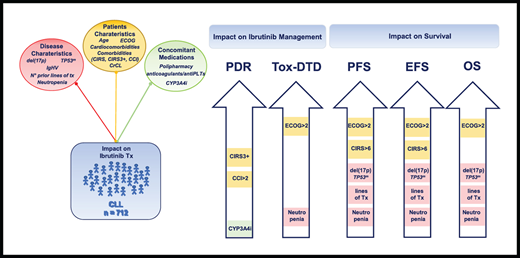
Key points
Age per se does not influence outcome in CLL patients on ibrutinib, whereas CIRS score is predictive of treatment management, PFS, and EFS.
ECOG-PS and neutropenia resulted as the only baseline parameters affecting overall survival.
Abstract
Functional reserve of organs and systems is known to be relevant in predicting immunochemotherapy tolerance. Age and comorbidities, assessed by the cumulative illness rating scale (CIRS), have been used to address chemotherapy intensity. In the ibrutinib era, it is still unclear whether age, CIRS, and Eastern Cooperative Oncology Group performance status (ECOG-PS) retain their predictive role on treatment vulnerability. In this series of 712 patients with chronic lymphocytic leukemia (CLL) treated with ibrutinib outside clinical trials, baseline ECOG-PS and neutropenia resulted as the most accurate predictors of treatment feasibility and outcomes. Age did not independently influence survival and ibrutinib tolerance, indicating that not age per se, but age-related conditions, may affect drug management. We confirmed the role of CIRS > 6 as a predictor of a poorer progression- and event-free survival (PFS, EFS). The presence of a severe comorbidity was significantly associated with permanent dose reductions (PDRs), not translating into worse outcomes. As expected, del(17p) and/or TP53mut and previous therapies affected PFS, EFS, and overall survival. No study so far has analyzed the influence of concomitant medications and CYP3A inhibitors with ibrutinib. In our series, these factors had no impact, although CYP3A4 inhibitors use correlated with Cox regression analysis, with an increased risk of PDR. Despite the limitation of its retrospective nature, this large study confirmed the role of ECOG-PS as the most accurate predictor of ibrutinib feasibility and outcomes, and importantly, neutropenia emerged as a relevant tool influencing patients’ vulnerability. Although CIRS > 6 retained a significant impact on PFS and EFS, its value should be confirmed by prospective studies.
Introduction
Chronic lymphocytic leukemia (CLL) is a disease of the elderly most frequently diagnosed among people aged 65 to 74 years.1 Aging brings about a reduction of the functional reserves of organs and systems translating to a reduced tolerance to treatment. Although aging and frailty are 2 concepts that have always gone hand in hand, chronological age alone is not a sufficient measure of fitness. Some older patients have no comorbidity and are functionally independent; others struggle with comorbidities, polypharmacy, functional dependence, or problems with cognition or mood.2 Patients of the same age can have a totally different ability to tolerate and benefit from treatment. To assess comorbidities that may reflect patients’ vulnerability toward immunochemotherapy, the cumulative illness rating scale (CIRS) has been the most frequently used in CLL.3 Age and CIRS, together with creatinine clearance (CrCl), given that purine nucleoside analogs are cleared by the kidney, have been used in large randomized trials to determine treatment elegibility.4-6 The most intensive treatments, such as fludarabine, cyclophosphamide, rituximab, bendamustine, and rituximab were considered a standard for the fit population, whereas unfit patients were addressed to low-intensity chemotherapy with chlorambucil in combination with obinutuzumab or ofatumumab.5,6 The first-generation BTK inhibitor (BTKi), ibrutinib, changed the treatment approach in naive and relapsed/refractory CLL. In monotherapy or in combination with monoclonal antibodies, it proved to be superior to comparators in 5 large randomized clinical trials addressed also to the elderly population.7-11 Despite the significant efficacy of ibrutinib, treatment is discontinued in a proportion of patients because of adverse events , and this is even more true in clinical practice where unselected patients are treated.12,13 Although the role of age, performance status (PS), and comorbidities is well known in patients receiving chemotherapy or chemoimmunotherapy, it is not clear whether the same parameters retain their predictive value with target agents. There is only 1 retrospective study on 145 patients specifically addressing the role of fitness on ibrutinib treatment outcome and discontinuation, in which the useful role of CIRS as a predictive tool was underlined.14 Furthermore, no study has analyzed the impact of concomitant medications and coadministration of drugs inhibiting the CYP3A system that may increase ibrutinib plasma levels potentially translating in an excess of toxicity.15 In the present study, we evaluated the impact of age, PS, comorbidities, concomitant medications, and baseline disease characteristics in a large population of patients who received ibrutinib therapy outside clinical trials.
Patients and methods
The population of this study included consecutive patients treated with ibrutinib monotherapy outside clinical trials in 15 Italian centers from March 2014 to May 2020. The study was institutional review board approved by each participating institution in accordance with the Declaration of Helsinki. All patients receiving at least 1 dose of ibrutinib treatment were considered. Medical records were reviewed to determine patients’ characteristics at the time of ibrutinib initiation, including age, Eastern Cooperative Oncology Group (ECOG) PS, CrCl calculated according to the Cockcroft-Gault equation, presence of grade 3 to 4 neutropenia, RAI stage, previous lines of therapy, IGHV mutational status, del(17p) by fluorescent in situ hybridization, and TP53mut by Sanger sequencing. Biological tests were locally performed. Concomitant medications assumed before the BTKi treatment initiation were recorded. As a univocal definition of polypharmacy does not exist, polypharmacy was numerically defined as the concomitant use of >3 prescribed drugs assumed on a regular basis.16 Short-term course reliever drugs were excluded. Among those, we specifically evaluated the administration of CYP3A4 inhibitors according to the ibrutinib brochure (supplement Appendix 1),17 cardiovascular drugs, antiplatelets, and anticoagulant agents.
Furthermore, comorbidities were evaluated at the time of ibrutinib initiation, and Charlson Comorbidity Index (CCI) and CIRS score were calculated (supplemental Appendices 1 and 2).18,19 As performed for other studies, medical conditions that were deemed to be complications of CLL (eg, anemia, thrombocytopenia, and splenomegaly) were not included as part of the total CIRS score.5,14
According to previous CLL studies, patients were considered as having a high comorbidity burden if the total CIRS score was >6. Patients were also assessed for the presence of CIRS3+ defined as a severe impairment (score 3 or 4) in any single organ system.20
The impact of age and patients’ fitness (including ECOG-PS, CIRS, CIRS3+, CCI) on definitive treatment discontinuation owing to toxicity (tox-DTD), permanent dose reduction (PDR), event-free survival (EFS; defined as time from treatment initiation to treatment discontinuation, disease progression, or death), progression-free survival (PFS; defined as time from treatment initiation to disease progression or death), and overall survival (OS; defined as time from treatment initiation to death) was analyzed. Survival functions for the time-to-event variables were estimated by Kaplan-Meier method and the related strata compared using the log-rank test.
To investigate the impact of selected patients’ characteristics (age, patients’ fitness, concomitant medications >3, CYP3A4 inhibitors, history of cardio-comorbidities, CrCl, neutropenia) before starting ibrutinib therapy and of disease characteristics (del(17p), and or TP53mut, IGHV unmutated status, previous lines of therapy) on the time to tox-DTD, PDR, PFS, EFS, and OS, a multiple (multivariable) Cox regression model was fitted. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were obtained for each outcome. Where applicable, the significance level for all analyses was set at α = 0.05. Statistical analysis was performed using the SAS version 9.4 (SAS Institute, Cary, NC).
Results
A total of 712 patients treated with ibrutinib monotherapy outside clinical trials was analyzed. These included 174 treatment-naive (24.4%) and 538 (75.6%) relapsed/refractory patients. Disease and patients’ characteristics before starting ibrutinib are presented in Table 1. Median age was 70.1 years with 68% of patients ≥65 years old. Overall, 15.6% of patients had an ECOG-PS > 1. Median CIRS was 5 with 34% of cases presenting with a high comorbidity burden (>6). In 20.7% of patients, a severe impairment of a single organ system was recorded (CIRS3+); 14.7% of them also had a CIRS > 6. A history of cardiologic disorder was present in 15.9% of cases.
Patients’ and disease characteristics at ibrutinib initiation
| Characteristic . | Value no. (%) . |
|---|---|
| Median age, y (range) | 70.1 (40-95) |
| <65 y/≥65 y | 228 (32.0)/484 (68.0) |
| Sex: male/female | 478 (67.1)/234 (32.9) |
| ECOG-PS 0-1/>1 | 601 (84.4)/111 (15.6) |
| CIRS median (range) CIRS ≤6/CIRS* > 6 | 5 (0-30) 470 (66.0)/242 (34.0) |
| CIRS3+ | 147 (20.7) |
| CIRS* > 6 and CIRS3+ | 105 (14.7) |
| CrCl mL/min ≥50/30-49/<30 | 548 (76.9)/147 (21.6)/17 (2.5) |
| Pts with cardio-comorbidity | 113 (15.9) |
| CCI median (range) CCI < 2/CCI ≥2 | 4 (0-35) 124 (17.4)/588 (82.6) |
| Median no. concomitant medications (range) Pts with >3 concomitant medications Pts treated with CYP3A4 inhibitors | 4 (0-14) 347 (48.7) 75 (10.5) |
| Pts treated with anticoagulants and/or antiplatelets Anticoagulants only Antiplatelets only Anticoagulant + antiplatelets Dual antiplatelet therapy | 165 (23.2) 49 (29.7) 110 (66.7) 6 (3.6) 2 (1.2) |
| RAI stage | |
| 0-2 | 397 (55.8) |
| 3-4 | 315 (44.2) |
| Prior Tx median (range) | 1 (0-10) |
| 0 | 174 (24.4) |
| 1-2 | 420 (59.0) |
| ≥3 | 118 (16.6) |
| IGHV unmutated | 473 (72.7) |
| del(17p) and TP53mut del(17p) TP53mut | 272 (38.7) 211 151 |
| High risk† del(17p) and/or TP53mut and/or unmutated IGHV and/or del(11q) | 580 (81.5) |
| Grade 3 to 4 neutropenia | 67 (9.4) |
| Characteristic . | Value no. (%) . |
|---|---|
| Median age, y (range) | 70.1 (40-95) |
| <65 y/≥65 y | 228 (32.0)/484 (68.0) |
| Sex: male/female | 478 (67.1)/234 (32.9) |
| ECOG-PS 0-1/>1 | 601 (84.4)/111 (15.6) |
| CIRS median (range) CIRS ≤6/CIRS* > 6 | 5 (0-30) 470 (66.0)/242 (34.0) |
| CIRS3+ | 147 (20.7) |
| CIRS* > 6 and CIRS3+ | 105 (14.7) |
| CrCl mL/min ≥50/30-49/<30 | 548 (76.9)/147 (21.6)/17 (2.5) |
| Pts with cardio-comorbidity | 113 (15.9) |
| CCI median (range) CCI < 2/CCI ≥2 | 4 (0-35) 124 (17.4)/588 (82.6) |
| Median no. concomitant medications (range) Pts with >3 concomitant medications Pts treated with CYP3A4 inhibitors | 4 (0-14) 347 (48.7) 75 (10.5) |
| Pts treated with anticoagulants and/or antiplatelets Anticoagulants only Antiplatelets only Anticoagulant + antiplatelets Dual antiplatelet therapy | 165 (23.2) 49 (29.7) 110 (66.7) 6 (3.6) 2 (1.2) |
| RAI stage | |
| 0-2 | 397 (55.8) |
| 3-4 | 315 (44.2) |
| Prior Tx median (range) | 1 (0-10) |
| 0 | 174 (24.4) |
| 1-2 | 420 (59.0) |
| ≥3 | 118 (16.6) |
| IGHV unmutated | 473 (72.7) |
| del(17p) and TP53mut del(17p) TP53mut | 272 (38.7) 211 151 |
| High risk† del(17p) and/or TP53mut and/or unmutated IGHV and/or del(11q) | 580 (81.5) |
| Grade 3 to 4 neutropenia | 67 (9.4) |
Pts, patients; Tx, therapy.
Medical conditions that deemed to be complications of CLL not included as part of the total CIRS score.
High risk defined as del(17p) and/or TP53mut and/or del(11q) and or unmutated IGHV.
Polypharmacy was reported in about half of patients (48.7%). Fifty-five patients (7.7%) were receiving anticoagulants (34 novel oral anticoagulant agents; 19 low-molecular-weight heparin; 2 warfarin), whereas 116 (16.3%) were receiving antiplatelets (dual antiplatelet therapy in 2). Both polypharmacy and use of antiplatelets and/or anticoagulants at univariate analysis were significantly associated with older age (≥65 years) and high comorbidity burden (CIRS > 6, CIRS3+, CCI, P < .0005 in all the analyses). Overall, 75 patients (10.5%) were receiving at least 1 CYP3A4 inhibitor at ibrutinib initiation, and this was significantly related to high CCI and age ≥65 years (P < .0005 and P = .010, respectively). A poor ECOG-PS was not associated with number or type of concomitant medications considered. Ibrutinib was administered in the second or third line in 59% of patients; 81.5% had a high-risk disease defined as the presence of del(17p) and/or TP53mut and/or unmutated IGHV and/or del(11q). At treatment initiation, 9.4% of cases had grade 3 to 4 neutropenia. Although heavily pretreated patients (0-1 vs ≥2) were more likely to present baseline neutropenia (P = .002), the same could not be observed in the elderly (<65 years vs ≥65 years, P = .410).
After a median follow-up of 26.6 months (range, 1-83.8), 440 (61.8%) patients are continuing treatment with the BTKi with a median time on treatment of 23.5 months (range, 1-83.8+). Overall, 272 (38.2%) permanently discontinued ibrutinib: 119 (16.7%) because of toxicity after a median of 10.2 months (range, 0.2-60.7); 130 (18.2%) because of progressive disease/Richter transformation; 23 (3.2%) for other reasons. Main toxicity leading to the 119 tox-DTD was infection in 36 cases (30.3%), 5 of them defined as fungal infections. Among the adverse events of special interest for BTKi, we recorded 20 cardiologic related (16.8%), 9 hemorrhages (8%), and 1 uncontrolled hypertension (0.8%).
In 325 (45.6%) patients, treatment was transiently discontinued for ≥7 days (median, 15 days; range, 7-160). At least 1 dose reduction occurred in 219 (30.8%) patients, toxicity being the main reason in 175 cases. In 123 (17.3%) cases, the BTKi was permanently administered at a lower dosage mostly for recurrent cytopenia (43 cases, 35%), followed by cardiologic toxicity (12 cases, 9.8%) and ibrutinib-drug interference (8 cases, 6.5%).
Cardiologic events leading to tox-DTD and/or PDR are detailed in supplemental Table 1.
Baseline neutropenia at univariate analysis significantly affected infection development, leading to PDR and/or tox-DTD (P = .006), whereas no impact was observed when only fungal infections were considered (P = .679).
As shown in Table 2, age, ECOG-PS, CIRS > 6, CIRS3+, and CCI were all associated with a significantly higher rate of tox-DTD and, all except age, with a permanent ibrutinib dose reduction.
Impact of age, ECOG-PS, CCI, CIRS, CIRS3+ on toxicity-related discontinuation and PDR
| . | Tox-DTD, % . | P . | PDR, % . | P . |
|---|---|---|---|---|
| Age | ||||
| <65 y vs ≥65y | 11 vs 19 | .003 | 15 vs 18 | .086 |
| ECOG-PS | ||||
| 0-1 vs >1 | 13 vs 37 | <.001 | 16 vs 22 | .020 |
| CCI | ||||
| <2 vs ≥2 | 8 vs 18 | .002 | 8 vs 18 | <.001 |
| CIRS | ||||
| ≤6 vs >6 | 13 vs 24 | <.001 | 12 vs 27 | <.001 |
| CIRS3+ | ||||
| No vs yes | 13 vs 29 | <.001 | 15 vs 26 | <.001 |
| Age <65 y | ||||
| CIRS ≤6 vs >6 | 11 vs 13 | .843 | 14 vs 18 | .537 |
| CIRS3+ no vs yes | 9 vs 31 | <.001 | 13 vs 29 | .018 |
| Age ≥65 y | ||||
| CIRS ≤ 6 vs >6 | 11 vs 26 | .020 | 11 vs 29 | <.001 |
| CIRS3+ no vs yes | 16 vs 29 | <.001 | 16 vs 26 | .010 |
| . | Tox-DTD, % . | P . | PDR, % . | P . |
|---|---|---|---|---|
| Age | ||||
| <65 y vs ≥65y | 11 vs 19 | .003 | 15 vs 18 | .086 |
| ECOG-PS | ||||
| 0-1 vs >1 | 13 vs 37 | <.001 | 16 vs 22 | .020 |
| CCI | ||||
| <2 vs ≥2 | 8 vs 18 | .002 | 8 vs 18 | <.001 |
| CIRS | ||||
| ≤6 vs >6 | 13 vs 24 | <.001 | 12 vs 27 | <.001 |
| CIRS3+ | ||||
| No vs yes | 13 vs 29 | <.001 | 15 vs 26 | <.001 |
| Age <65 y | ||||
| CIRS ≤6 vs >6 | 11 vs 13 | .843 | 14 vs 18 | .537 |
| CIRS3+ no vs yes | 9 vs 31 | <.001 | 13 vs 29 | .018 |
| Age ≥65 y | ||||
| CIRS ≤ 6 vs >6 | 11 vs 26 | .020 | 11 vs 29 | <.001 |
| CIRS3+ no vs yes | 16 vs 29 | <.001 | 16 vs 26 | .010 |
To better evaluate the impact of CIRS > 6 and CIRS3+ on tox-DTD and PDR, we stratified patients according to age with a cutoff of 65 years (Table 2). Although in the elderly both CIRS > 6 and the presence of CIRS3+ were significantly associated with the risk of tox-DTD (P = .020 and P < .001, respectively) and PDR (P < .001 and P = .010, respectively), in the younger population, only the presence of a severe organ impairment led to adverse events resulting in both discontinuations and dose reductions (P < .001 and P = .018, respectively).
WhileAlthough tox-DTD negatively affected OS (P < .001) (Figure 1), independently from age (<65 years vs ≥65 years; P = .351), PDR did not have any OS impact (P = .272). Furthermore, no difference in terms of PFS (P = .621) was observed in patients permanently reducing ibrutinib dosage. In our series, patients’ age and CCI did not influence survival outcomes. A high ECOG-PS (>1) was the only parameter significantly associated with a shorter PFS, EFS, and OS (Figure 2).
OS of the 712 patients according to tox-DTD. Overall survival of patients stratified according to the occurrence of discontinuation due to toxicity.
OS of the 712 patients according to tox-DTD. Overall survival of patients stratified according to the occurrence of discontinuation due to toxicity.
Outcomes in terms of PFS, EFS, and OS according to ECOG-PS. Impact of ECOG-PS on PFS (A), EFS (B), and OS (C).
Outcomes in terms of PFS, EFS, and OS according to ECOG-PS. Impact of ECOG-PS on PFS (A), EFS (B), and OS (C).
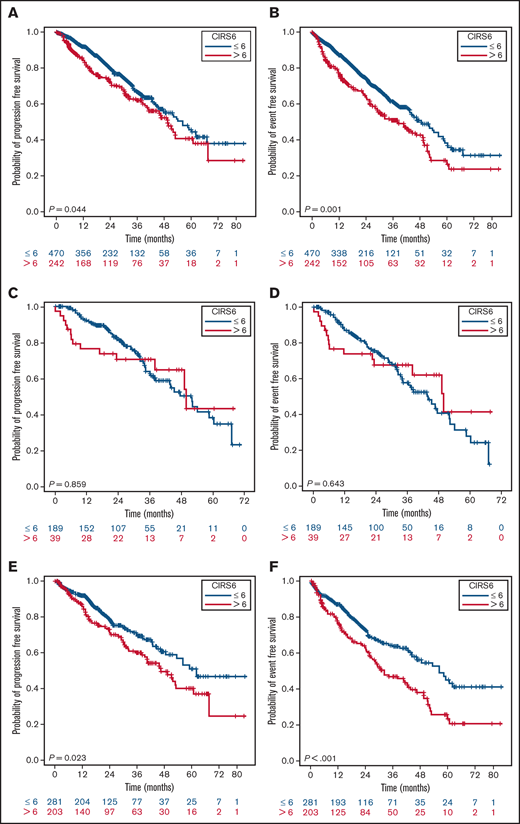
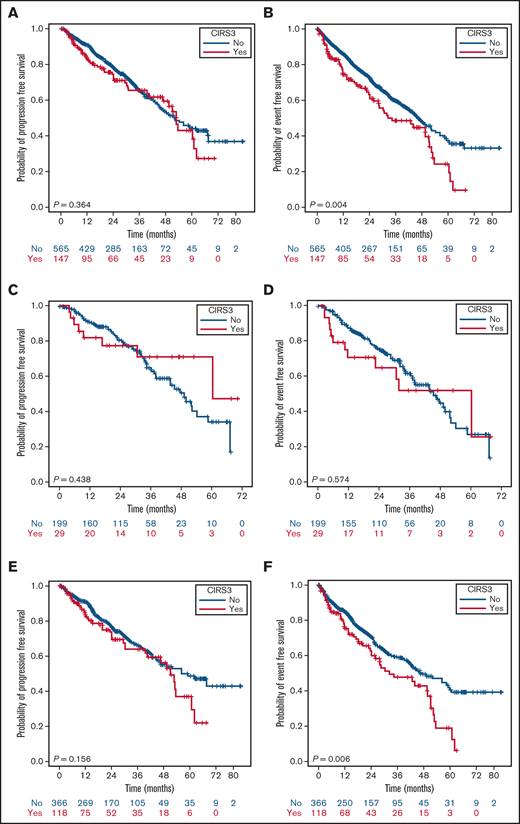
In the entire population, an inferior PFS (P = .044) and EFS (P < .001), but not OS, were observed in patients with a CIRS > 6 (Figure 3; OS curves not shown), whereas the presence of a CIRS3+ altered only EFS (P = .004) (Figure 4). The statistical significance of a high CIRS score was retained only in the elderly when patients were stratified according to the 65-years cutoff (Figure 3).
PFS and EFS stratified by CIRS and age. PFS (A) and EFS (B) of the whole population according to CIRS score; PFS (C) and EFS (D) in patients <65 years according to CIRS score; PFS (E) and EFS (E) in patients ≥65 years according to CIRS score.
PFS and EFS stratified by CIRS and age. PFS (A) and EFS (B) of the whole population according to CIRS score; PFS (C) and EFS (D) in patients <65 years according to CIRS score; PFS (E) and EFS (E) in patients ≥65 years according to CIRS score.
PFS and EFS stratified by CIRS3+ and age. PFS (A) and EFS (B) of the whole population according to CIRS3+; PFS (C) and EFS (D) in patients <65 years according to CIRS3+; PFS (E) and EFS (F) in patients ≥65 years according to CIRS3+.
PFS and EFS stratified by CIRS3+ and age. PFS (A) and EFS (B) of the whole population according to CIRS3+; PFS (C) and EFS (D) in patients <65 years according to CIRS3+; PFS (E) and EFS (F) in patients ≥65 years according to CIRS3+.
Table 3 shows patients’ and disease characteristics independently affecting at least 1 survival parameter and/or treatment management. Among age and patients’ fitness, only ECOG-PS independently affected tox-DTD (HR, 3.30; 95% CI, 2.09-5.20; P < .001), whereas PDR was significantly influenced by CIRS3+ (HR, 1.72; 95% CI, 1.08-2.75; P = .024) and CCI (HR, 3.88; 95% CI, 1.50-10.06; P = .005). A high comorbidity burden correlated with a shorter PFS (HR, 1.48; 95% CI, 1.02-2.15; P = .037) and EFS (HR, 1.44; 95% CI, 1.03-2.00; P = .033), whereas CIRS3+ did not alter any survival outcome. ECOG-PS resulted as the only factor independently affecting PFS (HR, 2.43; 95% CI, 1.72-3.42; P < .001), EFS (HR, 2.63; 95% CI, 1.92-3.61; P < .001), and OS (HR 3.90, 95% CI, 2.61-5.85; P < .001).
Cox proportional regression hazards model of factor on PFS, EFS, OS, Tox-DTD, and PDR
| . | PFS . | EFS . | OS . | Tox-DTD . | PDR . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . |
| Age | 0.82 (0.57-1.18) | .296 | 0.83 (0.59-1.15) | .254 | 0.85 (0.54-1.35) | .496 | 0.91 (0.53-1.54) | .722 | 0.73 (0.45-1.18) | .201 |
| ECOG-PS | 2.43 (1.72-3.42) | <.001 | 2.63 (1.92-3.61) | <.001 | 3.90 (2.61-5.85) | <.001 | 3.30 (2.09-5.20) | <.001 | 1.52 (0.91-2.55) | .112 |
| CIRS6 | 1.48 (1.02-2.15) | .037 | 1.44 (1.03-2.00) | .033 | 1.01 (0.63-1.62) | .964 | 1.33 (0.80-2.21) | .270 | 1.12 (0.70-1.81) | .638 |
| CIRS3+ | 0.79 (0.52-1.19) | .261 | 1.03 (0.71-1.48) | .894 | 0.95 (0.58-1.56) | .844 | 1.54 (0.94-2.51) | .084 | 1.72 (1.08-2.75) | .024 |
| CCI | 1.10 (0.71-1.72) | .662 | 1.19 (0.79-1.78) | .416 | 1.37 (0.75-2.52) | .306 | 1.53 (0.72-3.25) | .268 | 3.88 (1.50-10.06) | .005 |
| Neutropenia | 1.70 (1.09-2.67) | .020 | 1.51 (1.001-2.27) | .049 | 1.72 (1.01-2.91) | .044 | 1.83 (1.04-3.22) | .038 | 1.08 (0.57-2.02) | .814 |
| CYP3A4 | 1.07 (0.66-1.76) | .780 | 1.26 (0.82-1.94) | .285 | 1.09 (0.59-2.03) | .784 | 1.15 (0.59-2.25) | .670 | 2.05 (1.24-3.41) | .005 |
| del(17p) and/or TP53mut | 2.19 (1.57-3.04) | <.001 | 1.78 (1.32-2.40) | <.001 | 2.06 (1.35-3.15) | <.001 | 1.59 (0.98-2.57) | .059 | 0.94 (0.60-1.48) | .800 |
| Lines of previous Tx | 1.85 (1.17-2.95) | .009 | 1.65 (1.10-2.48) | .015 | 2.73 (1.33-5.60) | .006 | 1.80 (0.97-3.34) | .064 | 1.32 (0.79-2.22) | .289 |
| . | PFS . | EFS . | OS . | Tox-DTD . | PDR . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . |
| Age | 0.82 (0.57-1.18) | .296 | 0.83 (0.59-1.15) | .254 | 0.85 (0.54-1.35) | .496 | 0.91 (0.53-1.54) | .722 | 0.73 (0.45-1.18) | .201 |
| ECOG-PS | 2.43 (1.72-3.42) | <.001 | 2.63 (1.92-3.61) | <.001 | 3.90 (2.61-5.85) | <.001 | 3.30 (2.09-5.20) | <.001 | 1.52 (0.91-2.55) | .112 |
| CIRS6 | 1.48 (1.02-2.15) | .037 | 1.44 (1.03-2.00) | .033 | 1.01 (0.63-1.62) | .964 | 1.33 (0.80-2.21) | .270 | 1.12 (0.70-1.81) | .638 |
| CIRS3+ | 0.79 (0.52-1.19) | .261 | 1.03 (0.71-1.48) | .894 | 0.95 (0.58-1.56) | .844 | 1.54 (0.94-2.51) | .084 | 1.72 (1.08-2.75) | .024 |
| CCI | 1.10 (0.71-1.72) | .662 | 1.19 (0.79-1.78) | .416 | 1.37 (0.75-2.52) | .306 | 1.53 (0.72-3.25) | .268 | 3.88 (1.50-10.06) | .005 |
| Neutropenia | 1.70 (1.09-2.67) | .020 | 1.51 (1.001-2.27) | .049 | 1.72 (1.01-2.91) | .044 | 1.83 (1.04-3.22) | .038 | 1.08 (0.57-2.02) | .814 |
| CYP3A4 | 1.07 (0.66-1.76) | .780 | 1.26 (0.82-1.94) | .285 | 1.09 (0.59-2.03) | .784 | 1.15 (0.59-2.25) | .670 | 2.05 (1.24-3.41) | .005 |
| del(17p) and/or TP53mut | 2.19 (1.57-3.04) | <.001 | 1.78 (1.32-2.40) | <.001 | 2.06 (1.35-3.15) | <.001 | 1.59 (0.98-2.57) | .059 | 0.94 (0.60-1.48) | .800 |
| Lines of previous Tx | 1.85 (1.17-2.95) | .009 | 1.65 (1.10-2.48) | .015 | 2.73 (1.33-5.60) | .006 | 1.80 (0.97-3.34) | .064 | 1.32 (0.79-2.22) | .289 |
The presence of del(17p) and/or TP53mut and lines of therapy were both significantly associated with a poorer outcome in terms of PFS, EFS, and OS (Table 3) but did not interfere with drug management. Among all the other factors considered, only grade 3 to 4 neutropenia independently negatively impacted tox-DTD and the 3 survival parameters. Neither age, CrCl, cardio-comorbidity, nor number of concomitant medications influenced survival and ibrutinib management. PDR was associated with concurrent CYP3A4 inhibitors treatment.
Discussion
Many patients with CLL have comorbidities that affect their fitness. Conflicting data are available in the literature as to whether comorbid conditions predict prognosis in patients with CLL.21,22 However, recently, the largest CLL population-based study over a 20-year period highlighted the impact of individual comorbid conditions on both CLL-related and unrelated mortality.23
Although no comorbidity score has been prospectively validated in CLL, more consistent data on the role of CIRS are available. The value of CCI instead needs to be better defined. Nevertheless, in a recent cohort, CLL-related and unrelated mortalities were not associated with CCI score at multivariate analysis.24 More clear is the independent prognostic role exerted by comorbidities on chemotherapy-based treatment outcome.25-28
The relevance of careful evaluation of age, comorbidities, and CrCl to inform the optimal intensity of chemotherapeutic approach has been well established.4-6,29-32 Target therapy has changed the paradigm of CLL treatment. Considering that each agent presents unique and particular toxicities, there can be specific preexisting conditions that contraindicate their use regardless of age and fitness.33-39
Three ibrutinib-based randomized trials have been addressed to the elderly population or patients with coexisting conditions and showed a clear benefit of ibrutinib vs comparator and a favorable toxic profile.8,9,11 Furthermore, a pooled analysis of randomized trials confirmed that benefit was maintained in patients ≥75 years.40 Despite this, none of these studies evaluated whether, among patients treated with ibrutinib, age, PS, and comorbidities may have an impact on treatment management and outcomes.
The role of patients’ fitness may be better evaluated in patients treated outside of clinical trials considering that they are more likely to have poorer PS and more comorbidities and that a proportion of them would have been excluded from prospective trials.12,13,41,42 Notably, the discontinuation rate because of toxicity in our series was lower than reported in other common clinical practice analyses, possibly reflecting the low number of median previous lines of treatment (median 1) and an acquired improved experience in drug management.
In a pooled analysis of 308 patients enrolled in 4 sequential phase 2/3 ibrutinib trials, age emerged as the only significant independent risk factor for nonrelapse discontinuation,43 thus revealing that age-related vulnerability may exist also with target agents. Although in our larger unselected population age affected tox-DTD at univariate analysis, we could not confirm its role at multivariate analysis, thus indicating that not age per se but age-related conditions may influence drug management. Furthermore, age did not influence survival outcomes, suggesting that age alone cannot preclude the therapeutic decision to use ibrutinib.
CIRS represents a common tool of comorbidity assessment to determine eligibility in clinical randomized trials even with target agents.8,44
Although the ECOG-E1912 randomized trial was addressed to a younger and fit population, it is the only prospective study reporting that CIRS score predicts ibrutinib discontinuation for reasons other than PD or death.45 In the retrospective Gordon et al study, a high CIRS correlated with dose reductions, and therapy discontinuations was predictive of reduced OS and EFS even in younger patients (cutoff, 65 years).14 In our larger series, we can confirm the independent role of CIRS > 6 in reducing treatment tolerance, PFS, and EFS but not OS. However, when we stratified patients according to age, comorbidities resulted in being strong predictors of a shorter PFS and EFS only in the elderly. This seems reasonable, considering that this group of patients proved to be the least tolerant to ibrutinib treatment, giving strength to the fact that CIRS may reflect a worse vulnerability in the elderly in the setting of ibrutinib therapy. In the Swedish real-world experience, only on a longer observation (30 months), comorbidities weighed on OS.37,46 In our analysis, after a median follow-up of 26.6 months, the lack of divergence of survival curves does not suggest a similar impact.
Although CIRS in our series confirmed its predictive value on PFS and EFS, CCI did not. Although considered one of the most feasible scales in oncology, our series confirms the limit of CCI. This could be possibly related to the fact that this index is based on binary (yes/no) measures, and it does not necessarily account for the effects of fine gradations of comorbidity severity.
Although CIRS > 6 is emerging as a reliable tool even with ibrutinib, the role of CIRS3+ is still questionable. According to Gordon et al, CIRS3+ is a valuable predictor of survival. Notably, this category seems to be overrepresented (53.8%) in that study.14 Conversely, a severe comorbidity burden, recorded only in 20.7% of this 712-patient series, did not emerge as an independent factor affecting outcome. Noteworthy, a CIRS3+ resulted in the administration of a reduced ibrutinib dosage but not in a higher discontinuation rate because of toxicity. It could be argued that patients undergoing ibrutinib are negatively selected toward those conditions, potentially leading to BTKi-related adverse events of special interest, thus resulting in a bias in the evaluation of the role of CIRS3+.
The UK and Ireland series first outlined the role of a poorer pretreatment PS, as ECOG-PS > 1 was shown to be significantly associated with reduced discontinuation-free survival and OS.42 In our study, the ECOG-PS was shown to play a crucial role in treatment management as a significantly higher rate of patients with a poor level of functioning had to discontinue treatment. Importantly, among patients’ fitness, ECOG-PS was shown to be the best independent predictor of worse survival outcome.
As expected, together with ECOG-PS, when incorporating all the variables into the adjusted Cox model, del(17p) and/or TP53mut and prior lines of therapy showed their influence not only on PFS and EFS, but importantly, also on OS.
Among the other factors considered at baseline, patients with neutropenia more likely experienced toxicity-related discontinuations, as they were more susceptible to the development of major infections. Of importance, in this series, we highlighted the role of neutropenia as a parameter independently influencing all the survival outcomes.
Considering that ibrutinib is primarily metabolized by the CYP3A system,15 it has been suggested that concomitant medications and drug-to-drug interactions may contribute to the risk of an inadequate treatment. Polypharmacy is a common phenomenon increasing with age. This increase in medication use is in turn associated with increased morbidity.47 To our knowledge, this is the first study in which the impact of concomitant medications (>3), administration of drugs interfering with CYP3A, antiplatelet, or anticoagulants has been analyzed. None of these factors were detrimental to patients’ outcome. Not surprisingly, at the Cox regression analysis, the use of CYP3A4 inhibitors correlated with an increased risk of dose reductions, and this could be related to the improved experience in ibrutinib-to-drug interactions management.
Recently, Gordon et al reported on a simplified CLL-specific comorbidity scale, CLL-CI, which required assessment of only 3 organ systems (endocrine, vascular, and upper-gastrointestinal conditions) correlating with survival and tolerance of therapy in ibrutinib-treated patients. They also found that hypertension and cardiac comorbidities did not improve CLL-CI’s discriminatory power.48 Even in our series, cardio-comorbidities did not affect survival outcomes, possibly because of an a priori exclusion and accurate selection of patients before starting ibrutinib treatment.
Although the aim of our study was to evaluate only the variables assessable at baseline affecting survival and treatment management, we analyzed the impact of tox-DTD and PDR on PFS and OS. Importantly, discontinuation related to adverse events led to a worse survival, whereas no effect was seen with PDR, thus demonstrating that dose reductions in the case of ibrutinib intolerance do not have a detrimental implication on outcome. The understanding of management of patients with CLL with comorbidities in the target-agent era remains a challenging issue. Despite the limitation related to its retrospective nature, our study showed that CIRS > 6, even with ibrutinib, remains an informative tool for predicting outcome. Nevertheless, ECOG-PS resulted in being the most accurate predictor of treatment feasibility and outcomes. Greater awareness of the vulnerability of patients with baseline neutropenia should be recommended.
Acknowledgments
The authors thank Antonio Vita, Fondazione Malattie del Sangue, Rete Ematologica Lombarda, and AIRC IG 2018-ID.21352 project (P.S.) for their support.
Authorship
Contribution: A.T. conceived and designed the study, analyzed data, and wrote the manuscript; M.M. and G.D.P. analyzed data and detailed editing and discussion; A.M.F. designed the study, analyzed data, and wrote the manuscript; M.D. and G.Z. collected and analyzed data and wrote the manuscript; F.R.M., A.C., M.C., S.C., G.R., L.L., M.V., R.M., C. Barate, P.S., A.G., C. Borella, V.R., A.B., A.C.P., G.L., C.V., F.M., R. Cassin, A.F., C.C., M.P., C.I., R. Cairoli, and F.D.R. collected data; and all authors critically reviewed the manuscript and approved the final version.
Conflict-of-interest disclosure: A.T. has received honoraria and speakers bureau fees from Janssen, Abbvie, Beigene, and Sunesis. M.C. has membership on an entity's Board of Directors or advisory committees and has received research funding and speakers bureau fees from Abbvie; has received honoraria and has membership on an entity's Board of Directors or advisory committees and speakers bureau for Janssen; has received honoraria and has membership on an entity's Board of Directors or advisory committees for Shire and Gilead; and has received research funding Karyopharm Therapeutics. S.C. has received honoraria from Janssen and support for research funding from Abbvie. G.R. has membership on an entity's Board of Directors or advisory committees for Janssen, Abbvie, and Gilead. L.L. has received honoraria from Abbvie, Roche, Gilead, and Janssen. M.V. serves as consultancy for and has membership on an entity's Board of Directors or advisory committees for Janssen; receives travel/accommodations/expenses from Abbvie; serves as consultancy and has membership on an entity's Board of Directors or advisory committees for Roche. R.M. has membership on an entity's Board of Directors or advisory committees and serves on the speakers bureau for Abbvie, Janssen, and Gilead. P.S. receives honoraria from Abbvie and Janssen. C.V. has received honoraria from Janssen. F.D.R. has received research funding from Gilead and Incyte; has received honoraria from Amgen, Takeda, Novartis; and has received honoraria and fees for consultancy from Celgene, Janssen, GSK, Takeda, and Amgen. M.M. has received honoraria and research funding from Roche; has received honoraria and fees for speakers bureau from Abbvie, Janssen, and Gilead; and has received honoraria from Astrazeneca and Verastem. The remaining authors declare no competing financial interests.
Correspondence: Alessandra Tedeschi, Department of Hematology, Niguarda Cancer Center, ASST Grande Ospedale Metropolitano Niguarda, Piazza Ospedale Maggiore 3, 20162 Milano, Italy; e-mail: alessandra.tedeschi@ospedaleniguarda.it.
References
Author notes
For data sharing, contact the corresponding author: alessandra.tedeschi@ospedaleniguarda.it.
The full-text version of this article contains a data supplement.