P. is an employee and owns stock in Amgen GmbH. Y.W. and A.G. are employees and own stock in Amgen. J.C. is an employee and owns stock in Amgen; has received research funding from Amgen; and has patents, royalties, or other intellectual property from Amgen. C.P. has received honoraria from Amgen and Janssen; has served as a consultant for Amgen, Celgene, and Janssen; and has received travel expenses from Amgen, Celgene, and Janssen.
Correspondence: Evangelos Terpos, Department of Clinical Therapeutics, School of Medicine, National and Kapodistrian University of Athens, Alexandra General Hospital, 80 Vas. Sophias Ave, 11528 Athens, Greece; e-mail: eterpos@med.uoa.gr.
References
Author notes
The full-text version of this article contains a data supplement.