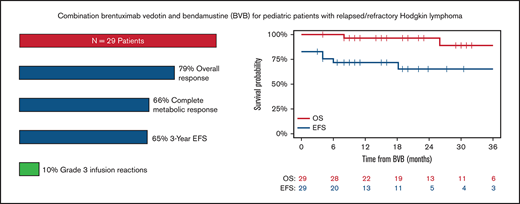
Key Points
BVB was an effective and tolerable retrieval regimen for pediatric patients with R/R HL and resulted in minimal toxicity.
Stem cell mobilization and collection was successful in patients before autologous stem cell transplant.
Abstract
In patients with relapsed/refractory (R/R) Hodgkin lymphoma (HL), achieving a complete metabolic response (CMR) after salvage therapy is associated with superior outcomes, and optimal treatments must be identified. The combination of brentuximab vedotin and bendamustine (BVB), although highly active in adult patients, has not been extensively evaluated in pediatric patients with R/R HL. We performed a multicenter, retrospective review of pediatric patients <21 years of age with R/R HL treated with BVB from January 2016 through July 2019. Response was assessed by local radiologists according to Lugano classification criteria. Twenty-nine patients (17 relapsed, 12 refractory) with a median age of 16 years (range, 10-20) were treated with BVB and received a median of 3 cycles of therapy (range, 2-7). Patients received an infusion of 1.8 mg/kg of BV on day 1 with bendamustine 90 mg/m2 on days 1 and 2 of 3-week cycles. Nineteen patients (66%) achieved a CMR (95% CI, 46-82). An objective response was observed in 23 patients (objective response rate, 79%; 95% CI, 60-92). The most common grade 3 and 4 toxicities were hematologic, and 3 patients (10%) experienced grade 3 infusion reactions. Seventeen of 18 patients underwent successful mobilization and collection of stem cells. Sixteen patients (13 autologous, 3 allogeneic) received a consolidative transplant after BVB. The 3-year post-BVB event-free and overall survival were 65% (95% CI, 46-85) and 89% (95% CI, 74-100), respectively. For pediatric patients with R/R HL, BVB was well tolerated and compared favorably with currently accepted salvage regimens.
Introduction
Hodgkin lymphoma (HL) is one of the most commonly occurring malignancies in adolescents and young adults.1 Overall, the prognosis is excellent with current first-line treatment approaches, but ∼15% of patients still experience relapse and will need salvage therapy.2,3 Most patients responsive to salvage treatments then receive high-dose chemotherapy followed by autologous stem cell transplant (ASCT) which typically provides prolonged disease-free survival.4,5 In patients with relapsed/refractory (R/R) HL, the ability to achieve a complete metabolic response (CMR) before ASCT dramatically affects long-term outcomes.6,7 Therefore, optimal salvage regimens must be identified, as conventional approaches result in complete responses (CRs) in only ∼24% to 52% of pediatric patients.8-11
The emergence of brentuximab vedotin (BV) has altered the landscape of salvage therapy for R/R HL. BV is an antibody-drug conjugate combining an anti-CD30 murine/human chimeric monoclonal antibody covalently linked by an enzyme cleavable peptide to monomethyl auristatin E. A phase 1/2 study in children demonstrated a favorable toxicity profile, compared with conventional myelosuppressive chemotherapy, with an overall response rate of 47%.12 Therapeutic studies evaluating the combination of BV with other chemotherapeutic agents as salvage therapy for R/R HL have been limited.13,14 Recent studies in adults with R/R HL that evaluated the combination of BV with bendamustine, a fusion hybrid molecule containing the purine analogue fludarabine and the alkylating nitrogen mustard, have reported impressive response rates with favorable toxicity profiles compared with traditional salvage regimens.15 -17 However, data on the use of BVB in the pediatric population are limited.18 To obtain a better understanding of its tolerability and efficacy in this population, we conducted a multicenter retrospective analysis of pediatric patients with R/R HL who received salvage treatment with BVB.
Methods
We performed a multicenter retrospective analysis including patient data from 4 academic centers (Cincinnati Children’s Hospital Medical Center, Children’s Healthcare of Atlanta, Children’s Hospital Colorado, and Memorial Sloan Kettering Cancer Center) on patients <21 years of age who were diagnosed with R/R HL and treated with BV and bendamustine as a part of salvage therapy. Refractory disease was defined as failure to achieve a remission or recurrence less than 3 months from completion of frontline chemotherapy. The institutional review boards of participating centers approved the study and patients were identified at individual sites by querying local databases. Data of diagnosis, treatment, outcome, and toxicity were collected locally and submitted for analysis. Positron emission tomography (PET) response was assessed by local radiologists according to Lugano classification criteria. CMR was defined as achievement of a Deauville score of 1, 2, or 3; partial metabolic response (PMR) was defined as a Deauville score 4 or 5 with reduced uptake compared with baseline.
Statistical analysis
The rates of CMR as well as objective response (defined as complete or partial response, i.e., CMR+PMR) were estimated with a 95% exact confidence interval (CI). Overall survival was defined as the time from BVB to death from any cause. Living patients were censored at their date of last follow-up. Event-free survival was defined as the time from BVB treatment to any of the following events: disease progression, start of a new therapy (other than ASCT), or death from any causes, whichever occurred first. Patients without events were censored at their date of last follow-up. Patients with unknown date of relapse were considered relapsed at time 0.
Results
Patient characteristics
Baseline patient characteristics are presented in Table 1. We identified twenty-nine patients (12 females and 17 males) with relapsed (n = 17) or refractory (n = 12) classic HL, who received salvage therapy with BVB. One patient with relapsed disease was initially diagnosed and treated for primary mediastinal B-cell lymphoma before experiencing a subsequent relapse in which repeat biopsy demonstrated nodular sclerosing HL. The median age at initial diagnosis was 15 years (range, 7-20 years). On initial presentation, there were 15 patients (52%) with stage II, 7 patients (24%) with stage III, and 5 patients (17%) with stage IV disease and 2 patients (7%) in whom the initial staging was unknown. Fourteen patients (48%) had bulky disease and 18 (62%) had B symptoms. The most common frontline therapy was doxorubicin, bleomycin, vincristine, etoposide, prednisone and cyclophosphamide (ABVE-PC) in 15 patients (52%). Thirteen patients (45%) received consolidative radiation therapy as a part of their initial therapy. After frontline therapy 11 (42%) patients initially achieved a CR, 3 patients (12%) had a partial response (PR), and 12 patients (46%) were classified as refractory. Initial response was not available for 3 patients. Relapse occurred less than 12 months from the end of initial therapy in 12 of 17 patients (71%).
Patient characteristics at initial diagnosis
| Characteristics at diagnosis . | Data (n = 29) . |
|---|---|
| Age, median (range) | 15 (7-20) |
| Female (%) | 12 (41) |
| Male (%) | 17 (59) |
| Disease stage at diagnosis, n (%) | |
| II | 15 (52) |
| III | 7 (24) |
| IV | 5 (17) |
| Unknown | 2 (7) |
| Baseline disease characteristics, n (%) | |
| Bulk | 14 (48) |
| B symptoms | 18 (62) |
| Extranodal disease | 4 (14) |
| Frontline treatment, n (%) | |
| ABVE-PC ×4-6 | 15 (52) |
| OEPA-COPDAC/OPPA-COPP | 4 (14) |
| ABVD/A-AVD ×6 | 3 (10) |
| ABVD ×3-4 | 2 (7) |
| Other | 5 (17) |
| Radiation therapy | 13 (45) |
| Characteristics at diagnosis . | Data (n = 29) . |
|---|---|
| Age, median (range) | 15 (7-20) |
| Female (%) | 12 (41) |
| Male (%) | 17 (59) |
| Disease stage at diagnosis, n (%) | |
| II | 15 (52) |
| III | 7 (24) |
| IV | 5 (17) |
| Unknown | 2 (7) |
| Baseline disease characteristics, n (%) | |
| Bulk | 14 (48) |
| B symptoms | 18 (62) |
| Extranodal disease | 4 (14) |
| Frontline treatment, n (%) | |
| ABVE-PC ×4-6 | 15 (52) |
| OEPA-COPDAC/OPPA-COPP | 4 (14) |
| ABVD/A-AVD ×6 | 3 (10) |
| ABVD ×3-4 | 2 (7) |
| Other | 5 (17) |
| Radiation therapy | 13 (45) |
A-AVD, brentuximab vedotin, doxorubicin, vinblastine, dacarbazine; ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; COPDAC, cyclophosphamide, vincristine, prednisone, dacarbazine; COPP, cyclophosphamide, vincristine, procarbazine, prednisone; OEPA, vincristine, etoposide, prednisone, doxorubicin; OPPA, vincristine, procarbazine, prednisone, doxorubicin.
Treatment response
Characteristics at relapse are presented in Table 2. After the diagnosis of relapse or refractory disease 21 patients (72%) received BVB as first line salvage therapy. Five patients (17%) had received BV, either as a part of initial therapy (n = 1) or as salvage therapy (n = 4). Four patients (14%) had previously undergone consolidation with high-dose chemotherapy and ASCT. Patients received an infusion of 1.8 mg/kg of brentuximab vedotin on day 1 with bendamustine 90 mg/m2 on day 1 and 2 of 3-week cycles. One patient received an alternate BVB regimen in which BV was administered at a dose of 1.8 mg/kg on day 1, and bendamustine was administered on days 2 and 3 at a dose of 120 mg/m2. Twenty-six patients (90%) received premedication before each cycle of BVB to decrease the risk of infusion-related reactions (IRR): diphenhydramine/glucocorticoid ± acetaminophen (n = 14), diphenhydramine/acetaminophen (n = 8), glucocorticoid/loratadine (n = 2), diphenhydramine alone (n = 1), and glucocorticoid alone (n = 1). The median age at the time of BVB treatment was 16 years (range, 10-20). Patients received a median of 3 cycles of BVB (range, 2-7 cycles). In total, 19 of 29 (66%; 95% CI, 46-82) patients achieved a CMR as determined by PET. An objective response was observed in 23 patients (objective response rate [ORR], 79%; 95% CI, 60-92). Of note, 1 patient had a PR after 2 cycles of BVB, but had an IRR attributed to BV. This individual achieved a CMR after an additional 2 cycles of single-agent bendamustine. CMR and ORR in patients receiving BVB as the first-line salvage therapy were 67% (95% CI, 43-85) and 81% (95% CI, 58-95) in comparison with those receiving it as a second-line therapy or greater, where CMR and ORR were 62% (95% CI, 24-91) and 75% (95% CI, 35-97), respectively. Response rates were comparable among patients with relapsed (CMR, 71%; 95% CI, 44-90, and ORR, 82%; 95% CI, 57-96) and refractory disease (CMR, 58%; CI, 28-85; ORR, 75%; 95% CI, 43-95). Among responders, 15 (79%) achieved best response within 2 cycles. Responses were also observed in patients who received prior treatment with ASCT or BV. This group included 3 patients who underwent ASCT and treatment with BV (2 CMR, 1 PMR), 1 patient with prior ASCT (CMR), and 1 patient treated with prior BV (PMR). Deauville scores were available for 27 of 29 patients. The median Deauville score for all patients was 2, with 14 of 18 responders (78%) having a Deauville response of 1 to 2.
Patient characteristics at relapse
| Characteristics at relapse . | Data (n = 29) . |
|---|---|
| Age at R/R disease, median y, (range) | 16 (10-20) |
| Refractory disease | 12 |
| Relapsed disease | 17 |
| Time to initial relapse, median mo (range)* | 9.5 (4-31) |
| Relapse ≤12 mo, n (%) (n = 17) | 12 (71) |
| Prior salvage attempts, n (%) | |
| 0 | 21 (72) |
| ≥1 | 8 (28) |
| Prior BV | 4 (14) |
| Prior stem cell transplant | 4 (14) |
| Characteristics at relapse . | Data (n = 29) . |
|---|---|
| Age at R/R disease, median y, (range) | 16 (10-20) |
| Refractory disease | 12 |
| Relapsed disease | 17 |
| Time to initial relapse, median mo (range)* | 9.5 (4-31) |
| Relapse ≤12 mo, n (%) (n = 17) | 12 (71) |
| Prior salvage attempts, n (%) | |
| 0 | 21 (72) |
| ≥1 | 8 (28) |
| Prior BV | 4 (14) |
| Prior stem cell transplant | 4 (14) |
Includes patients with relapsed disease, except for 1 patient for whom the exact time to initial relapse was unknown (n = 16).
Toxicity
Overall, BVB proved to be a well-tolerated outpatient regimen. Two patients (7%) discontinued therapy secondary to toxicity. The most common grade 3 or 4 adverse events were hematologic and included neutropenia (grade 3, n = 9; grade 4, n = 4), anemia (grade 3, n = 4), and thrombocytopenia (grade 3, n = 3; grade 4 = 1). Two patients experienced grade 3 hyperbilirubinemia. Additional notable adverse events included nausea and vomiting (grade 2, n = 1), leading to discontinuation of treatment, culture-negative sepsis (grade 3, n = 1), and pericardial effusion (grade 3, n = 1). There were no reports of significant neuropathy or transaminitis. Three patients experienced grade 3 IRR, including 1 patient who did not receive premedication. Subsequent to IRR, 1 patient continued treatment with BVB after a desensitization protocol; in another, the reaction was believed to be primarily related to BV, and treatment was continued with single-agent bendamustine.
Stem-cell mobilization and ASCT
Eighteen patients underwent mobilization and collection of CD34+ stem cells after treatment with BVB. One patient receiving BVB as second-line therapy (previously treated with 5 cycles of ABVE-PC) had an insufficient number of stem cells collected after 2 attempts and ultimately declined ASCT, despite plans for future attempts. Mobilization was achieved with granulocyte colony-stimulating factor (GCSF) alone in 4 patients, plerixafor alone in 3 patients, and GCSF+plerixafor in 10 patients. The median number of stem cells collected was 5.4 × 106 cells per kilogram. Three patients needed >1 mobilization event.
Of 23 patients who demonstrated a response to BVB, 13 (12 CMR, 1 PMR) went on to consolidation with an ASCT. Three patients underwent consolidation with an allogeneic transplant because of a history of ASCT. Reported reasons for not having a consolidative ASCT after CMR included being transplant ineligible (n = 1) and decision of the patient or family (n = 2). Conditioning regimens before ASCT varied according to institution and included cyclophosphamide, carmustine, and etoposide (CBV; n = 5); carmustine, etoposide, cytarabine, and melphalan (BEAM; n = 4); gemcitabine, busulfan, melphalan, and vorinostat (n = 3); and gemcitabine, busulfan, and melphalan (n = 1). A subset of patients received additional consolidation therapy after ASCT with radiation therapy (n = 5) or BV (n = 10).
Outcomes
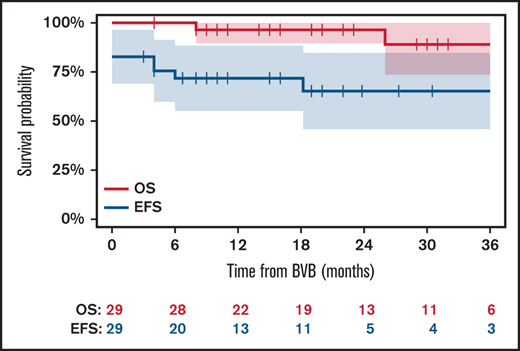
The 3-year post-BVB event-free and overall survival were 65% (95% CI, 46% to 85%) and 89% (95% CI, 74% to 100%), respectively (Figure 1). Two patients died secondary to disease progression, and another died secondary to complications related to allogeneic transplant. After CMR was not achieved with BVB, 3 of 10 patients had a CMR after salvage therapy: ifosfamide, carboplatin, and etoposide (ICE; n = 1), pembrolizumab (n = 1), and allogeneic transplant (n = 1).
Post-BVB event-free and overall survival. EFS, event-free survival; OS, overall survival.
Post-BVB event-free and overall survival. EFS, event-free survival; OS, overall survival.
Discussion
The combination of BVB proved to be a highly effective and well-tolerated salvage regimen for this multi-institutional cohort of pediatric patients. This study included 29 patients: 17 with relapsed disease and 12 with refractory disease, from 4 academic centers. Complete metabolic responses were achieved in 66% of patients and enabled 16 patients to proceed to consolidation with transplant. Notably, there were comparable responses in patients receiving BVB for relapsed or refractory disease, as well as those receiving it as first-line salvage therapy or later.
Primary progressive or refractory disease, time to relapse (<12 months), and chemoresistance have been identified as poor prognostic factors at the time of relapse.8 More recently, functional imaging before ASCT has been noted to dramatically affect posttransplant outcomes, with patients achieving negativity according to PET with a long-term relapse-free survival of ∼75% compared with ∼25% in patients with metabolically active disease. Therefore, normalization of pretransplant functional imaging should be the goal of salvage therapy.6,7,19
Currently, there is no standard salvage regimen for pediatric and adolescent patients with relapsed/refractory HL. Recent trials have sought to optimize response and CMR rates, while trying to minimize additional toxicity, both short and long-term, before ASCT. Regimens such as gemcitabine/vinorelbine and ifosfamide/vinorelbine, with or without bortezomib, demonstrated comparable responses and are therefore preferred among pediatric oncologists compared with the more toxic regimens, such as ICE. The complete response rates observed in all these studies are between 24% and 52%.9-11,20,21
The use of BV as a single agent for R/R HL proved to be safe and effective for heavily pretreated adults and children, resulting in CMR rates of 34% and 33%, respectively.12,22 Subsequent studies have sought to combine BV with traditional cytotoxic chemotherapy to further improve responses. One of the first pediatric studies to investigate such an approach was sponsored by the Children’s Oncology Group (COG) and sought to evaluate the effectiveness of combination therapy with BV and gemcitabine in patients <30 years of age with R/R HL. The median age of patients enrolled in this trial was 17.6 years, and the combination resulted in an ORR of 74% with a promising CMR rate of 67% after 4 cycles of therapy.13
Like BV, bendamustine has been shown to be highly potent as a monotherapy in heavily pretreated patients with R/R HL, making these agents an attractive combination for salvage therapy.23-27 Two large phase 2 studies have been conducted to date involving adult patients receiving BVB for first relapse of HL or initial salvage therapy for refractory disease and reported ORR and CMR rates of 84.2% to 92.5% and 73.6% to 78.9%, respectively.15,17 O’Connor et al included a more heavily treated adult population with patients treated in the phase 2 portion having a median of 3 prior therapies and 8% having received prior BV. The reported ORR and CMR rates in this trial were 78% and 43%, respectively.16 Although therapy in all of these studies was well tolerated, the incidence of IRRs was the most notable nonhematologic toxicity. LaCasce et al reported IRR in 56.4% of patients with the introduction of premedication, including high-dose corticosteroids and antihistamines, which appeared to dampen the severity of these reactions if not the overall incidence.15 Reported IRRs were less prevalent in the remaining trials (10.8-13.1%). Overall, these studies supported the tolerability and potency of this regimen in heavily pretreated and newly relapsed adult patients with HL.
The data for the use of BVB in pediatric patients are limited. The COG recently completed a trial investigating BVB in pediatric patients after suboptimal response to initial salvage with nivolumab and BV (registered on www.clinicaltrials.gov, as NCT02927769). Early results are promising, with all patients (n = 6) with residual metabolically active disease after initial reinduction with nivolumab and BV achieving CMR after BVB.28 Most recently, McMillan et al published their single-center experience with BVB in pediatric and young adult patients in which the median age of patients treated was 21 years and CMR and objective response rates were 79% and 83%, respectively.18
Our study represents the first multicenter effort to evaluate the safety and efficacy of BVB in a true pediatric cohort of patients (median age at time of BVB, 16 years). We are unable to make direct comparisons of our results with those of previously published treatments for children, but these initial results compare favorably with those of existing salvage regimens. In addition, the combination of BVB was primarily administered in the outpatient setting and with minimal toxicity. In our study, only 10% of patients experienced grade 3 or 4 IRRs, but it remains advisable that any future trials in which BVB is used follow premedication guidelines. Last, with the assistance of either GCSF or a combination of plerixafor/GCSF, nearly all patients underwent successful mobilization and collection of stem cells in preparation for ASCT.
Data on the long-term toxic effects from BVB are limited, but the use of bendamustine, a bifunctional alkylating agent, raises the concern for treatment-related secondary malignant neoplasms (SMNs). Studies of the use of bendamustine in the treatment of recurrent non-Hodgkin lymphomas have contributed to these concerns, but the risk remains unclear.29-31 Pooled analyses have suggested that exposure to bendamustine may be associated with cumulative incident rates of SMNs as high as 6.2%; however, these findings are confounded by patients’ prior exposure to multiple prior lines of therapy and subsequent hemapoietic stem cell transplantation conditioning regimens, including other alkylating agents.32 Clinical trials evaluating the use of BVB for relapsed or refractory HL have not demonstrated an increased rate of SMN in this population, but follow-up on these trials was relatively short.15,16 Additional long-term toxicity data are needed to clarify this risk, but with nonalkylating–based regimens for pediatric patients, such as BV/gemcitabine, having comparable response rates, these potential effects should be taken into consideration when selecting a salvage regimen.
This study is limited by the retrospective nature of the analysis, but provides important data on the tolerability and usefulness of this regimen before autologous or allogeneic stem cell transplant and compares favorably with previously published pediatric studies. Although the risks of IRR are legitimate, they appear manageable relative to some of the high rates of IRRs noted in prior studies in adults. The use of BV in frontline therapy is increasing, and it remains to be seen how the efficacy of this regimen will be affected by prior exposure to BV. These results support further investigation of these agents in the context of large, multicenter, prospective trials involving pediatric patients.
Acknowledgments
The authors thank Joseph Olechnowicz for editorial assistance.
This work was supported by the National Institutes of Health, National Cancer Institute Cancer Center Support Grant P30 CA008748.
Authorship
Contributions: C.J.F., N.G., A.M., M.J.A., S.M.C., A.F., F.G.K., and N.S. all substantially contributed to conception and design of the work; acquisition, analysis, and interpretation of the data; drafting and critical review and revision of the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christopher J. Forlenza, Department of Pediatrics, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: forlenzc@mskcc.org.
References
Author notes
For original data, please contact the corresponding author (forlenzc@mskcc.org). Individual participant data will not be shared.